Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital heart disease (CHD) is a common birth defect with continually improving survival of neonates with complex lesions requiring heart surgery.
Neurodevelopmental (ND) abnormalities are common in school-age children and adolescents after neonatal heart surgery.
Magnetic resonance imaging (MRI) studies have demonstrated evidence of brain injury and delayed brain development, even before having a corrective operation, in patients with hypoplastic left heart syndrome and transposition of the great arteries.
Fetal MRI studies suggest this delayed development begins in the third trimester.
Aberrant fetal physiology resulting in decreased oxygen and substrate supply to the brain may contribute to these imaging and ND abnormalities in addition to other perioperative risk factors.
Children with complex CHD have a prevalence of pervasive but subtle cognitive problems termed the neurodevelopmental signature of complex congenital heart disease .
Severe congenital heart disease (CHD) requiring a corrective operation occurs in 6 to 8 per 1000 live births, with up to half of the cases requiring an operation in the neonatal period to survive. With improved surgical techniques, mortality for CHD has steadily declined. In fact, the number of adults living with CHD has surpassed the number of children with CHD in the United States.
Given the changing epidemiology of CHD, considerable effort has been devoted to evaluating both neurodevelopmental (ND) outcomes and quality of life for patients with CHD. Evidence suggests that although children with CHD may no longer have overt signs of neurologic dysfunction, they may exhibit deficits in multiple domains, including visual-spatial skills, memory, executive function, speech and language, and gross and fine motor function. Long-term follow-up data suggest that these deficits continue into adolescence and young adulthood with potentially significant impacts on societal contribution.
A natural assumption has been that these adverse outcomes were directly related to a brain injury sustained during neonatal cardiac surgical interventions. However, in the recent era it has become apparent that patient-specific risk factors play essential roles in determining the ultimate ND outcome and, furthermore, that the interplay between the brain and the circulation is complex, occurring at many levels throughout fetal and postnatal development. This chapter will review mechanisms influencing neurologic outcomes in CHD, including (1) the physiologic effects of congenital heart lesions on brain blood flow, (2) brain development in the context of CHD, (3) the timing, appearance, and mechanism of acquired brain injuries, and (4) current knowledge on ND outcomes in critical CHD in the short and long term.
Human cardiac development is largely complete by gestational week 7. In contrast, brain development extends over a much longer time period, with morphologic events (cell proliferation, migration, axon pathfinding, and target selection) occurring predominantly in the first two trimesters, followed by a prolonged period of refinement of circuits that begins in the third trimester and extends into infancy. This stage of brain development includes dramatic growth, myelination, and increasing neuronal electrical activity and depends upon receiving an adequate supply of nutrients and oxygen. Consequently, blood flow to the developing brain increases and is estimated to be a quarter of the combined ventricular output in the third trimester, demonstrating the unique heart–brain interplay critical for normal brain development.
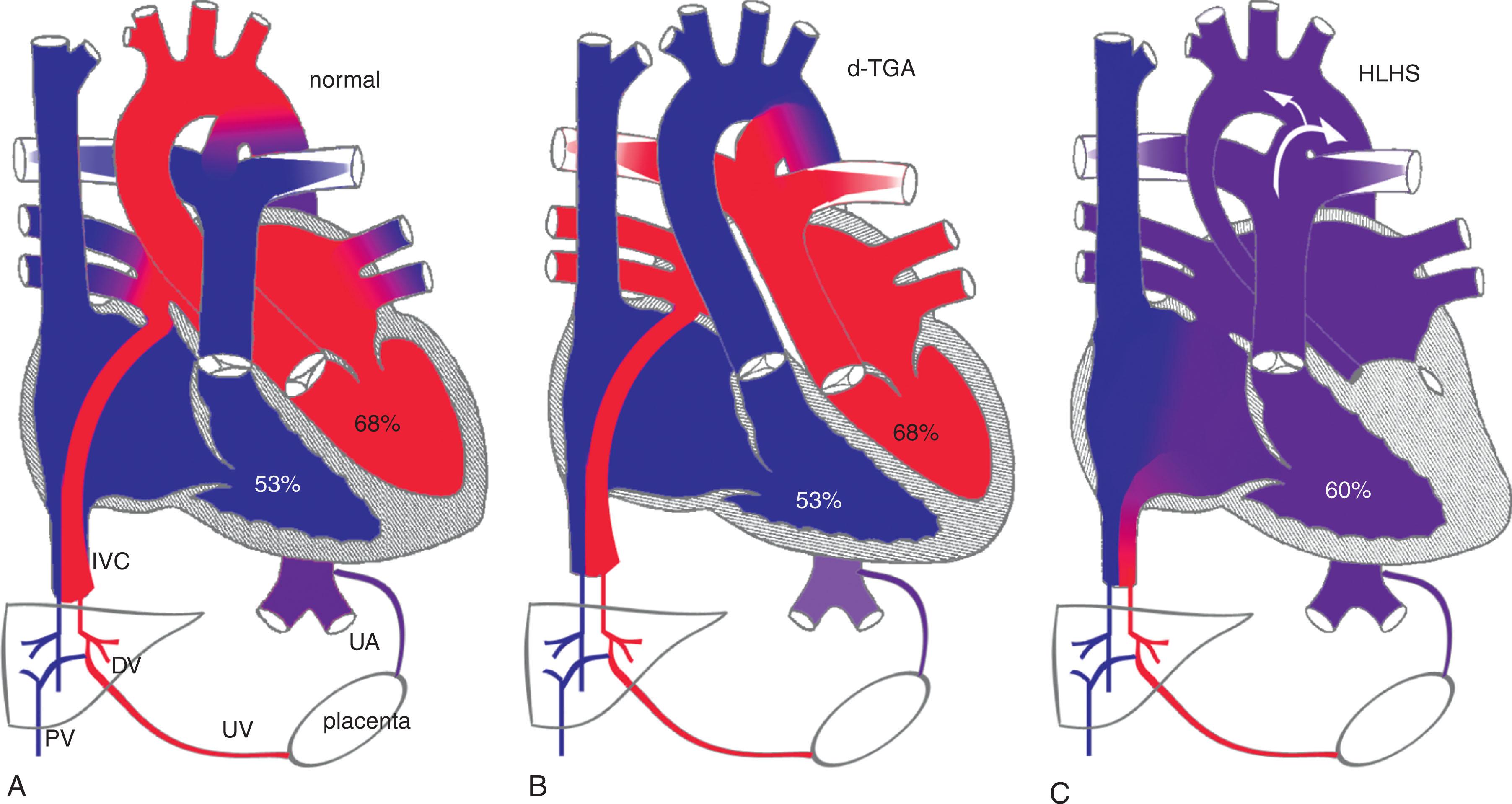
Fetal circulation is unique in many respects that can impact cerebral blood flow and development. In the normal fetus, cerebral blood flow is supplied by highly oxygenated blood from the ductus venosus preferentially streaming across the foramen ovale to the left atrium and ventricle ( Fig. 51.1 ). In contrast, in fetuses with transposition of the great arteries (TGA), the aorta and pulmonary artery are transposed. Thus the higher oxygenated blood reaches the pulmonary vasculature as opposed to the cerebral vasculature. Similarly, in hypoplastic left heart syndrome (HLHS), inadequate left heart structures lead to a reversal of blood flow in the foramen ovale with the mixing of oxygenated and deoxygenated blood in the right ventricle and, in cases of aortic atresia, retrograde flow in the ascending aorta. The effects of these abnormal flow patterns on brain development are uncertain but may involve different mechanisms; despite that both decrease the oxygen content of the blood delivered to the brain. Other mechanisms may be involved, such as inadequate substrate delivery (glucose) because of decreased perfusion pressure and flow to the brain. In the d-transposition of the great arteries (dextroposition [d]-TGA), the pulsatility and perfusion pressure of the cerebral circulation are normal. However, in HLHS, the hypoplastic isthmus and aortic arch may function as resistors, potentially decreasing the pulsatility and perfusion pressure to the cerebral circulation. In contrast, in d-TGA, decreased pulsatility and perfusion to the brain can result from preferential blood flow to the pulmonary vasculature because of a lower pulmonary vascular resistance than usual.
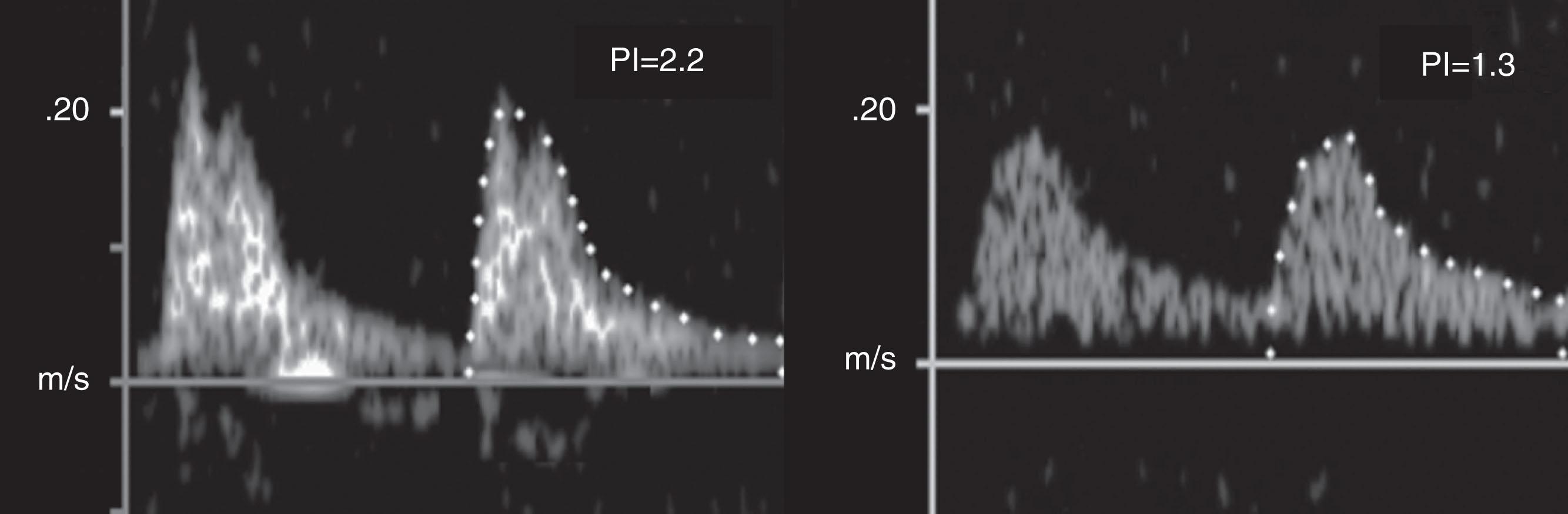
Cerebral Doppler ultrasound can assess fetal cerebral vascular resistance in the middle cerebral artery (MCA) and provide insight into fetal cerebral blood flow patterns. By calculating the MCA pulsatility index (PI, a measure of vascular resistance in the circulatory bed downstream from the point of Doppler sampling), studies have identified a pattern of "brain-sparing" in fetuses with intrauterine growth restriction and placental insufficiency as a consequence of autoregulation of fetal cerebral blood flow. In normal pregnancies, the cerebral/umbilical PI ratio is more than 1.0, whereas, in many growth-restricted fetuses, the ratio is less than 1.0 and predicts adverse perinatal and neurologic outcomes. This autoregulatory mechanism is thus paradoxically a harbinger of poor outcomes in the setting of fetal growth restriction.
There have been several studies examining in utero blood flow patterns in human fetuses with CHD. These have demonstrated lower MCA PI in fetuses who have lesions with the most intracardiac mixing, such as HLHS ( Fig. 51.2 ). In fact, fetuses with HLHS have been shown to have the lowest cerebral/umbilical PI ratio among different types of CHD. This is likely secondary to the lower oxygen content of blood delivered to the brain and abnormalities in cerebral perfusion with a hypoplastic aortic isthmus.
Cerebral blood flow characteristics have been shown to predict ND outcomes in fetuses with CHD. In a retrospective multicenter study of infants with HLHS, a lower MCA PI in utero predicted a better ND outcome at 14 months of age as assessed by the Bayley Scales of Infant Development (BSID) II. These findings suggest that the autoregulatory response of cerebral vasodilation in the setting of HLHS may be sufficient and adaptive to a state of chronic hypoxemia, which is in contrast to what is seen in the context of fetal growth restriction. Further large prospective studies are needed to understand the predictive utility of cerebral blood flow patterns in fetuses with CHD.
Structural brain malformation in neonates with CHD has been identified, even in the absence of a defined genetic syndrome. Autopsy studies have revealed multiple congenital brain anomalies in neonates with HLHS, including microcephaly, abnormal cortical mantle formation, and overt central nervous system malformations such as agenesis of the corpus callosum or holoprosencephaly.
In addition to structural abnormalities, there is magnetic resonance imaging (MRI) evidence that brain development is delayed in CHD prior to corrective surgery. Quantitative MRI techniques such as diffusion tensor imaging (DTI) measure the direction and magnitude of water movement and thus microstructural brain development. During normal brain development, the magnitude of water diffusion motion decreases (apparent diffusion coefficient [ADC]), and regional directionality increases in white matter (fractional anisotropy [FA]). Similarly, metabolic brain development can be measured with magnetic resonance spectroscopy (MRS) by measuring major metabolic compounds such as N -acetylaspartate (NAA), choline (Cho), creatine (Cr), and lactate. Using these techniques, researchers have discovered that newborns with CHD (d-TGA and single ventricle lesions) have findings suggesting an immature brain with abnormal DTI (4% higher ADC and 12% lower FA) and MRS (10% lower NAA/Cho ratio). Comparing these findings with those obtained in premature newborns without CHD, full-term newborns with CHD appear approximately 1 month delayed. These observations have been replicated in studies assessing brain development by semiquantitative morphologic scoring, specifically the brain total maturation score (TMS). The TMS was found to be significantly lower in full-term newborns with d-TGA and HLHS compared with a normal cohort of newborns. Finally, morphometry studies have demonstrated lower total and regional brain volumes in newborns with CHD compared with controls. These findings help explain the abnormal somatic growth seen in the newborn with CHD. Specifically, those with d-TGA and HLHS have smaller head circumferences that are out of proportion to their weight. These brain MRI findings in the neonate with CHD, seen before any corrective operations are performed, have led to the theory that abnormal brain development and susceptibility to acquired injury begin in utero.
Technical advances in fetal MRI have made it an important tool in the clinical evaluation of fetuses with suspected cerebral abnormalities. A study comparing brain volumes and MRS between normal fetuses and fetuses with CHD between 25 and 37 weeks' gestation showed definitive evidence for delayed fetal brain development. During the third trimester, a progressive impairment of brain volumes was observed, particularly in those fetuses with left-sided obstruction. Additionally, larger delays in the expected increase in NAA/Cho ratio and greater impairment of growth in brain volume were noted in fetuses with aortic atresia, who have no antegrade blood flow in the aortic arch. These observations support the concept that brain development is altered during fetal life because of impaired fetal cerebral blood flow, oxygen, and substrate delivery and that compensatory mechanisms (i.e., brain-sparing effect) may not be adequate.
Recently, novel fetal cardiac MRI techniques have been utilized to understand the heart–brain interplay in CHD. These techniques enable measurements of flow and oxygen saturation in fetal blood vessels. By combining fetal brain MRI and cardiovascular magnetic resonance, researchers found a correlation between fetal cerebral oxygen consumption and brain size (estimated brain weight) among 30 fetuses with CHD in late gestation. There was a direct correlation between estimated brain weight and cerebral oxygen consumption. In addition, there was a modest association between cerebral oxygen delivery and brain size. Other novels and efficient imaging techniques have been developed to understand cerebrovascular physiology while accommodating significant motion artifacts from fetal movement and maternal breathing. These studies revealed that fetuses with both left sided obstructive lesions and d-TGA have lower fetal cerebral oxygenation compared to controls. The predictive value of these findings for postnatal outcomes such as brain injury and ND outcomes remains unknown. Further exploration to potentially identify targets and methods of intervention to modify ND outcome in CHD is warranted.
Although much of the literature has been focused on the identification of abnormal brain development in the fetus and newborn with CHD, studies on older patients have emerged suggesting that these delays continue into adolescent years. In a study of single ventricle patients who had undergone a Fontan operation, the frequency of any structural abnormality on MRI was 11 times higher than in a normative cohort. Similarly, in a brain MRI study of adolescents with d-TGA, FA was significantly reduced in several regions of the white matter compared with a normative cohort. Many have postulated that the trajectory of brain development over time may be dependent on the specific cardiac lesion and whether the surgical management is corrective (i.e., d-TGA) or largely palliative (i.e., HLHS). In particular, patients with aortic atresia have been found to have the least robust microstructural brain development. In a direct comparison of HLHS to d-TGA, neonates with d-TGA had a faster rate of global and regional brain growth in the perioperative time period. Another study demonstrated, at 2.5 years of age, more cortical atrophy and lower brain volumes in those with HLHS compared with those with d-TGA, suggesting a lesion-specific influence on the long-term trajectory of brain development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here