Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
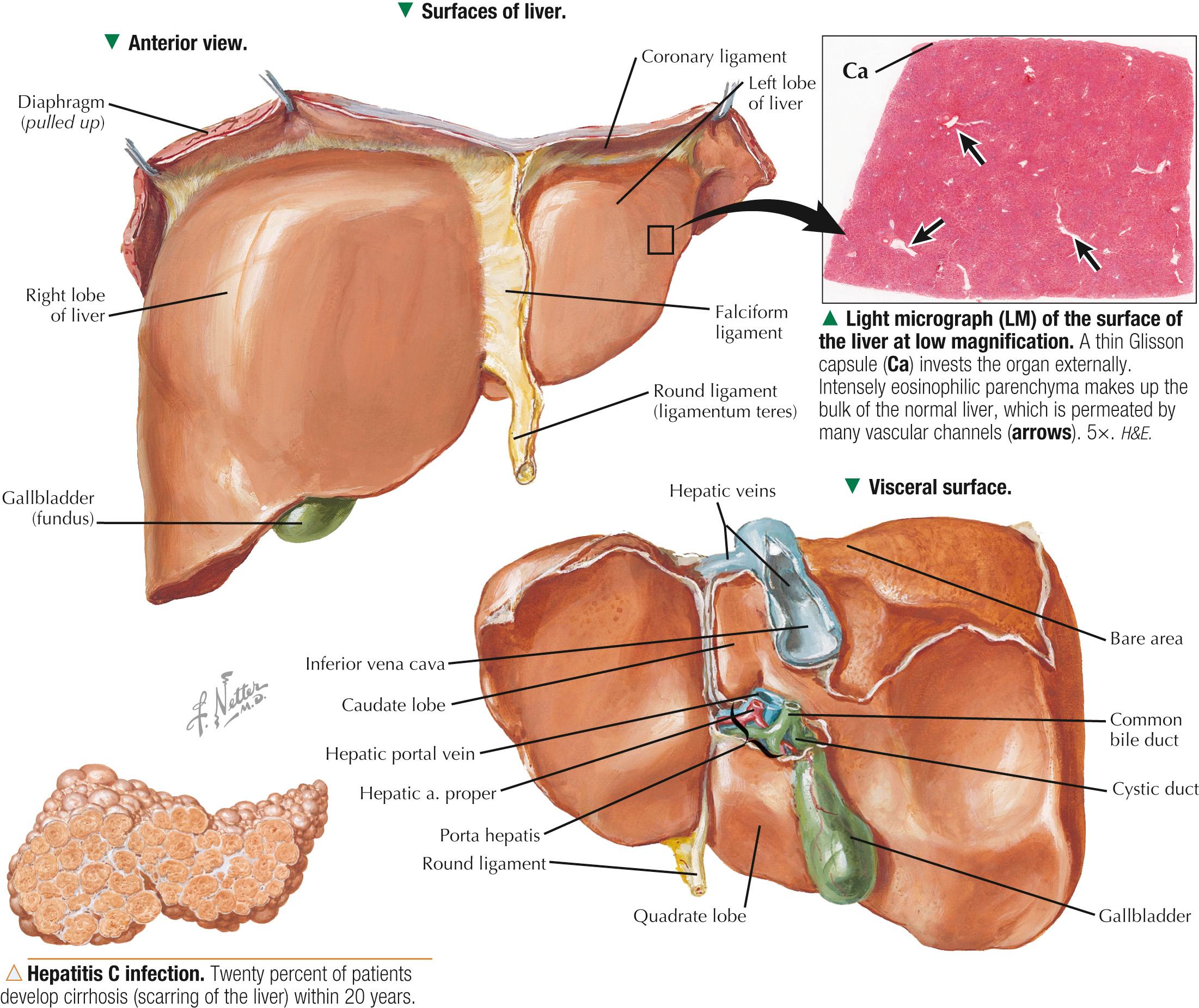
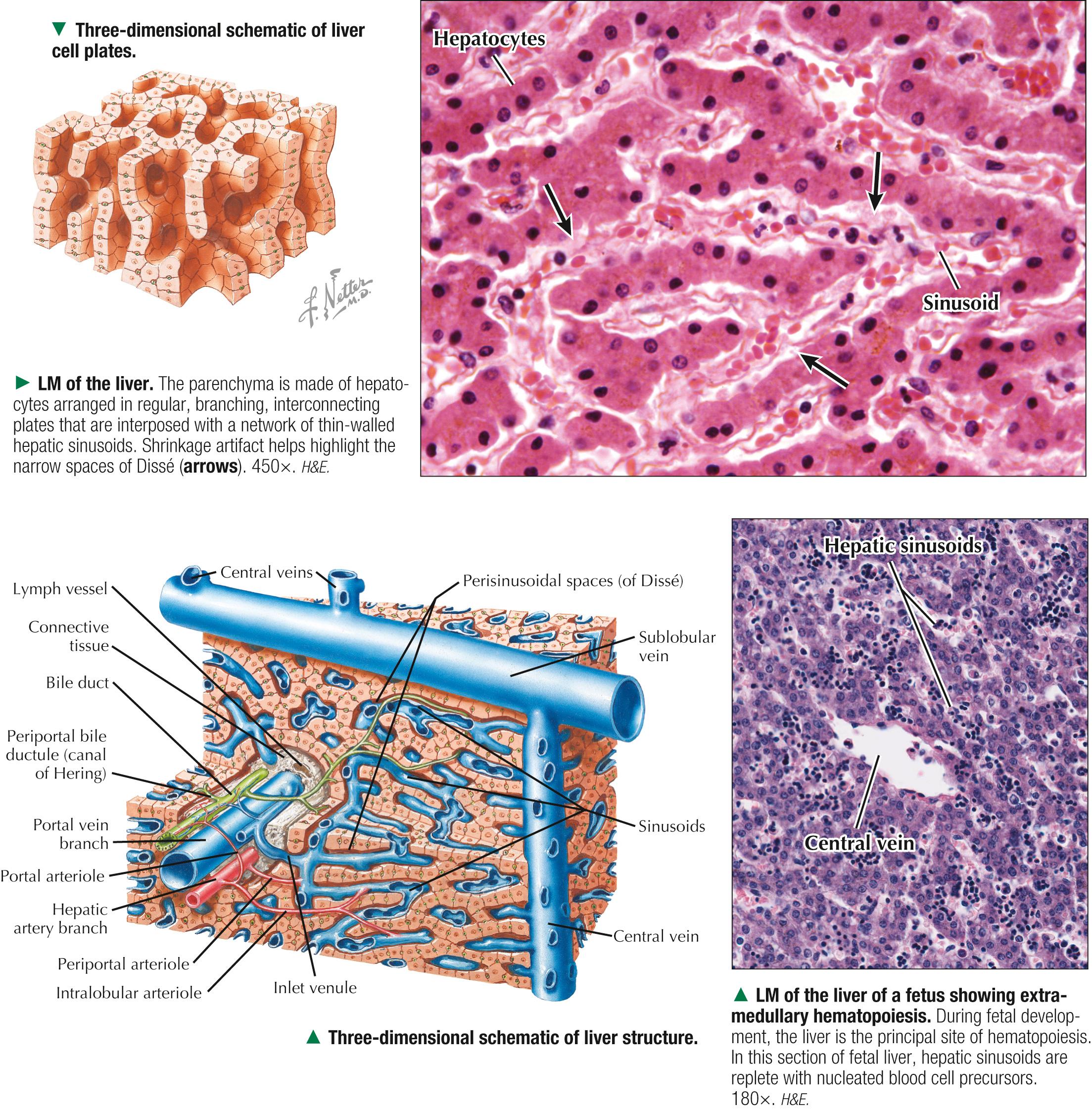
The wedge-shaped liver, the largest and heaviest internal organ (weighs about 1.5 kg in an adult), is essential to life and is the most versatile and vascular organ. It sits just below the diaphragm in the upper right quadrant of the abdominal cavity and is protected completely by the rib cage. It comprises two main lobes of almost equal size— right and left —separated by a falciform ligament and a round ligament (ligamentum teres). Two smaller lobes — caudate and quadrate —are seen on the inferior (visceral) surface but are poorly demarcated. Under a peritoneal serous covering, a thin connective tissue ( Glisson ) capsule surrounds the lobes. On the visceral surface is the porta hepatis , the gateway for the hepatic ducts, portal vein, hepatic artery, lymphatics, and nerves . The liver arises in the embryo as a diverticulum of foregut endoderm. Parenchymal cells proliferating from this diverticulum pass into mesenchyme of the septum transversum and occupy meshes of a network of capillaries related to vitelline and umbilical vessels. The liver is an accessory gland—both exocrine and endocrine —of the digestive tract and plays a central role in three broad functional categories: metabolism of carbohydrates, proteins, and fats; modification of exogenous substances such as drugs and alcohol; and formation and exocrine secretion of bile , which is critical for fat digestion.
Hepatitis —inflammation of the liver—is a serious health risk worldwide. Caused by hepatotropic viruses (A to G), viral hepatitis encompasses a broad array of acute or chronic inflammatory liver disorders. Epstein-Barr virus, herpes simplex virus , and cytomegalovirus may also cause acute hepatitis. Modes of transmission are fecal-oral, food/waterborne, sexual, parenteral, and perinatal. Acute hepatocellular injury, elevated serum aminotransferase and bilirubin levels, and the presence of serum IgM and anti–hepatitis A virus antibody characterize hepatitis A . The histologic hallmark of hepatitis B is the presence of ground-glass hepatocytes caused by accumulation of hepatitis B surface antigen in endoplasmic reticulum. Hepatitis C is the leading cause of death from liver disease in North America. Often transmitted via blood or blood products, it may become chronic, which may lead to cirrhosis and hepatocellular carcinoma.
About 80% of liver tissue in the adult is parenchyma consisting of hepatocytes arranged as a labyrinth of cellular plates. The remaining 20% is stroma , a delicate supportive framework of connective tissue that forms the outer Glisson capsule. At the porta hepatis, this capsule is continuous with the arborization of connective tissue that accompanies the branching pattern of the entering hepatic artery and portal vein and the emerging bile duct. Branches of these three structures are the portal triads . They travel together throughout the liver's interior and divide repeatedly through 17-20 orders of branches. Their size progressively decreases to the terminal ramifications. Connective tissue in the liver indistinctly divides hepatic parenchyma into classic hepatic lobules , which are the liver's structural units. In humans, the lobules are poorly defined, the amount of connective tissue between lobules being scanty. In each lobule, a delicate stroma of reticular fibers forms a supportive network for hepatocytes and surrounding sinusoids.
Malignant tumors of the liver have high morbidity and mortality rates; they may be classified as primary or metastatic . Hepatocellular carcinoma —the most common primary hepatic neoplasia —usually arises from hepatocytes. Chronic liver disease—most often associated with persistent hepatitis B or hepatitis C infection —is the leading cause. Clinical signs may include hepatomegaly, jaundice, fatigue, and elevated serum levels of certain liver enzymes. More commonly, the liver is involved in metastatic (or secondary) spread of tumors from other sites. In most patients with metastatic liver disease , such malignancies mainly arise from the lung, colon, pancreas, and breast. However, other kinds of tumors (e.g., leukemias, lymphomas, melanomas) may also spread to the liver. Depending on the stage of disease, treatment options vary and include surgical resection , targeted radiation therapy , percutaneous hepatic perfusion of chemotherapeutic agents, and liver transplant surgery .
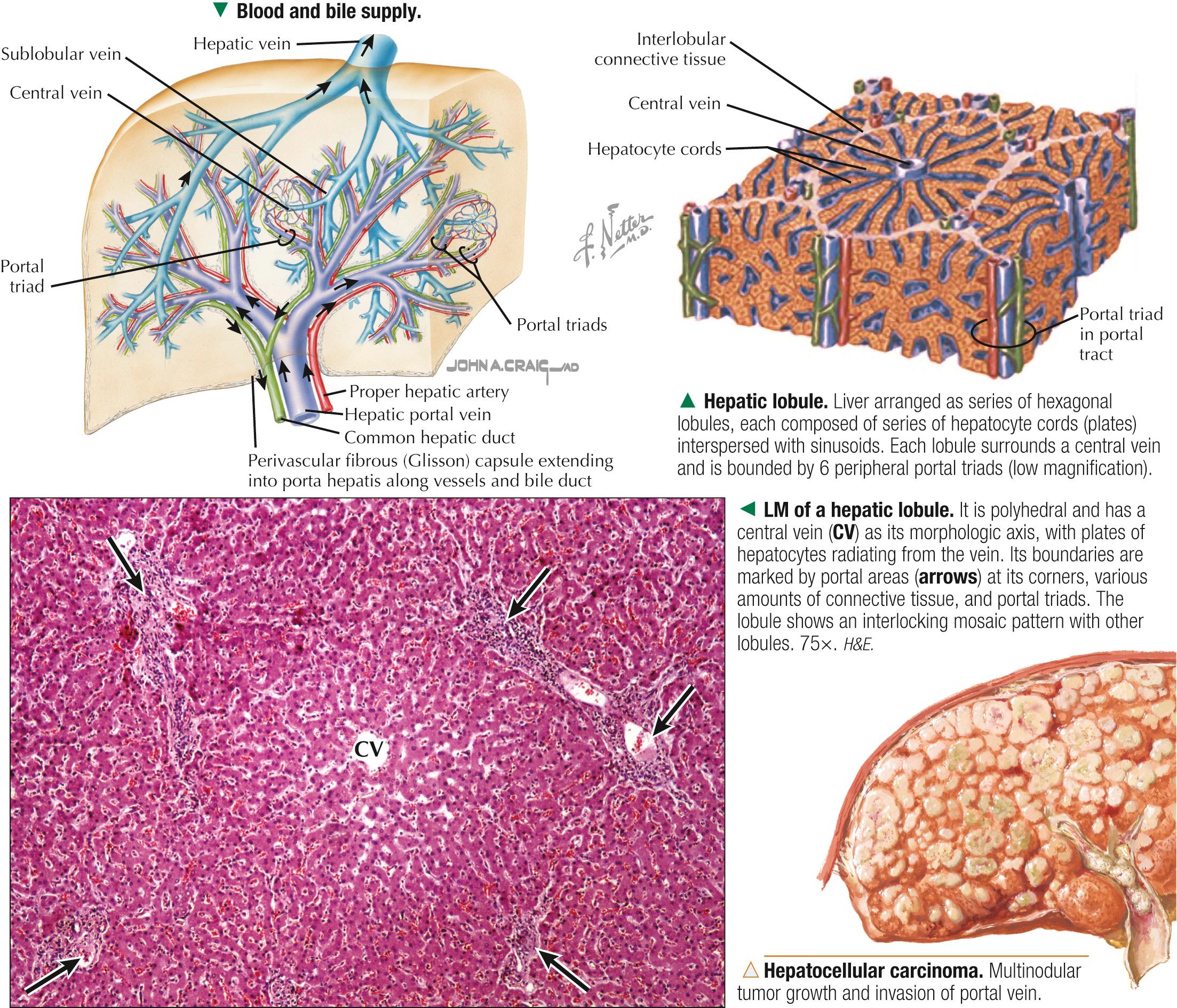
Each classic hepatic lobule has a prism form of about six sides, about 1 mm in diameter and 2 mm long. Portal triads surrounded by small amounts of interlobular connective tissue sit at corners of each lobule in areas called portal tracts , around which are limiting plates of hepatocytes. Triads also mark peripheral meeting places of adjacent lobules , which look like a mosaic of interlocking tiles. In transverse section, each lobule consists of plates of hepatocytes, one or two cells thick, which are separated by hepatic sinusoids and appear to radiate out from a small central vein. Hepatocyte arrangement resembles that of a sponge, with sinusoids represented by the spaces. Each triad consists of a branch of the bile duct, portal vein, and hepatic artery, which divide into smaller branches. Small lymphatic vessels often accompany them. A unique, unusual feature of the liver is a dual blood supply. The portal vein brings nutrient-rich blood from the gastrointestinal tract—75% of the total blood to the liver; hepatic arteries provide 25% of oxygenated blood. The liver receives about 1.5 L of blood each minute, and at least 20% of its volume is occupied by blood. Terminal branches of portal veins , about 300 µm in diameter, regularly give off inlet venules, which empty into thin-walled, fenestrated hepatic sinusoids that are in intimate contact with hepatocytes. Terminal branches of hepatic arteries, which ramify with portal vein branches, end as arterioles that drain into sinusoids, which thus receive a mixture of arterial and venous blood. Sinusoids converge toward a central vein , also called a terminal hepatic venule, and empty into it in the center of each lobule. A central vein is about 50 µm in diameter. Central veins unite to form sublobular veins , which lead into larger hepatic veins that travel alone and branch repeatedly. Hepatic veins coalesce to join the inferior vena cava , the main drainage route of blood from the liver. Important for understanding lobule organization and hepatocyte function, blood and bile flow through lobules in opposite directions.
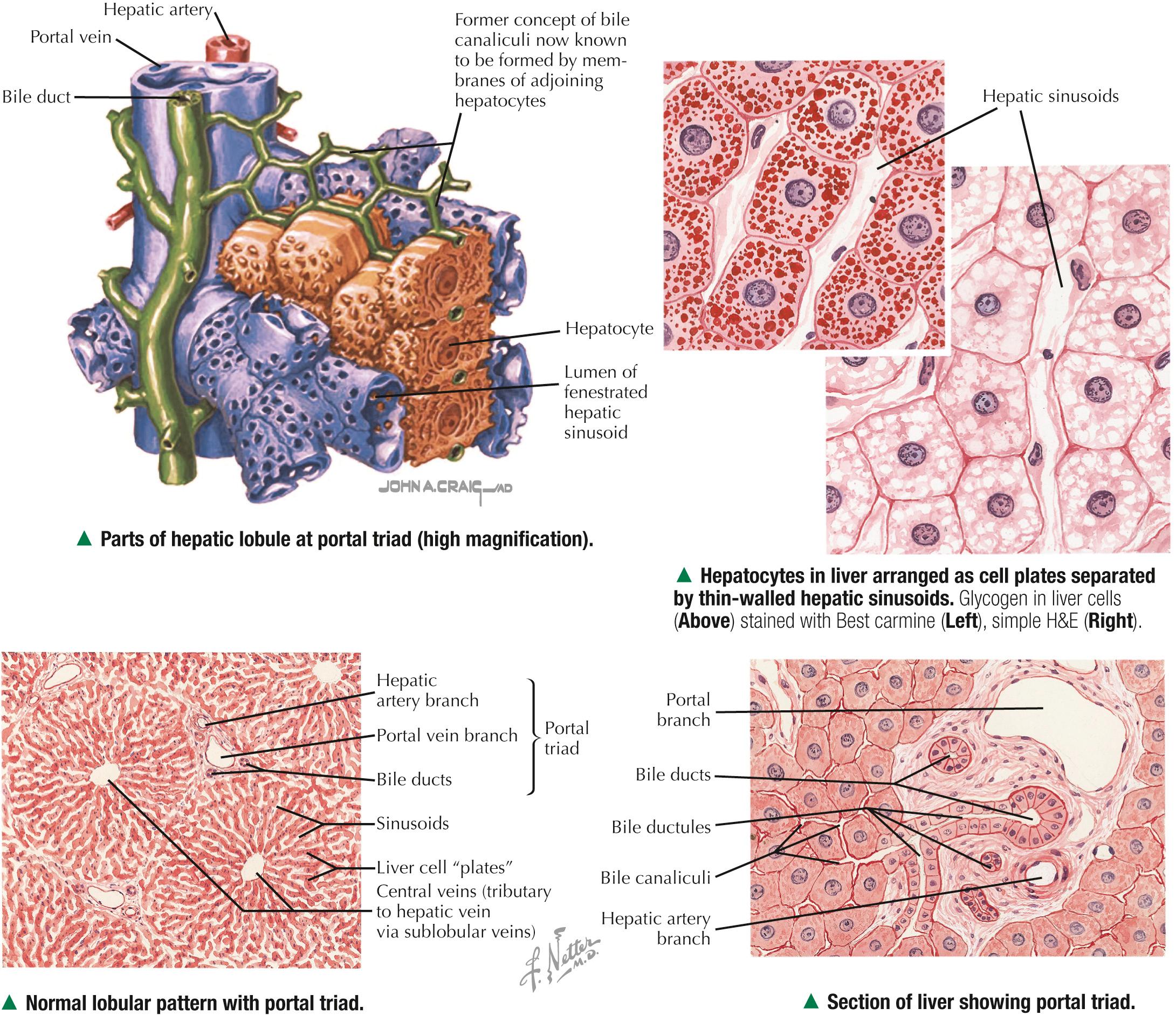
At their smallest branches, the three components of the portal triad are accompanied by small lymphatic vessels. Connective tissue stroma known as the portal tract encloses them all. In transverse section, the hepatic arteriole consists of one to three layers of smooth muscle cells and a relatively small lumen. The portal venule has a larger, often collapsed lumen with a more attenuated wall. The bile ductule is lined by simple cuboidal to columnar epithelium and drains exocrine secretions of hepatocytes from the liver. Biliary passages start with tiny bile canaliculi between hepatocytes. Best seen by electron microscopy, they are small intercellular channels formed by groove-like invaginations of adjacent hepatocytes. As canaliculi approach the periphery of each lobule, they are drained by small ducts, known as canals of Hering , lined by a low simple cuboidal epithelium. These canals drain to larger bile ducts in portal tracts. As the ducts widen, their simple columnar epithelium becomes taller. Typical central veins are thin-walled venules with an attenuated endothelium. They normally lack significant investment of connective tissue stroma. The lumen of each central vein has numerous openings, which allows several hepatic sinusoids to drain freely into them.
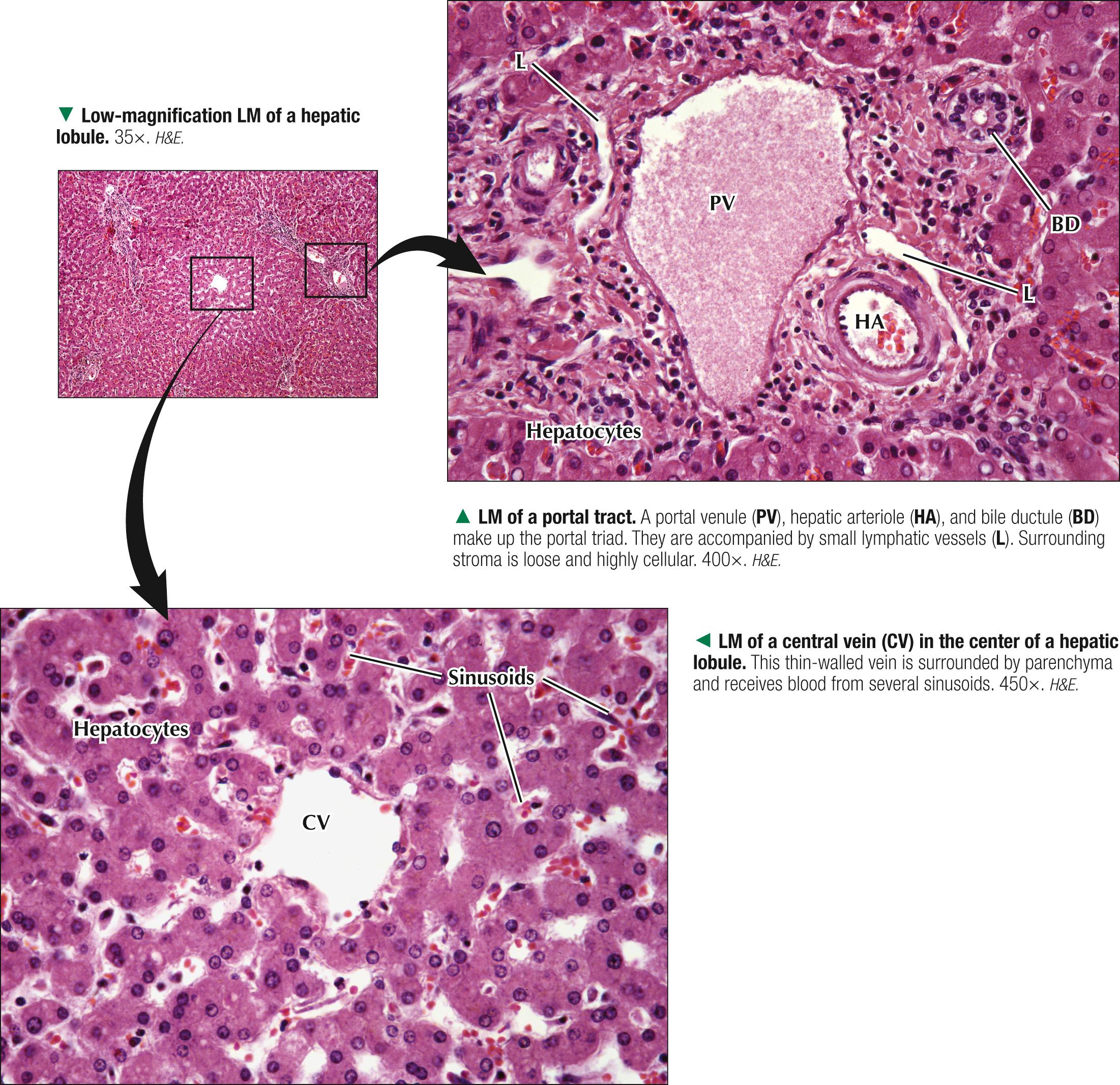
Three-dimensional reconstructions of liver from serial sections provide insights into the arrangement of hepatic parenchyma and its relationship to vascular and biliary duct systems. The parenchyma consists of an anastomosing network of interconnecting plates, one or two cells thick, which resemble walls of a building with spaces in between. The hepatocytes in each plate can be likened to the building's bricks, and the hepatic sinusoids appear suspended within the spaces. In humans, one-cell-thick plates are most common in the normal adult liver; two-cell-thick plates occur in the embryo and in adults during regeneration in certain diseases. Via electron microscopy or special light microscopic techniques, narrow fluid-filled perivascular spaces—spaces of Dissé (or perisinusoidal spaces)—can be seen separating the endothelial lining of sinusoids from the hepatocyte surfaces. These spaces allow plasma to flow between sinusoidal lumina and hepatocyte surfaces, which permits rapid exchange of soluble, noncellular substances between blood and parenchyma. In the fetus and in chronic anemias, these spaces are sites of extramedullary hematopoiesis. Hepatic lymph originates in these spaces and eventually drains to small lymphatic vessels in portal tracts.
Another concept of liver lobulation is the liver acinus —an oval to diamond-shaped area of hepatic parenchyma defined in relation to blood supply from terminal branches of the portal vein and hepatic artery . It is smaller and harder to see than the classic hepatic lobule, but it is useful functionally and clinically because it is best for describing metabolic and pathologic changes in relation to many diseases. Its short axis runs along the border of two classic hepatic lobules; its long axis is an imaginary line between two central veins closest to the short axis. Hepatocytes in the acinus are arranged in three concentric, elliptical zones around the short axis. Zone 1 , most central, is closest to the terminal distributing branches of the portal venule and hepatic arteriole. This zone first receives oxygen, hormones, and nutrients from the bloodstream, and most glycogen and plasma protein synthesis by hepatocytes occurs here. Zone 3 is furthest from the distributing vessels; between zones 1 and 3 is the intermediate zone 2 . A gradient of metabolic activity exists for many hepatic enzymes in the three zones. Zone 3 is poorly oxygenated, is the first to show ischemic necrosis and fat accumulation if metabolism is altered, and is the site of most drug and alcohol detoxification . The classic hepatic lobule and liver acinus are not contradictory concepts of lobulation but rather complement each other.
A broad panel of laboratory tests—known collectively as liver function tests —is used clinically for diagnosis of liver disorders. They also assess disease severity, prognosis, and treatment outcome. Serum aspartate aminotransferase (AST) and alanine transaminase (ALT)—cytosolic enzymes released into bloodstream in response to hepatocyte injury—are sensitive indicators of liver damage. The highest elevations occur in patients with acute viral hepatitis and toxin-induced hepatic necrosis . The AST-to-ALT ratio may also be useful in distinguishing different causes of hepatotoxicity. A high ratio suggests advanced alcoholic liver disease; lower values are seen in those with viral hepatitis . Whereas chronic disorders, such as cirrhosis, lead to decreased serum levels of albumin (a protein synthesized exclusively in the liver), bile duct obstruction and intrahepatic cholestasis cause elevations in alkaline phosphatase (an enzyme present in the biliary duct system).

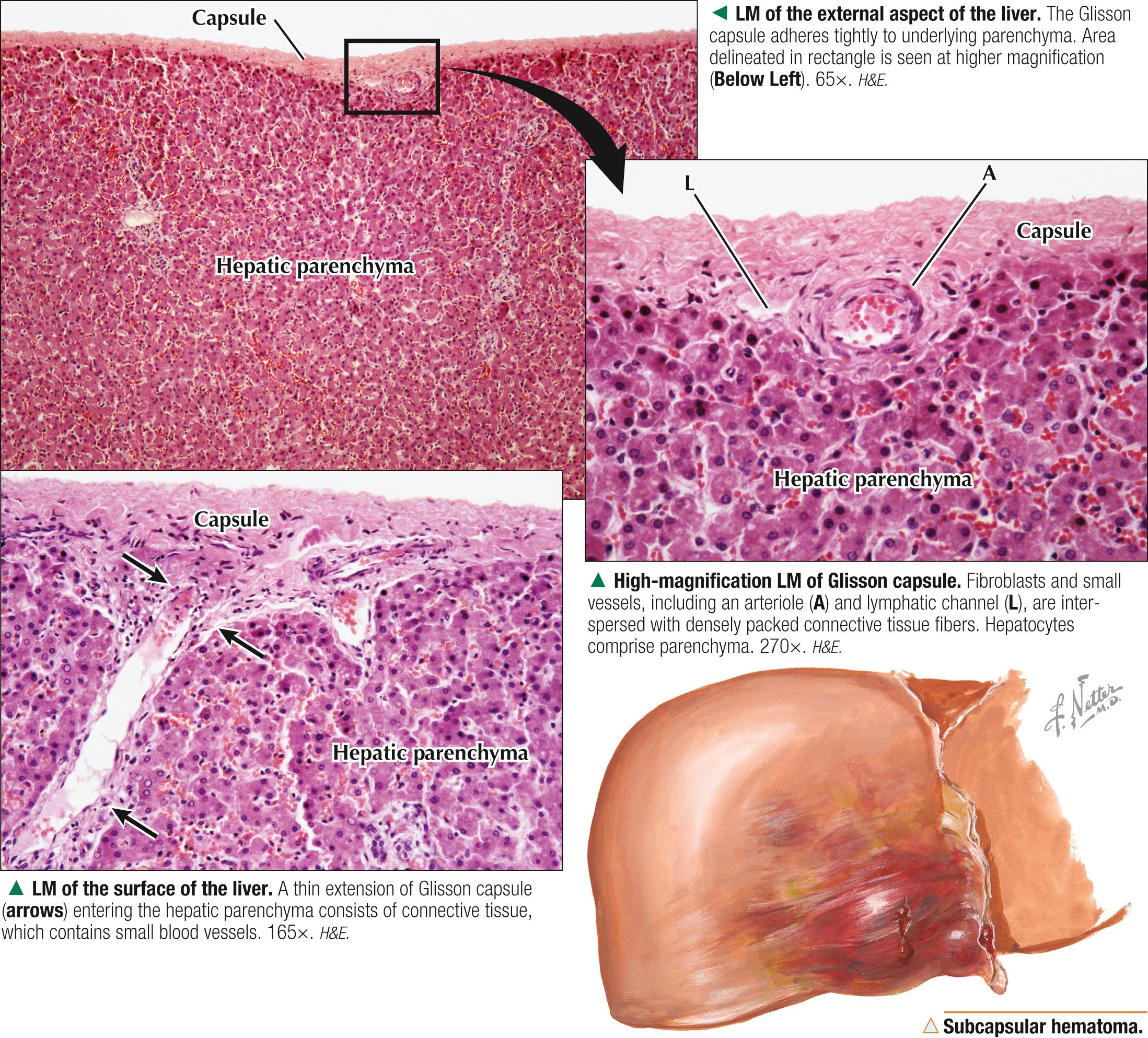
Except where the liver attaches to the diaphragm, it is surrounded by a dense fibrous connective tissue capsule , 70-100 µm thick. Serous mesothelium covers this capsule where it faces the peritoneal cavity. The mesothelium acts as a shield, especially against entry of pathogenic bacteria and other potentially harmful substances. The capsule comprises regularly arranged collagen and elastic fibers and provides external support and shape to the liver. It also contains a few small blood vessels and sends anchoring extensions into hepatic parenchyma, which contributes to its supportive stroma. Continuations of the capsule penetrate through the porta hepatis for support of blood and lymph vessels, ducts, and nerves. In part because of the thinness of the capsule, the liver, which has the consistency of table jelly, damages easily. Being richly vascularized, it bleeds profusely after injury. The capsule increases in thickness with aging and may undergo excessive proliferation in response to certain diseases. The stroma also proliferates after hepatocellular injury.
Because of its strategic location, large mass, and fragile capsule (that provides relatively little protection to the organ), the liver is prone to many injuries. Its complex vascular supply and enormous blood reserve also make it vulnerable to bleeding with extensive blood loss ( exsanguination ) into the abdominal cavity. Blunt and penetrating traumas are the most common causes of subcapsular hepatic hematomas —localized collections of extravasated blood contained by Glisson capsule—that may subsequently rupture and be life threatening. Subcapsular hematomas may also occur as a complication of preeclampsia in pregnancy (a leading cause of maternal death) or in some parasitic infestations (e.g., amebiasis , schistosomiasis ) of the liver, which cause hemorrhage, liver necrosis, inflammation, and subsequent fibrosis. Although most patients are managed conservatively, some require urgent surgical intervention.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here