Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ability of the liver to regenerate was recognized by the Greeks in the ancient myth of Prometheus, the Titan god of forethought, who gave fire to the mortals and angered Zeus. Prometheus was chained to the Caucasus mountains and each day he would be tormented by Zeus’ eagle Ethon as it devoured his liver. Each night, the damaged liver would be restored so the eagle could begin anew, illustrating the liver’s unique power to regenerate. This regenerative capacity is what allows transplant surgeons to successfully remove or transplant portions of a liver, with the remnant portion then rapidly growing to the original volume, and also allows for restoration of function after hepatocyte mass loss from toxic injury or inflammation.
The terms regeneration , hyperplasia, and hypertrophy are used synonymously in literature, but hyperplasia is the most precise from a cellular standpoint. The damaged or resected hepatic lobes do not grow back in the same way that a lizard’s tail regrows, but rather there is a hyperplastic response (defined as increasing in cell number) in the remnant liver, leading to its hypertrophic appearance (defined as enlarging liver size). This process is highly regulated and involves multiple cell types, extrahepatic signals, complex molecular pathways, and cellular interactions. A delicate balance is required for initiation of regeneration, exerting a growth response, and maintaining normal metabolic function. The inability to maintain this process leads to poor liver function and ultimately liver failure after surgery, whereas a successfully orchestrated response results in restoration of normal liver function.
Hepatobiliary surgery is now routinely and safely accomplished for malignant and benign disease. This technical success in both resection and transplantation relies on the remarkable ability of the liver to regain most of its functional mass within a matter of weeks (see Chapters 101 and 109 ). Factors that limit the achievement of curative tumor resection and small-for-size (SFS) transplantations make up the high morbidity and mortality rates associated with insufficient volume of the liver remnant or transplanted graft. As hepatobiliary and transplant surgeons continue to expand the magnitude and complexity of liver resection and explore the limits of living donor liver transplantation (LDLT), understanding the mechanisms behind liver regeneration is essential for clinical practice. Many tumors that were previously considered to be unresectable are now amenable to complete resection through induction chemotherapy and innovative treatment strategies to increase liver remnant volume. There are several techniques, including portal vein embolization (PVE) or portal vein ligation (PVL), additional hepatic vein embolization, and (the most extreme) associating liver partition and PVL for staged hepatectomy (ALPPS; see Chapter 102D ). They cause atrophy of the ipsilateral hemiliver and hypertrophy of the contralateral side and are particularly valuable in patients who have underlying liver disease. Many patients with underlying liver disease are now considered suitable candidates for liver resection, even with Child-Pugh grade A cirrhosis and minimal portal hypertension.
Regeneration also is crucial in liver transplantation. In deceased donor transplantation, hepatocyte loss occurs in the form of ischemia/reperfusion (I/R) injury because of the necessary preservation period from procurement to implantation and damage that may have occurred in the donor (see Chapters 105 and 111 ). Because of the scarcity of organs, more “marginal” organs are accepted for transplantation, which have increased need for regeneration and recovery in the environment of I/R injury (see Chapter 109 ). One of the landmark advances in liver transplantation is the ability to use segmental liver grafts obtained from either a deceased donor or a living donor. In the latter situation, success of the procedure relies on relatively rapid hepatic regeneration in both donor and recipient. The minimal amount of functional liver necessary for successful transplantation or for safe recovery in the donor is a major concern. Donor graft size, recipient weight, portal hypertension, I/R injury, and the recipient’s disease severity all contribute to the amount of post-transplant regeneration and recovery needed. Efforts to decrease the amount of liver removed from the donor to minimize donor risk results in smaller grafts for the recipients and real challenges in postoperative recovery.
Liver regeneration is most clearly shown in the experimental model that was pioneered in 1931 by Higgins and Anderson. In this model, a simple two-thirds partial hepatectomy (PHx) is performed, without damage to the lobes left behind. This leads to enlargement of the residual lobes to make up for the mass of the removed lobes in five to seven days. Other well-known models of liver regeneration are associated with extensive tissue injury and inflammation and include the use of hepatic toxins, such as ethanol (EtOH), carbon tetrachloride (CCl 4 ), and galactosamine (GalN) ; bile duct ligation or PVL ; and I/R injury. Newer models include transgenic albumin promoter urokinase-type plasminogen activator (u-PA) fusion constructs, Fah/Rag2 knock-out mice, , and PHx in zebrafish. In each model, the different toxic agents injure specific liver cell subpopulations. Therefore, PHx is the preferred in vivo model to study the regenerative response. Debonera demonstrated that regenerative signaling observed in a rat liver transplant model of I/R injury is similar to that observed after PHx. The most recent instrument to study liver regeneration, when proliferation is impaired, is lineage tracings using cyclization recombinase (Cre) recombinase–mediated cell labeling. In its classical setting, a traced cell population harbors two transgenes. The first expresses Cre under the control of cell-specific regulatory elements. The Cre activity is modulated by fusing a mutated ligand-binding domain of the estrogen receptor (ER), which is sensitive to tamoxifen but insensitive to estrogen. Upon addition of tamoxifen, CreER eliminates a locus of X-over P1 (loxP)–flanked stop cassette and induces transcription of the second transgene, coding for a reporter protein. Although this represents the gold standard for defining cell fate, this strategy is not without pitfalls, which may explain the sometimes-controversial results.
It is well established that liver regeneration after surgical resection is carried out by growth and proliferation of existing mature hepatocyte populations. These include hepatocytes, biliary and fenestrated endothelial cells, Kupffer cells, platelets, and Ito cells (stellate cells; Fig. 6.1 ). The kinetics of cell proliferation and the growth factors produced by proliferating hepatocytes suggest that hepatocytes provide the mitogenic stimuli leading to proliferation of the other cells. The degree of hepatocyte proliferation is directly proportional to the degree of injury. , Immediately after liver resection, the rate of DNA synthesis in hepatocytes begins to increase as they exit the resting state of the cell cycle (G 0 ) and enter G 1, traverse to DNA synthesis (S phase), and ultimately undergo mitosis (M phase). The first peak of DNA synthesis occurs at 40 hours after resection in rodents and at seven to 10 days in primates. In small animals, the regenerative response returns the liver to the pre-resection mass in one week to 10 days. Clinical studies from living donor transplantation suggest that a significant amount of regeneration occurs in human within two weeks after resection and is nearly complete at three months after resection.
After resection, hepatocyte proliferation starts in the periportal areas of the lobules and then proceeds to the pericentral areas by 36 to 48 hours. Liver histology at day three to four after PHx is characterized by clumps of small hepatocytes surrounding capillaries, which change into true hepatic sinusoids. The hepatic matrix composition also changes from high laminin content to primarily containing fibronectin and collagen types IV and I. After a 70% hepatectomy, restoration of the original number of hepatocytes theoretically requires 1.66 proliferative cycles per residual hepatocyte. In fact, most of the hepatocytes (95% in young and 75% in very old rats) in the residual lobes participate in one or two proliferative events. Hepatocytes have an almost unlimited capacity to regenerate as transplantation of several hundreds of healthy hepatocytes can repopulate a whole damaged liver in a calculated minimum of 69 doublings. Interestingly, the mechanisms associated with how a liver knows when to stop regenerating are much less clear than the starting mechanisms.
In contrast to other regenerating tissues (bone marrow, skin), primary liver regeneration after surgical trauma is not dependent on a small group of progenitor cells (stem cells). However, in response to toxic liver damage inflicted by agents such as galactosamine, hepatocytes are unable to replicate. In this situation a population of cells known as “oval cells” proliferates to replace the hepatic parenchyma. In distinct approaches to determine whether cells other than hepatocytes themselves could be the source of new hepatocytes in oval cell injury, two groups found no evidence of such liver stem/progenitor cells. ,
In the human situation, hepatic progenitor cells (HPCs) were presumed to participate in repopulation of the liver after acute massive necrosis and have also been identified in chronic liver disease. The human HPCs originate from the canals of Hering and play an important role in acetaminophen-induced injury. Huch et al. described conditions allowing for the long-term expansion of these adult bile duct–derived bipotent progenitor cells from human liver, which enables disease modeling, toxicology studies, and regenerative medicine. More recent studies have shown the contribution of HPC is very much context dependent, with hepatocyte senescence after injury being a major driver for HPC expansion and their hepatocytic differentiation. The rapidity of regeneration after PHx suggests a minimal involvement of stem cells in this response, but new stem/progenitor hepatocytes have been located either randomly throughout the lobule or at opposite ends of the portal vein-hepatic vein axis. It was reported that different regeneration stimuli trigger different regenerative responses; after toxic liver damage, stem cell–dependent proliferation is seen along the central vein, , whereas homeostatic proliferation is present throughout the whole liver. The presence of regenerative stem cells at the portal rim has refueled the streaming liver debate.
Altogether, there remains considerable disagreement of the exact role of stem/progenitor cells in liver regeneration and homeostasis. ,
Within minutes after PHx, specific immediate early genes are activated in remnant hepatocytes. , These 70 to 100 genes include proto-oncogenes, which play an important role in normal cell-cycle progression, such as c-jun, c-fos, c-myc, and K-Ras, and the transcriptional factors nuclear factor (NF)-κB, signal transducer and activator of transcription 3 (STAT3), activator protein-1 (AP-1), and CCAAT enhancer binding protein beta (C/EBPβ). ,
Historically, the onset of liver regeneration has been attributed to a flow-dependent response by which increased relative flow after PHx resulted in hepatocyte proliferation and hyperplasia. A more recent experimental partial liver transplant model demonstrates that increased portal flow is essential for liver regeneration. Nevertheless, portal hyperperfusion (flow that exceeds 250 mL/100 g/min) completely abolishes the process (see “Portal Inflow and Hepatic Outflow”).
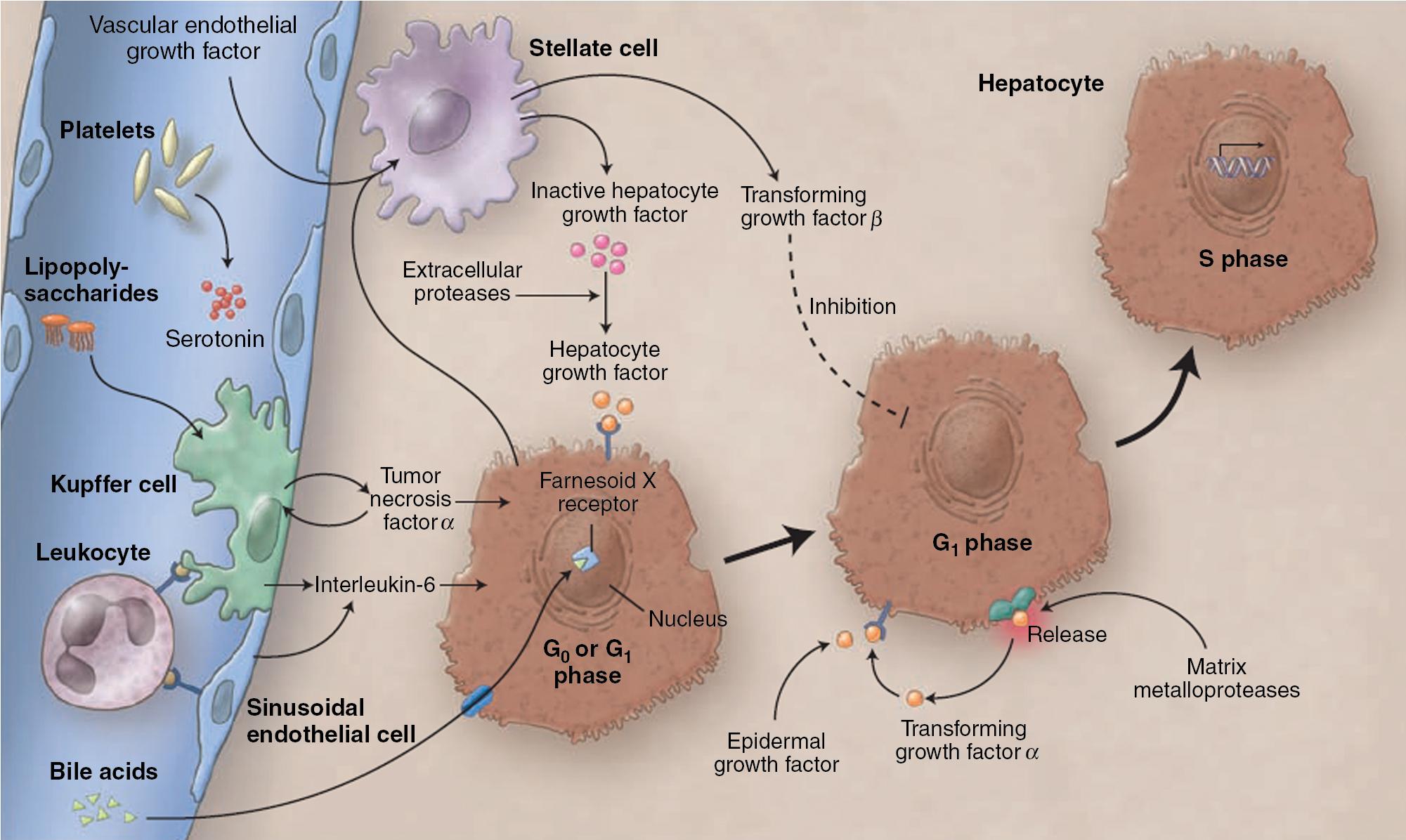
Very early experiments in parasymbiotic rats demonstrated the existence of humoral factors in the induction of liver growth after PHx. Interleukin (IL)-6 and tumor necrosis factor (TNF)-α have since been identified as the earliest factors triggering activation of several transcription factors during regeneration , ( Fig. 6.2 ). In IL-6 deficient or TNF-α receptor deficient mice, liver regeneration after hepatectomy is delayed but not completely abolished. , Therefore other blood-derived mitogens, such as hepatocyte growth factor (HGF), were identified as putative hepatic growth factor during liver regeneration. Hepatocytes in normal liver are not ready to respond to mitogenic signals without a set of “priming” events that switch them into a responsive state. This has been described by Fausto, who identified the priming factors involved in initiating and triggering the hepatic response to injury and concomitant growth factors and their receptors, which allow for competent hepatocytes to progress through the cell cycle. Priming is accomplished by the release of preformed cytokines that subsequently activate transcription factor complexes and allow the cell to exit G 0 into G 1 of the cell cycle. This group includes TNF-α and IL-6.
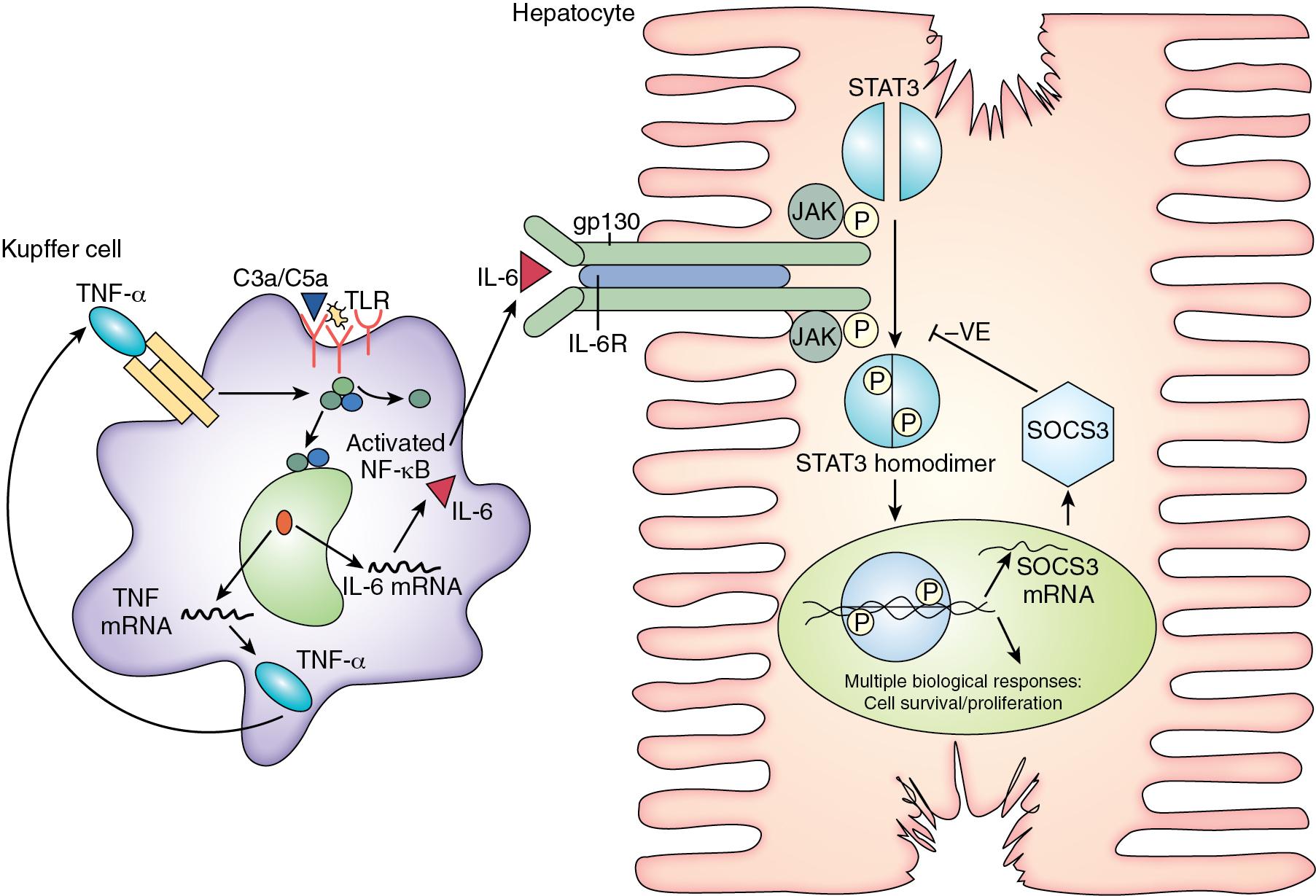
TNF signaling through TNF receptor (TNFR)-I initiates liver regeneration after PHx with IL-6 as the key target. Knockout mice that lack TNFR-I showed an almost complete inhibition of NF-κB binding and a severe defect in hepatocyte replication after PHx. IL-6 reverses the deficiency in hepatocyte replication imposed by the lack of TNFR-I and corrects the defects in STAT3 and AP-1 binding but does not reverse the inhibition of NF-κB binding. This solved a long-standing riddle in the understanding of liver regeneration by identifying TNF as the initiator of IL-6.
IL-6 activates the Janus kinase (JAK)/STAT3 and MAPK signaling pathways via the gp130/IL-6R complex. This leads to activation of an array of immediate and delayed early genes required for normal liver-specific metabolic functions, repair, and hepatoprotection from injury. STAT3 is crucial for cells to progress from G 1 to S phase and for activating the c-myc gene, a gene required for cell-cycle progression.
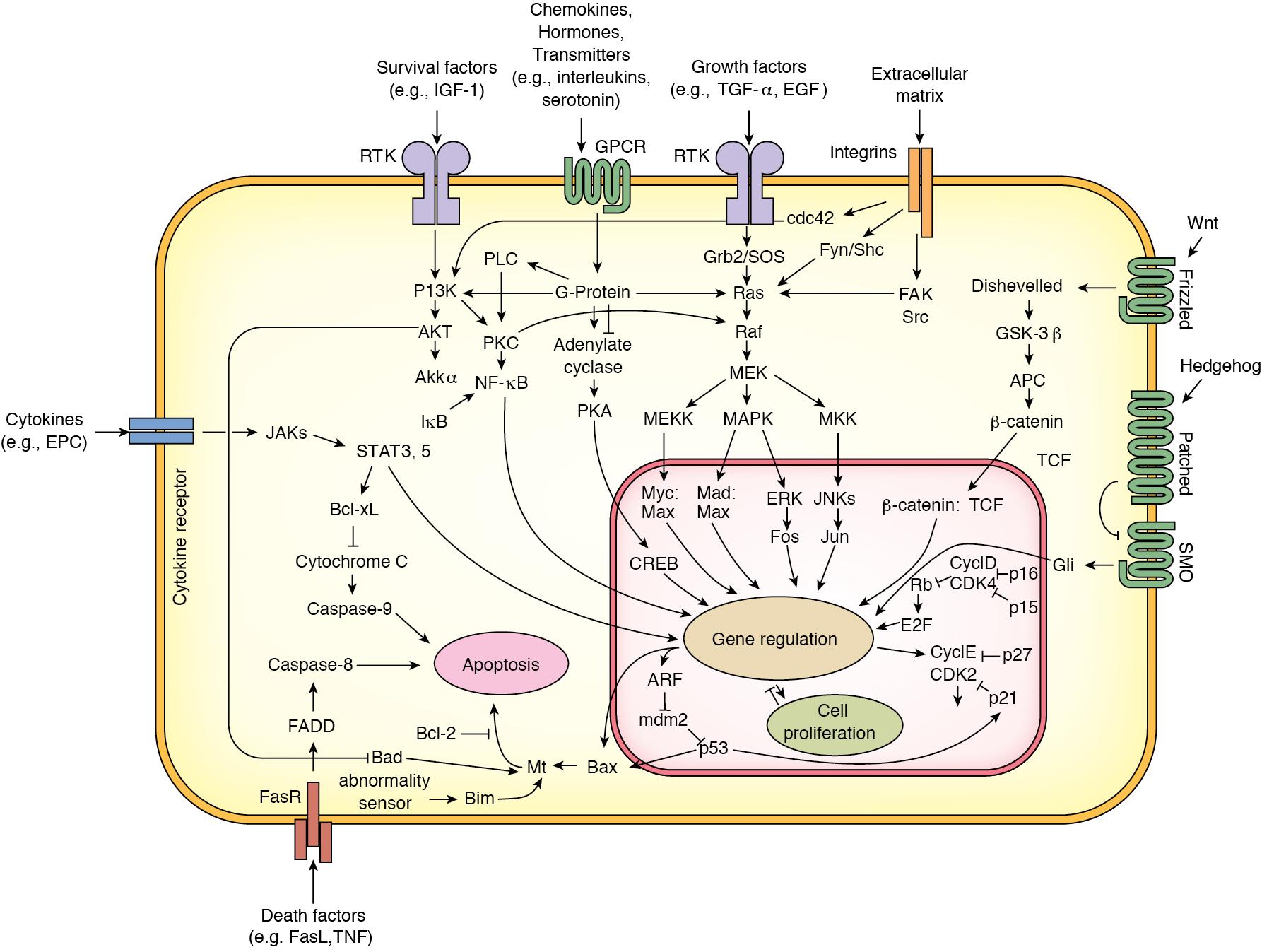
Other intracellular signaling pathways that involve the receptor tyrosine kinases p38 mitogen-activated protein kinase (MAPK), protein kinase R-like ER kinase (pERK), and c-jun-NH2-terminal (JNK) are also rapidly activated. Progression through the cell cycle is regulated by cyclins and cyclin-dependent kinases (CDKs). Various combinations bind to form kinase complexes that are active at distinct points within the cell cycle and tightly controlled by several mechanisms, including binding by CDK–inhibitory proteins, such as p21. Feedback signals to this process are provided by suppression of cytokine signaling-3 (SOCS3) and transforming growth factor (TGF)-β, also regulated by IL-6. Other studies confirmed the importance of NF-κB and showed immediate upregulation of apoptotic genes (Fas and caspases) in livers that failed because of excessive resection ( Fig. 6.3 ).
So far, a few factors have been identified to be possibly responsible for the release of these priming cytokines and growth factors in the onset of liver regeneration. The first is endotoxin lipopolysaccharide (LPS), produced in the gut by Gram-negative bacteria. Circulating LPS is an extremely strong signal for Kupffer cells to produce TNF and start the cascade, resulting in hepatocyte replication. Rats treated with antibiotics and germ-free rodents have a delayed peak of DNA replication after PHx, confirming the importance of LPS.
Another major finding is the demonstration that cytokine activation and DNA replication are severely impaired in mice lacking the complement components C3a and C5a. In particular, mice lacking both C3a and C5a have impaired production of TNF and IL-6 after PHx and poor activation of NF-κB and STAT3.
After the priming phase, concomitant growth factors are essential to progress hepatocytes into cell division. Growth factors include the potent hepatocyte mitogens HGF, TGF-α, and heparin-binding epidermal growth factor (HB-EGF). This process is further controlled by co-mitogens, such as insulin, glucagon, steroid and thyroid hormone, and epinephrine, which facilitate activity of the mitogens, and by downregulation of growth factor inhibitors, such as activin A and TGF-β.
The HGF/c-Met pathway is important for sustaining DNA synthesis after injury and activates various downstream pathways that involve PI3K, ERK, and AKT. This pathway cross-talks with the Wnt/β-catenin signaling pathway, which has come to the forefront in liver biology over the last several years. Increased levels of HGF result in β-catenin dissociation along with nuclear translocation and upregulation of downstream targets of this pathway such as cyclin D1, c-myc, uPAR, matrix metalloproteinases (MMPs), and epidermal growth factor receptor. Vascular endothelial growth factor (VEGF) interacts with endothelial cells in the liver to increase HGF production from nonparenchymal cells.
In the initiation of HGF, urokinase-type plasminogen activator (uPA) appears to play an important role. uPA and its downstream effector, plasminogen, increase within one to five minutes after PHx and rapidly cleave the HGF precursor, pro-HGF. Blocking uPA delays the appearance of HGF, and thereby delays liver regeneration, whereas blocking plasminogen-activator inhibitor (PAI) accelerates the release of HGF and liver regeneration.
Another humoral factor that triggers the concerted regenerative response in hepatocytes has been discovered; extracellular adenosine triphosphate (ATP) has emerged as a rapidly acting signaling molecule that after PHx leads to rapid and transient activation of JNK signaling, induction of immediate early genes c-Fos and c-Jun, and activator protein-1 (AP-1) DNA-binding activity. Recent studies directly link mitochondrial bioenergetics to several markers of postoperative liver function after resection; early lactate clearance and postoperative alanine aminotransferase (ALT) strongly correlated with mitochondrial energy state, as well as with enhanced growth of the future liver remnant (FLR).
The control of inflammatory signals is also necessary to allow for the progression of the regenerative pathways. The NF-κB inhibitory and ubiquitin-editing A20 protein (tnfaip3) plays a key role in the liver’s protective response to injury, particularly its antiinflammatory effects. A20 is significantly upregulated in the liver after PHx and protects hepatocytes from apoptosis and ongoing inflammation by inhibiting NF-κB. , A20 also allows for proliferation and optimizes metabolic control and energy production after liver regeneration, as demonstrated by increased enzymatic activity of cytochrome c oxidase or mitochondrial complex IV. A20-based therapies could be beneficial in future prevention and treatment of hepatic failure after liver resection. The cytokine induced form of nitric oxide synthase (iNOS) also seems to play an important role in scavenging oxygen radicals and protecting from apoptosis, caused by an uncontrolled inflammatory reaction mediated through IL-6 and TNF-β.
The mechanisms of regeneration were also studied in the transplant setting, where microarray analysis of SFS rat liver grafts showed upregulation of vasoconstrictive and adhesion molecule genes at early time points after reperfusion, with later increases in genes associated with inflammation and cell death and downregulation of genes related to energy metabolism. These pathways have been confirmed in the situation of clinical deceased donor and LDLT. ,
Remodeling of the newly regenerated liver tissue begins with the repopulation and maturation of nonparenchymal cells, such as endothelial, stellate, and biliary epithelial cells (see Chapter 1 ).
Newly formed hepatocytes form clusters into which replicating endothelial cells invade to form new sinusoids. To restore normal architecture, stellate cells, which are located between endothelial cells and hepatocytes, synthesize extracellular matrix (ECM) proteins and TGF-γ1, which can regulate the production of hepatic ECM. VEGF, angiopoietins 1 and 2, TGF-α, fibroblast growth factor (FGF)-1 and FGF-2, and HGF all are likely involved in the angiogenic process. Angiostatin, an inhibitor of angiogenesis, causes delayed and suppressed liver regeneration in mice. , Remodeling of the ECM is associated with the activation of the urokinase/plasminogen pathway and the MMP pathway. MMPs not only remodel the ECM but also regulate immune responses and participate in modulation of vascular integrity at the endothelial cell–cell junctions in steatotic livers after I/R injury. MMPs, in combination with HGF, EGF, and TGF-β1, act to remodel the ECM, changing the levels of several ECM proteins, such as collagen, fibronectin, laminin, and entactin. The maturation and thickening of the ECM seems to have an inhibitory effect on proliferating hepatocytes, potentially signaling the end of rapid regeneration.
The role of the ECM has been increasingly studied and plays an important role in regeneration, influencing proliferation, differentiation, and termination signals that regulate the regenerated liver size. The ECM was regarded as “that stuff between cells” but now is considered to be the dictator of metabolic liver zonation and is a hepatic growth/size rheostat during development, homeostasis, and regeneration. The interaction between LGR4/5 receptors and their cognate RSPO ligands potentiate Wnt/β-catenin signaling and promote proliferation and tissue homeostasis. Also, the role of mechanical forces and mechanosensing in regulating liver regeneration is being increasingly studied. Increased shear stress after liver resection is picked up by the cells through mechanosensors on their membranes, which include glycocalyx, primary cilia, caveolae, ion channels, receptor tyrosine kinases, and G proteins and G protein-coupled receptors. These mechanosensing mechanisms either generate molecular signals that further activate downstream signaling pathways, such as Yes-associated protein (YAP), or directly transduce mechanical signals by regulating the actomyosin cytoskeleton. α-Catenin is now considered a key mechanosensor for direct cell–cell tension and pressure, leading to proliferation. Additionally, the ECM maintains the differentiation state of hepatocytes. Increased rigidity favors hepatocyte proliferation, with hepatocytes remaining differentiated on softer support of fibrillar collagen meshwork and committing into dedifferentiation on stiffer support of monomeric collagen-coated dish. On rigid surfaces, hepatocytes exhibit epithelial to mesenchymal transition and switch into fibroblast-like morphology. In this context, it is interesting that temporary fibrosis is seen during normal liver regeneration and resolves over time. ,
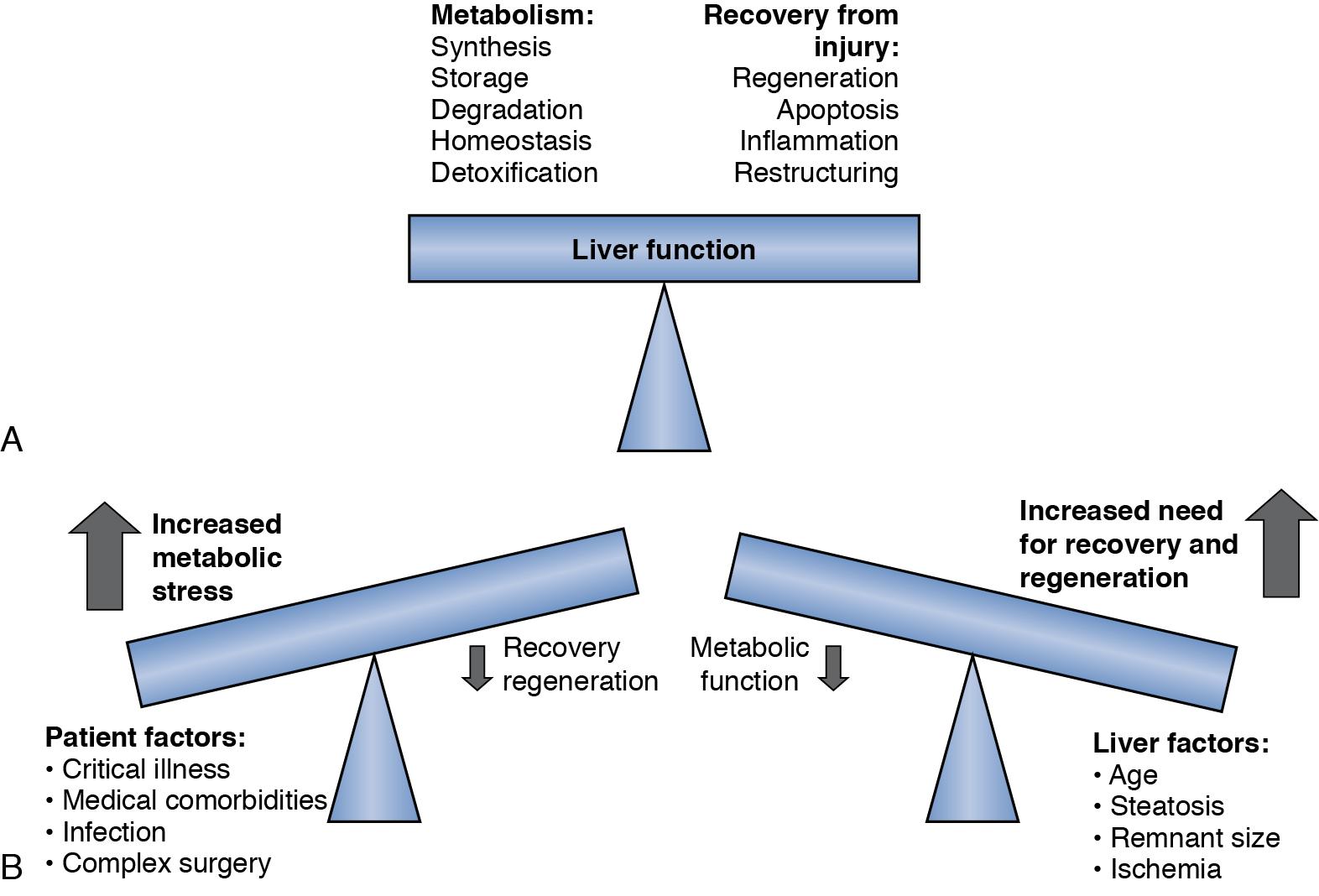
After volume loss, hepatocytes must adapt rapidly and seek a compromise between maintenance of continued differentiated function and cellular replication to permit survival. After toxic injury, resection, or transplantation, the balance is dramatically shifted to the crucial tasks of recovery and regeneration at the expense of normal hepatic metabolism. The success of restoring lost liver mass, repairing tissue injury, and resolving inflammation determines the ability of the liver to support normal metabolic function and determines the ability of the liver to recover. ( Fig. 6.4 ). Several of the expressed immediate early genes encode enzymes and proteins that are involved in regulating gluconeogenesis, a very important process after PHx to compensate for the lost glycogen content and to produce sufficient glucose for the whole organism. , There is rapid increased expression of genes involved in glucose homeostasis after PHx. Most notably, these include phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), and hepatic insulin–like growth factor binding protein 1 (IGFBP-1), controlled at the level of transcription by insulin (downregulation), glucagon/adenosine 3’,5’-cyclic monophosphate (cAMP; upregulation), and glucocorticoids (upregulation). , Insulin itself can be a potent growth factor mediated through the insulin receptor, and insulin and glucagon have long been established as important “gut-derived” growth factors. Liver-specific transcription factors (hepatocyte nuclear factors [HNFs]) have an important role in determining the level of glucose production, fatty acid metabolism, and liver-specific secreted proteins. C/EPBα regulates expression of genes involved in hepatic glucose and lipid homeostasis, has antiproliferative properties, and is downregulated during liver regeneration after hepatectomy. ,
During early regeneration, the liver accumulates fat before the major wave of parenchymal growth. Suppression of hepatocellular fat accumulation is associated with impaired hepatocellular proliferation after PHx, indicating that hepatocellular fat accumulation is specifically regulated during, and may be essential for, normal liver regeneration. Unlike pathologic steatosis, this transient regeneration-associated steatosis in hepatocytes is a physiologic process observed in every regenerating liver. Acute energy deprivation provokes hypoglycemia, mobilization of peripheral fats, and a switch to lipid usage. In mice, indirect calorimetry revealed that lipid oxidation is the primary energy source early after hepatectomy. This was regulated by downregulation of phosphatase and tensin homolog (PTEN), a key inhibitor of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) axis that regulates growth and metabolic adaptations after hepatectomy.
Some data show that decreasing lipid peroxidation levels by vitamin treatment after PHx produces an attenuation of cellular apoptosis and a marked increase in the proliferation process, suggesting that the modulation of lipid peroxidation also has a role in the liver regeneration process. Hepatocytes in the periportal regions that divide and replicate after PHx require mitochondrial fatty acid beta oxidation. Peroxisome proliferator–activated receptor (PPAR)-α may be a crucial modulator controlling energy flux important for repair of liver damage and regeneration. , Data from experimental models of liver transection, combined with PVL to promote liver regeneration, showed an overwhelming inflammatory response that interfered with the peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) mitochondrial biogenesis pathway. This resulted in the accumulation of immature and malfunctioning mitochondria in hepatocytes during the early phase of liver regeneration and showed close association with growth of the FLR. Also, increased expression of augmenter of liver regeneration (ALR), a potent hepatotrophic factor with important regulatory functions in cellular respiration, was shown. ALR is one of the strongest hepatic cell mitogens and also modulates mitochondrial biogenesis and ATP synthesis.
In a microarray analysis of gene expression profiles after LDLT, it was demonstrated that C/EBPα was downregulated, as was HNF-4α and PPAR-α. Expression of many other liver-specific genes, such as IGFBP1 and G6Pase, is regulated in the basal state by HNF1. The transcriptional activity of HNF1 is upregulated during liver regeneration by binding of HNF1 to the growth-induced transcription factors STAT3 and AP-1.
New insights into how the liver fulfills the adaptive response to metabolic needs during regeneration may come from the tight regulation of lipid, glucose, and bile acid (BA) metabolism through the class III NAD1-dependent histone deacetylase SIRT1 114. The role of SIRT1 as a key regulator of the regenerative response of the liver, controlling BA homeostasis, protein synthesis, and cell proliferation through deacetylation of farnesoid X receptor (FXR) and histones, and regulation of mTOR was established. SIRT1 is activated in situations of low energy availability and links nutritional status with metabolic homeostasis. It regulates adenosine monophosphate–activated protein kinase (AMPK). Contrary to SIRT1, mTOR is activated in high-energy conditions and controls cell growth and proliferation. mTORC1 promotes protein synthesis, and this axis is essential to regulate the cell cycle during liver regeneration after PHx. BA is also essential for the regeneration of the liver after PHx, although, when present in excess, BA can be toxic and promote hepatocyte death. Therefore a fine regulation of BA metabolism is essential to preserve liver homeostasis and a proper response to injury. FXR (NR1H4) is the master regulator of BA, lipid, and glucose metabolism. Through the activation of FXR, BAs regulate their hepatic metabolism and also promote hepatocellular proliferation. FXR is also expressed in enterocytes, where BAs stimulate the expression of FGF15/19, which is released to the portal blood. Through the activation of FGFR4 on hepatocytes, FGF15/19 regulates BA synthesis and finely tunes liver regeneration as part of the “hepatostat.” ,
The size of the liver is highly regulated and is controlled by the functional needs of the organism. This observation implies the existence of a master regulator of the liver/body mass ratio (i.e., a “hepatostat”).
From LDLT we know that differences are present between donors and recipients in the percentage reconstitution of the standard liver volume (80 vs. 93% at 3 months), which is probably caused by the need for functional liver mass to compensate for long-standing liver disease.
The most well-known antiproliferative factors within the liver are TGF-β and related family members such as activin. TGF-β is produced mainly by hepatic stellate cells, but in the early phase it forms inhibitory complexes with SKI proto-oncogene (SKI) and SKI-like proto-oncogene (SnoN), rendering hepatocytes initially resistant to TGF-β. The downregulation of miR23b may further contribute to activation of the TGF-β1/Smad3 signaling pathway during the termination stage. Upon activation, Smad2, Smad3, and Smad4 assemble in a common complex, translocate into the nucleus, and activate target genes that negatively regulate the cell cycle. Reactive oxygen species (ROS) enhance synthesis and activation of TGF-β, which may account for the reduced regeneration after ischemia and reperfusion. Interacting with the TGF-β/Smad signaling could restore regeneration in a model of SFS liver grafts.
Similarly, activin A blocks hepatocyte mitogenesis and shows diminished signaling during liver regeneration when its cellular-receptor level is reduced. Its receptor level is restored once liver regeneration is terminated. The level of activin receptor mRNA expression was shown to be an important determinant in the magnitude of regeneration in PVL and PHx. , Suppressors of cytokine signaling (SOCS) are important negative regulators of cytokine signaling that prevent the tyrosine phosphorylation of STAT proteins.
Of the cytokines, both IL-1 and IL-6 are involved in the termination of proliferation. The administration of exogenous IL-1β suppressed DNA synthesis post-PHx, and increased expression of IL-1β has also been observed in a shrinking liver lobe of a rat PVL model, indicating that IL-1β is involved in the process of cellular atrophy. The suppression of IL-1β was shown to promote liver regeneration in rat models of classic 70% PHx and 90% extended hepatectomy.
The IL-1 is secreted by Kupffer cells, regulated by prostaglandin E2 (PGE2), and suppressed by heparin and PGE1 after PHx. The effect is mediated through the IL-1 receptor as its antagonist (IL-1Ra), a competitive inhibitor of IL-1α and IL-1β and antiinflammatory protein, inhibits facilitated liver regeneration. IL-1 appears to contribute to the cessation of liver regeneration in a reduced-size liver transplantation model by reducing HGF and promoting TGF-β release. Another study showed that IL-1β inhibits the FGF19 signaling pathway, which regulates cell growth and metabolism of hepatocytes in liver regeneration.
IL-6 signaling in the liver causes the rapid upregulation of SOCS3, which correlates with a feedback loop and the subsequent downregulation of phosphorylated STAT3, thereby terminating the IL-6 signal. Also the role of C/EBPα, a key regulator of liver proliferation, in the termination of regeneration has been demonstrated. Complex formation of C/EBPα and chromatin remodeling protein HDAC1 represses other key regulators of liver proliferation: C/EBPα, p53, FXR, SIRT1, PGC1α, and TERT. The C/EBPβ-HDAC1 complexes also repress promoters of enzymes of glucose synthesis PEPCK and G6Pase. Proper cooperation of C/EBP and chromatin remodeling proteins seems essential for the termination of liver regeneration after surgery and for maintenance of liver functions. Additional work strongly implicates the detection of blood BA levels by nuclear receptors as a regulator of liver growth.
The Hippo signaling pathway and its downstream effectors, YAP and transcriptional coactivator with PDZ-binding motif (TAZ), have also been identified as key regulators of cell proliferation and organ size. Overexpression of YAP in a transgenic mouse model leads to massive liver hyperplasia, reaching 25% of body weight. The core component of the mammalian Hippo pathway is a kinase cascade in which mammalian Ste20-like kinases 1/2 (MST1/2) phosphorylates and activates large tumor suppressor 1/2 (LATS1/2). LATS1/2 then phosphorylates the transcriptional coactivators, YAP and TAZ, downregulating their function and increasing their degradation by the proteasome. During regeneration in a rat model, YAP was activated one day after PHx through decreased activation of core kinases MST1/2, as well as LATS1/2 by three days after PHx. At day seven, reaching normal liver size, YAP nuclear levels and target gene expression returned to baseline.
In aged mice, it was shown that MST1 and LATS1 activity was increased, leading to anomalous Hippo signaling and nonregenerating livers. It is therefore suggestive that the Hippo kinase pathway has a decisive role in determining overall liver size.
Classically, atrophy is triggered by an obstruction of portal venous blood flow and/or results from chronic obstruction of the bile duct. When atrophy occurs unilaterally, the opposite lobe of the liver responds with a hypertrophic response. This response has been capitalized on by hepatobiliary surgeons and interventional radiologists, who perform selective PVE to induce hypertrophy of potentially small remnant lobes before resection, or ligate the portal vein intra-operatively in the two-staged ALPPS procedure (see Chapters 102C and 102D ). In slow-onset atrophy, the liver frequently is significantly distorted, and anatomic landmarks can be markedly changed, most commonly seen accompanied by a rotation of the liver and portal triad structures ( Fig. 6.5 ).
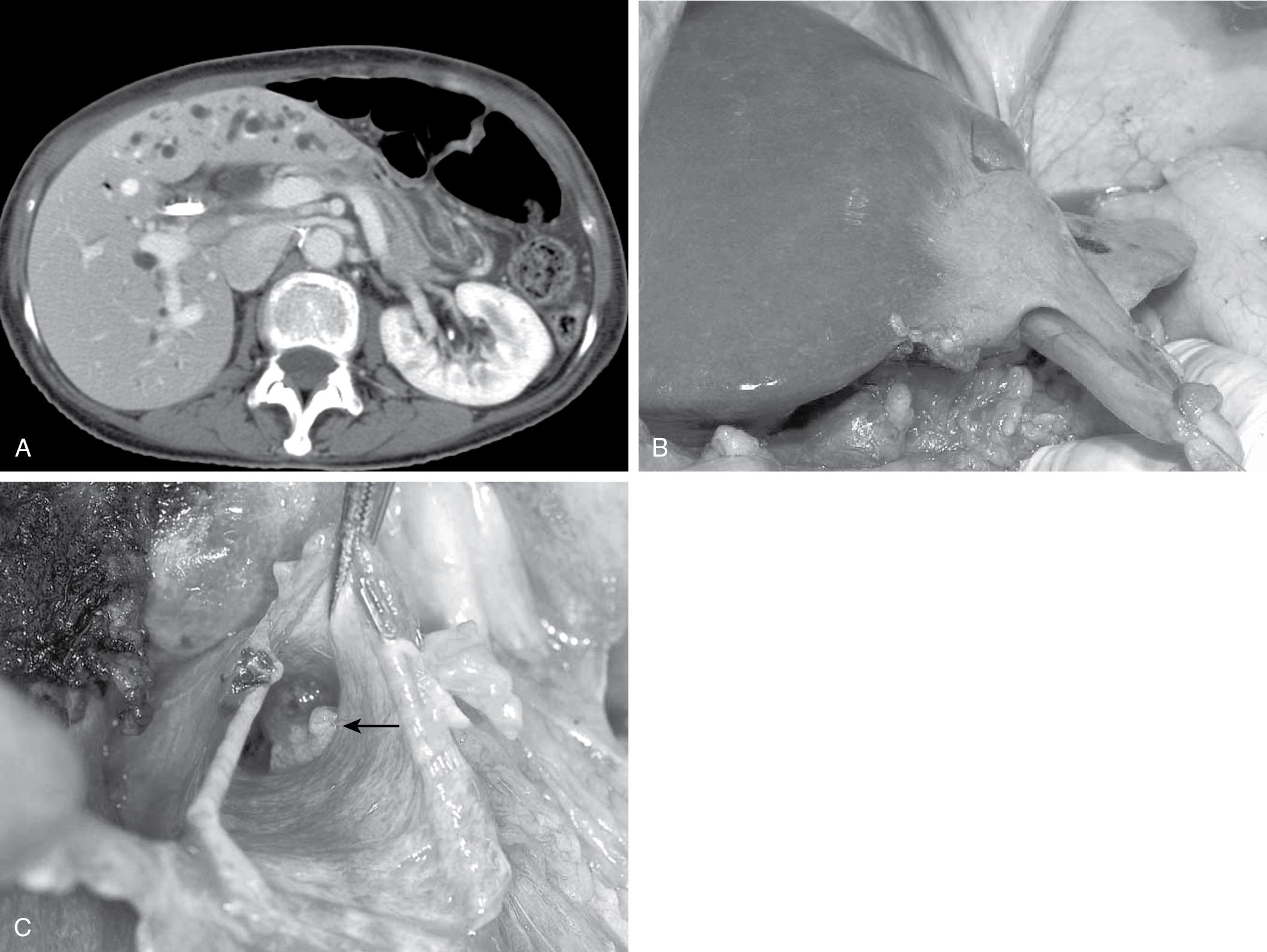
The death of liver cells in atrophy generally is divided into necrosis and apoptosis. The distinction is important because necrosis is a nonregulated traumatic disruption of a cell that occurs when it encounters overwhelming injury, whereas apoptosis is an inducible, highly orchestrated cascade of events that is physiologic. Necrotic cells lose membrane integrity, leak lysosomal enzymes, and induce a large inflammatory response. Apoptosis is energy dependent and allows cells to shrink and die without inducing inflammation.
For more information, see Chapter 102C .
The liver has the remarkable potential to maintain its total volume by adjusting lobes differently in response to extrahepatic stimuli; atrophy of the ligated lobe is the result of apoptosis, whereas increased portal flow in the nonembolized lobe induces proliferation and activates several cytoplasmic growth–promoting signal transduction pathways. , Ischemic necrosis of centrilobular areas of the liver predominates in the first three days of cell death. Areas peripheral to the necrotic liver cells predominantly undergo apoptotic cell death, and apoptosis persists long after necrosis subsides. Oxygen levels and mitochondrial function help determine which cells will undergo necrosis or apoptosis. Models of portal vein ischemia in rats have confirmed a caspase-dependent apoptosis and have indicated that Kupffer cells are involved in generating reactive oxygen substrates and other acute-phase reactants, culminating in mitochondrial dysfunction and apoptosis. ,
Interestingly, in a research environment when the contralateral lobes are resected after PVL, the regenerative stimulus of a 70% hepatectomy can counteract the atrophy of the ipsilateral liver, leading to a low but prolonged regenerative response of the portally deprived liver lobe.
The molecular mechanisms involved in biliary obstruction leading to hepatic atrophy are much more centered on apoptosis, with little or no involvement of acute necrosis. Cholestasis results in the accumulation of toxic bile salts, which induce apoptosis through the Fas-mediated pathway. In this case, TNF-α and Fas ligand bind to the Fas death receptors, leading to a cascade of intracellular events, including cytochrome c release from mitochondria and activation of apoptosis-mediating caspases. In Fas-deficient mice, bile duct ligation resulted in impaired apoptosis and less injury and fibrosis compared with wild-type mice. More recent data, however, suggest a nonischemic model of necrosis/oncosis as the predominant process leading to cell death after common bile duct ligation, with cell swelling and without apoptotic caspase 3 activation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here