Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aneurysms occurring in the arteries of the lower extremity are second in frequency only to aneurysms of the infrarenal aorta and iliac arteries. Historically, lower extremity aneurysms were typically mycotic, syphilitic, or traumatic in origin. However, most aneurysms in the femoral, popliteal, and tibial arteries are currently either degenerative aneurysms or posttraumatic pseudoaneurysms related to catheterization or instrumentation. True aneurysms occur far more commonly in men than in women, at a ratio of 30:1. , Furthermore, true aneurysms of the femoral or popliteal arteries are often associated with aortic aneurysms and aneurysms in the contralateral lower extremity. The association with aortic aneurysms ranges from 50% to 90% for femoral aneurysms, from approximately 30% to 50% for popliteal aneurysms, and as high as 70% for bilateral popliteal aneurysms. , , , Bilaterality is also common, occurring in approximately 25% to 50% of femoral aneurysms and 50% to 70% of popliteal aneurysms. , , Conversely, up to 14% of men with aortic aneurysms have been found to have femoral or popliteal aneurysms, but this combination is a rare finding in women. Additional imaging, such as duplex ultrasound (DUS), may therefore be indicated to evaluate for concomitant aneurysmal disease when an aortic or lower extremity aneurysm is diagnosed.
Aneurysms in the lower extremity are of clinical significance because of their potential to cause limb-threatening ischemia. More rarely, such aneurysms can rupture, particularly false aneurysms of the common femoral artery. Treatment is preferable when they are asymptomatic – hence the importance of detection and a thorough knowledge of the association with other aneurysms. Symptomatic true aneurysms have a much higher incidence of limb loss because of the prevalence of embolization of thrombus from within the aneurysm sac to more distal arteries. Clinicians must always consider embolization from an aneurysm when evaluating lower extremity ischemia and when planning interventions. There have been significant changes in the treatment of lower extremity aneurysms, along with additional treatment options, including various endovascular therapies.
Femoral artery aneurysms occur primarily in the common femoral artery and less commonly in the profunda femoris and superficial femoral arteries. The common femoral artery (CFA) can be the site of true aneurysms and of pseudoaneurysms related to prior instrumentation or previous revascularization procedures. True aneurysms are less common and are often associated with popliteal artery and aortic aneurysms. In any artery, an aneurysm is defined as a focal, fusiform dilation of the artery to 1.5 times the normal diameter of the adjacent segment of artery. The normal size of a CFA in men is approximately 1.0 cm, and 0.8 cm in women. Repair has been considered clinically indicated for aneurysms more than 2.5 cm in diameter, but data from 2014 has 3.5 cm as an indicated diameter for repair. ,
Pseudoaneurysm of the CFA usually appears as a saccular outpouching of the vessel and has a narrow neck representing a defect in the vessel wall due to instrumentation. The pseudoaneurysm is actually a rim of fibrous tissue containing thrombus and arterial flow in continuity with a defect in the CFA. Iatrogenic and mycotic pseudoaneurysms are addressed separately from true aneurysms.
In a large study of true aneurysms of the femoral arteries, 57% were in the CFA, 26% in the superficial femoral artery (SFA), and 17% in the profunda femoral artery (PFA); 26% were bilateral and 48% were associated with additional aneurysms. They are found predominantly in older men (70 years or older) and are associated with smoking and hypertension. The majority are degenerative, but they have also been reported with atriomegaly and other conditions such as Behçet disease, Parkes Weber syndrome, and Wegener granulomatosis.
In addition, a recent study on true aneurysms in the femoral arteries by Lawrence et al., in which eight institutions pooled data in the Vascular Low Frequency Disease Consortium, showed similar results, with a higher percentage (81%) in the CFA, and lower percentage (14%, 5%) in the SFA and PFA, respectively.
Isolated true femoral aneurysms are asymptomatic in 30% to 40% of patients, and are often found on physical examination or DUS scan. Another 30% to 40% cause localized pain and tenderness or compressive symptoms, resulting in neuropathic pain or leg edema. The most common presentation in up to 65% of cases is lower extremity ischemia, including claudication or critical ischemia resulting from embolization. , , Rupture is a rare occurrence – occurring in approximately 4% of cases. Duplex ultrasound is the modality of choice for diagnosis and assessment of femoral artery aneurysms. It is cost-effective and accurate. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) can also be used and may be of additional value in the planning of endovascular repair and when specific measurements are needed to identify normal artery proximal and distal to the identified aneurysm. CTA and MRA can also be useful to look for additional aneurysms such as aortic, iliac artery, contralateral femoral artery, and popliteal artery aneurysms (PAAs).
All symptomatic femoral aneurysms should be treated to prevent embolization, thrombosis, worsening of local compressive symptoms, and rupture. Although the natural history of asymptomatic femoral artery aneurysms is unclear, it had previously been suggested that the repair of asymptomatic femoral aneurysms more than 2.5 cm diameter is indicated in “good-risk” patients. This suggestion is based on one of the largest reported series of 172 femoral aneurysms in 100 patients; 40 were asymptomatic, and 105 small (<2.5 cm) aneurysms were managed nonoperatively. In the more recent study by Lawrence et al., it was noted that acute complications such as ischemia or rupture did not occur in patients with asymptomatic femoral artery aneurysms less than or equal to 3.5 cm. The size threshold for elective repair may be reduced in the presence of intraluminal thrombus. Smaller asymptomatic aneurysms can be managed with observation by duplex scan and do not require intervention unless they expand or become symptomatic.
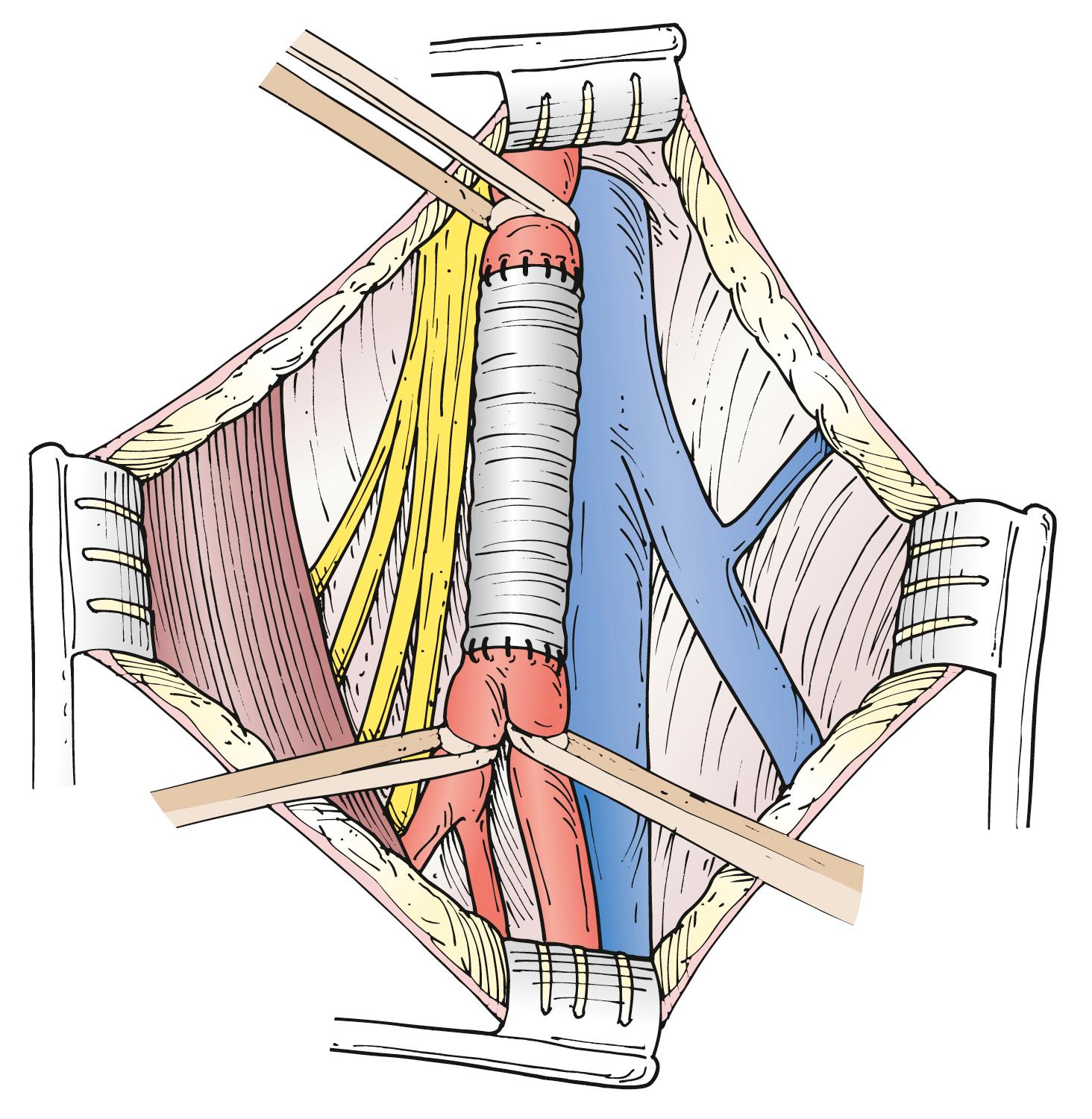
Treatment of CFA true aneurysms usually consists of open repair, in which the aneurysm is excluded with an interposition graft. To prevent injury to surrounding structures that may be adherent to it, the aneurysm sac is usually not resected. The preferred graft material is synthetic – either polytetrafluoroethylene (PTFE) or Dacron. , , The prosthetic grafts are better size matches and have equivalent or better patency rates than vein grafts in this positon. Preoperative assessment of the aorta and the iliac, superficial femoral, and popliteal arteries should be performed to detect additional aneurysmal disease. In both elective procedures and emergency procedures for rupture, open repair can be achieved with minimal morbidity and excellent long-term patency. , , Proximal control can usually be achieved with a clamp on the distal external iliac artery or proximal CFA via a longitudinal groin incision. In cases of large aneurysms, proximal control can be achieved with balloon occlusion placed via the contralateral femoral approach. If this is not possible, a suprainguinal retroperitoneal exposure of the external iliac artery may rarely be required to obtain proximal control of a large, proximal CFA aneurysm.
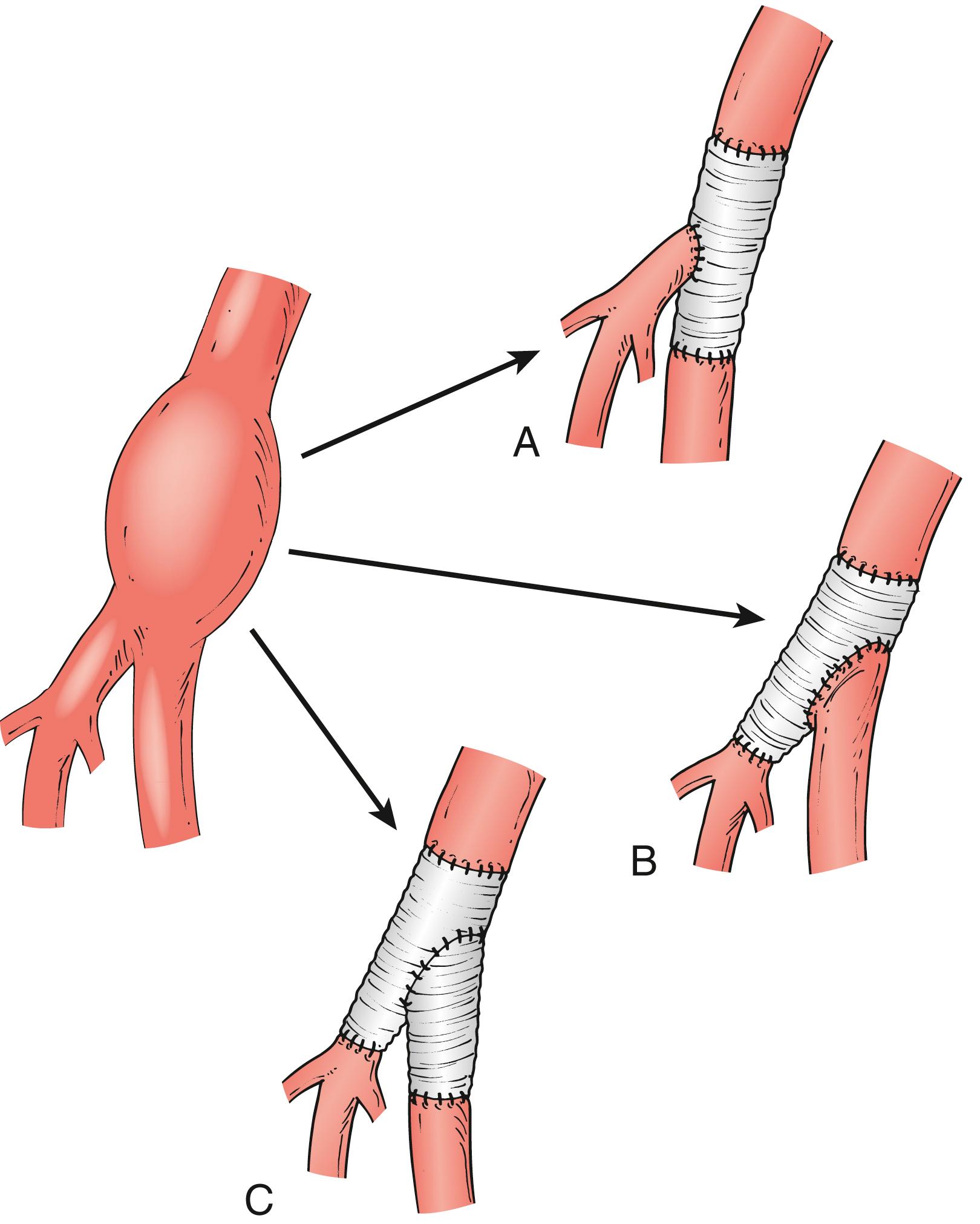
An aneurysm confined to the CFA can be treated with a short interposition graft ( Fig. 85.1 ). An aneurysm extending into the PFA or SFA requires more complex reconstruction with an interposition graft from the proximal CFA to the PFA or SFA, and a jump graft to the other femoral branch ( Fig. 85.2 ). It is important to preserve flow to the PFA to prevent future severe ischemia.
Endovascular treatment of CFA aneurysms has largely been limited to emergency situations or performed as part of a hybrid approach with an open surgical procedure. Bakoyiannis et al. described such a procedure using a stent-graft to exclude a distal external iliac artery aneurysm and a traditional Dacron graft for the femoral portion of a large aneurysm extending from the distal external iliac artery into the common femoral artery. Percutaneous endovascular treatment of a ruptured femoral artery aneurysm has also been described. Contralateral femoral access is often required in these cases for delivery of the stent-graft. However, caution should be used in the consideration of placement of a stent-graft in the CFA, particularly when it crosses the inguinal ligament, because of possible stent fracture or dislodgement in this location.
Endovascular techniques can be used as adjuncts to open procedures. For example, proximal control can be obtained via a balloon placed under fluoroscopic guidance through the opened aneurysm sac in standard surgery, or percutaneously via the contralateral CFA when necessary.
Pseudoaneurysms in the CFA occur most commonly from iatrogenic causes such as instrumentation for cardiac or peripheral catheterization and after open reconstructions. , Iatrogenic false aneurysms can be treated with open surgery or ultrasound-guided therapy, or with observation alone if they are small in diameter. Pseudoaneurysms may also occur as a result of infection that causes focal erosion of the arterial wall, usually from external sources such as contaminated needles used during intravascular substance abuse. The management of infected pseudoaneurysms is difficult and requires ligation either alone or with extra-anatomic bypass. , Infected aneurysms are discussed in detail in Ch. 49 (Graft Infection). Management of anastomotic pseudoaneurysms is discussed in Ch. 50 (Anastomotic Aneurysms).
The most common clinical manifestation of a femoral pseudoaneurysm is a painful pulsating mass in the groin, usually with an associated hematoma. Femoral bruits are heard frequently, and if an arteriovenous fistula is present, the bruit has a characteristic continuous to-and-fro quality. Patients may also complain of femoral neuropathic pain, paresis of hip flexion, or edema from compression of adjacent structures. Progressive enlargement can result in overlying skin ischemia and necrosis. In our experience, distal embolization or limb ischemia is rare. Rupture can lead to cardiovascular collapse and death from hemorrhagic shock and is a surgical emergency. Significant bleeding can occur without overt clinical findings if the initial puncture is in the external iliac artery or through the inguinal ligament, and results in occult bleeding into the retroperitoneal space.
Aneurysm size and the presence of anticoagulation determine which pseudoaneurysms require treatment. Most aneurysms less than 2 or 3 cm in diameter undergo spontaneous thrombosis and may be safely observed with periodic DUS examinations. Continued anticoagulation therapy greatly decreases the likelihood of spontaneous closure of such a pseudoaneurysm. , In one prospective series, 18 pseudoaneurysms were identified after 1838 femoral catheterizations over an 8-month period (0.98% incidence). Aneurysms that were less than 1.8 cm in diameter and causing no symptoms were observed. Surgical repair was carried out if the aneurysms enlarged by 100% or did not close within 2 months. Of the 16 that were managed expectantly, half underwent spontaneous thrombosis and the other half required repair. Although the difference did not reach statistical significance, the likelihood of spontaneous closure was lower for aneurysms more than 1.8 cm in diameter, and no aneurysm closed spontaneously in the presence of continued anticoagulation, regardless of size.
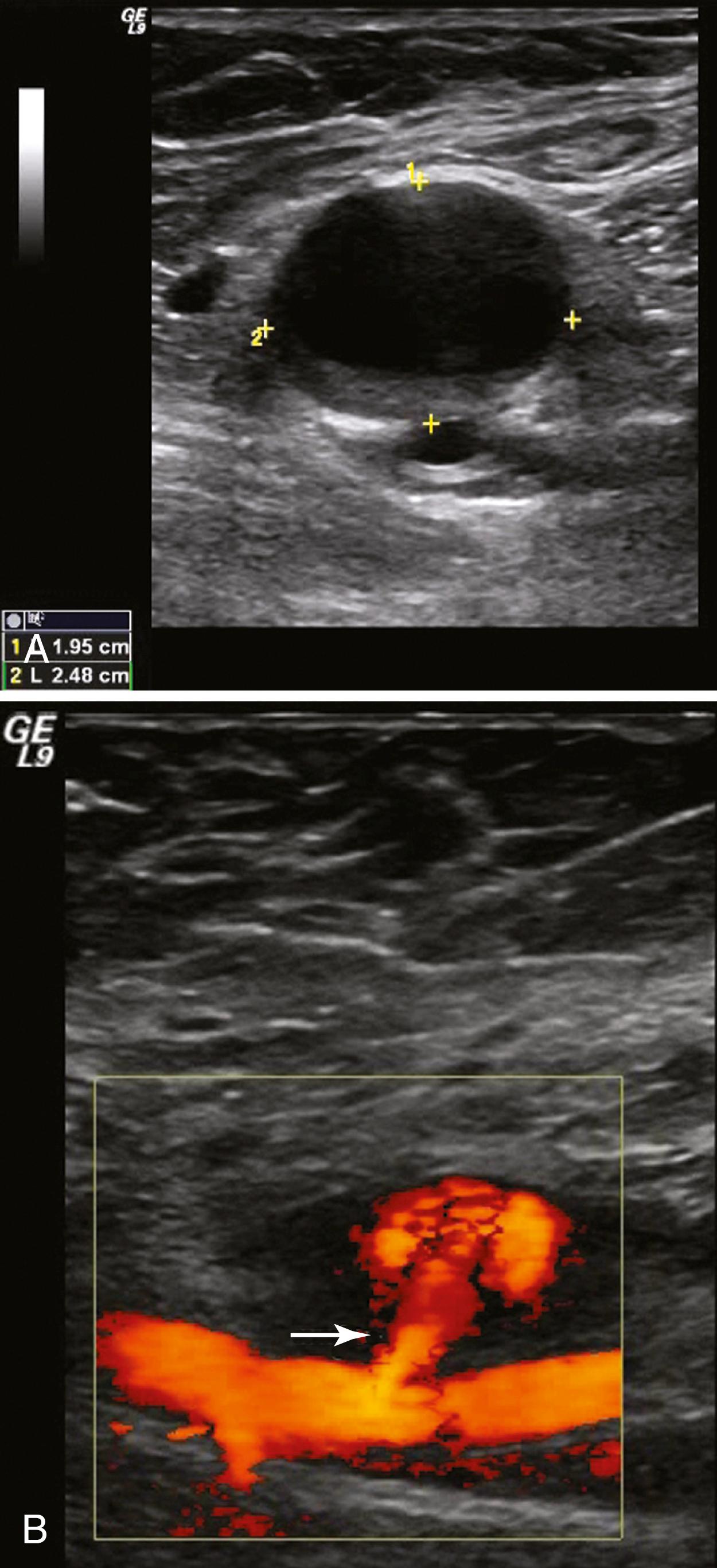
Duplex ultrasound is the preferred imaging method. The sensitivity and specificity of DUS for femoral false aneurysms are 94% and 97%, respectively. Duplex ultrasound also provides important information on diameter, morphology, and anatomy of the neck and location of the femoral artery defect. On B-mode imaging, an aneurysm appears as a characteristic echolucent mass adjacent to the femoral artery and a channel, usually narrow, with arterial flow into the mass ( Fig. 85.3 ).
Compression of pseudoaneurysms to induce thrombosis was proposed as a less invasive alternative to surgery in 1991. Duplex ultrasound is used to locate the aneurysm, and suitable pressure is applied with the transducer to stop flow within the aneurysm cavity while maintaining flow in the adjacent femoral artery. Excessive pressure can result in femoral artery thrombosis. Compression is maintained for 10 to 20 minutes, after which time flow is reassessed. If flow persists, the procedure is repeated one or more times until flow within the aneurysm cavity is noted to have stopped. The patient is kept on bed rest for 6 hours, being reevaluated with DUS at 24 and 48 hours after the procedure to rule out recurrence. If the procedure is performed in the outpatient setting, the patient may be discharged home after the initial 6 hours.
Success rates for ultrasound-guided compression (UGC) range from 66% to 86%, with required compression times averaging 30 to 44 minutes. Recurrence has been reported in 4% of cases. The presence of anticoagulation greatly reduces the likelihood of success to less than 40%. UGC is contraindicated in patients with ischemic skin changes or infection, and when the puncture site originates above the inguinal ligament. Moreover, UGC is not usually possible in patients with severe pain and very large hematomas. Imaging and compression are technically more difficult in obese patients, thus decreasing the likelihood of success. Aneurysm rupture, femoral vein thrombosis, limb ischemia from femoral arterial thrombosis, and hypotension from vasovagal events are all potential complications that have occurred in 2% to 4% of cases. , ,
From a practical standpoint, UGC has the disadvantage of being time-consuming and placing demands on the vascular laboratory, time, equipment, and personnel. Operator and patient fatigue and management of the often significant pain that patients experience during compression are common problems. The use of mechanical compression devices can obviate some of these issues, but requires a cooperative and immobile patient and repeated reevaluation during compression. Such devices offer little advantage and have not generally been widely accepted.
Angiographically directed thrombin injection via percutaneous access into the aneurysm cavity to induce immediate thrombosis was first described by Cope et al. more than 20 years ago. Kang et al. modified the technique by performing direct percutaneous aneurysm puncture under duplex ultrasound guidance. Thrombin directly converts fibrinogen to fibrin, thereby leading to immediate clot formation and “short-circuiting” the upstream mechanism of the coagulation cascade, where heparin and warfarin interact. Consequently, thrombin injection is effective even in patients receiving anticoagulation. Bovine and human thrombin are commercially available in powder form that is reconstituted in normal saline immediately before use.
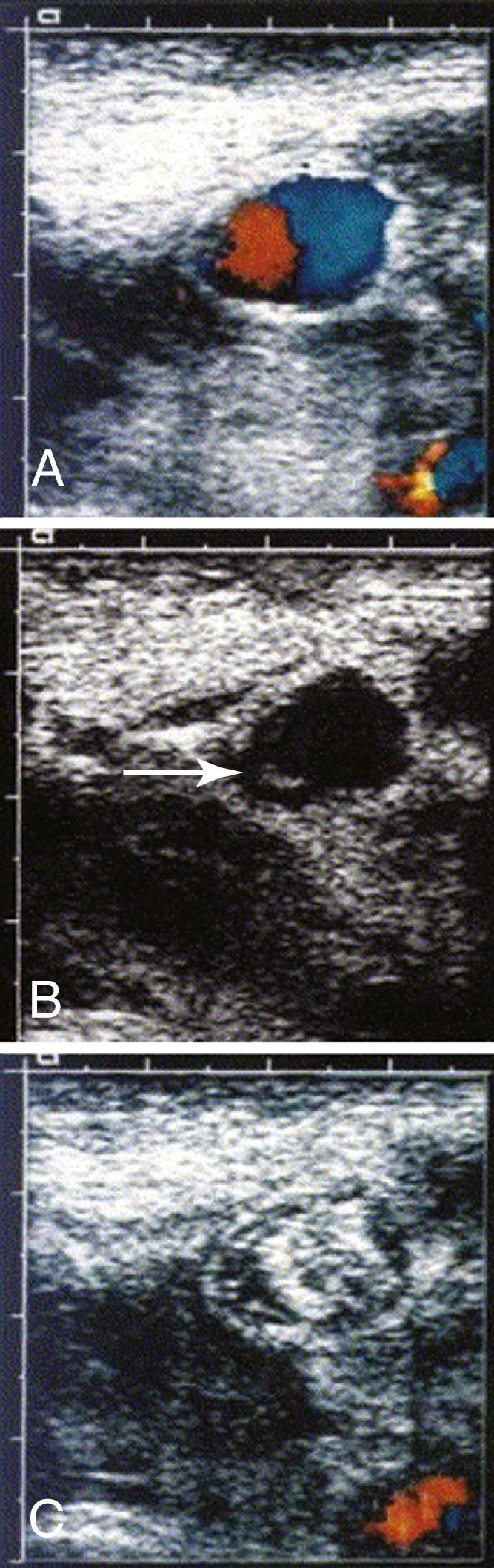
Ultrasound-guided thrombin injection (UGTI) is quick, simple, and relatively painless. Duplex ultrasound is used to identify the aneurysm cavity. After infiltration with local anesthetic, the cavity is punctured with either a 22-gauge catheter or a 25-gauge hypodermic needle. Spinal needles can be used for deeper aneurysms. Identification of the needle or catheter within the aneurysm sac is a critical step ( Fig. 85.4 ) and can be facilitated with the use of a commercially available echodense needle. The needle is filled with thrombin, which when inserted causes a small clot to form at the needle tip that is readily visualized on ultrasound. Once proper positioning of the needle is confirmed, thrombin (1000 IU/mL) is injected slowly through a 3-mL syringe for a period of about 10 to 15 seconds, until flow within the cavity ceases. In our experience and that of others, a dose of approximately 1000 units of thrombin is required to induce thrombosis. It is important to inject slowly and to stop immediately once thrombosis has occurred to minimize the chance of thrombin entering the circulation. A second injection is sometimes required. Patients are given bed rest for 1 hour. Duplex ultrasound is repeated 24 hours later to confirm permanent thrombosis of the aneurysm. UGTI has a success rate of 96% to 100%. , Second injections of thrombin are necessary in up to 7% of cases to achieve complete thrombosis.
Most writers prefer to use UGTI to treat pseudoaneurysms and to use UGC only in patients in whom thrombin products are contraindicated. Contraindications to bovine thrombin include known allergy, infection, and pregnancy. A relative contraindication to UGTI is a short, wide channel or “neck” of flow into the pseudoaneurysm. In these cases there can be a higher incidence of embolization of thrombus into the CFA with thrombin injection. An update of a Cochrane review compared multiple no-surgical treatments, including ultrasound-guided compression, blind compression, and ultrasound-guided thrombin injections. Compression was effective in achieving pseudoaneurysm thrombosis. Ultrasound-guided application did not confer an additional benefit. Percutaneous thrombin injection was more effective than a single session of ultrasound-guided compression within individual randomized controlled trials.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here