Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver is the largest solid organ in the human body. It has a unique structure with a dual blood supply, being approximately one-third from the hepatic artery and two-thirds from the portal venous system. Within the liver substance, blood flows through sinusoids between plates of hepatocytes into central veins, which in turn join the hepatic veins draining into the vena cava. The liver is a major site of protein synthesis exporting plasma proteins to maintain oncotic pressure and coagulation factors. Acute-phase proteins that act as antiproteases, opsonins and metal ion carriers are synthesised by the liver in response to injury or infection. Numerous immune cells populate the liver and the resident tissue macrophages, the Kupffer cells, form an important component of the innate immune system. Nutrients are extracted from portal blood by the liver and processed, and the liver acts as an important reservoir for glycogen. Waste products are either modified in the liver for excretion by the kidneys or are excreted into bile. Many drugs are taken up by the liver and metabolised, giving either active metabolites or inactive metabolites for excretion. In humans, as in many vertebrates, the liver’s capacity for metabolism and clearance far exceeds what is required for day-to-day life. It is possible that this ability offers a significant advantage in terms of survival from poisoning, starvation or trauma.
In the acute setting, liver failure can present with a number of symptoms, but it is important to note that not all of these may be present at the same time. Typically, a patient with acute liver failure after surgery, transplantation or due to acute poisoning will be confused or cognitively impaired as a result of encephalopathy, which may progress to loss of consciousness and a need to protect the airway by intubation and mechanical ventilation. Patients are often not immediately jaundiced, but jaundice may develop over the course of several days. Patients may be hypoglycaemic, and the requirement for intravenous infusion of dextrose is a sinister development and an indicator of severe acute liver failure. Coagulopathy may develop, with evidence of bruising or bleeding from line sites or surgical scars. Severe acute liver failure can be assessed using the King’s College Hospital criteria, which were designed to predict mortality in paracetamol- and non-paracetamol-dependent acute liver failure. Later, this scoring system was adopted in the UK to determine criteria indicating likely benefit from liver transplantation. In the surgical patient, the development of acute liver failure is usually more gradual and less dramatic; a useful scoring system for liver dysfunction in the acute setting has been reported by Schindl et al. ( Box 2.1 ).
Total bilirubin (μmol/L)
<20 (0 points)
21–60 (1 point)
>60 (2 points)
Prothrombin time (seconds above normal)
<4 (0 points)
4–6 (1 point)
>6 (2 points)
Serum lactate (mmol/L)
≤1.5 (0 points)
1.6–3.5 (1 point)
>3.5 (2 points)
Encephalopathy grade (West Haven Criteria)
None (0 points)
1 and 2 (1 point)
3 and 4 (2 points)
Severity of hepatic dysfunction
None = 0 points; mild = 1–2 points; moderate = 3–4 points; severe > 4 points
Liver resection has the potential to cure patients with cancers that have originated in the liver itself (primary liver cancer) or that have originated elsewhere and have subsequently spread to the liver (metastatic liver cancer). Equally, it is a preferred therapy in patients with benign liver tumours that have the potential of malignant transformation (uncertain benign primary liver tumours). Resection of up to 70% of the liver is feasible, because the liver has a remarkable capacity to regenerate. Within 6–8 weeks following 60–70% hepatectomy, the liver has regained nearly all of its original size and weight.
The most common cause of liver metastases is from primary colorectal cancer, and it is estimated that in the West there is a yearly incidence of 300 new cases of colorectal liver metastases per million population. The current estimate is that this should lead to approximately 100–150 patients per million eligible for liver resection for this indication. To this should be added the patients with primary benign and malignant liver tumours, and hence about 150–200 liver resections should probably be performed per million population each year.
Ever since the first liver resection by Langenbuch in 1887, this procedure has remained a major undertaking and even in the recent past, liver resection was still a dangerous surgical procedure with a high mortality of 20–30% in the 1970s. This was mainly due to excessive intraoperative bleeding; however, over the subsequent decades, the procedure has become increasingly safer due to improvements in surgical and anaesthetic techniques. At present, mortality rates are reported to be well below 5%. Currently, the single most important cause of lethal outcome following hepatic resection is liver failure. For this reason, many researchers and clinicians have attempted to design methods to identify patients at risk of liver failure (and hence mortality) following liver surgery. However, the development of such a method has been hampered by several factors, as outlined below.
The critical point determining lethal outcome following liver resection has been a failure of the residual liver to function properly. Focus in this research area has been to determine a single liver function test that identifies patients with impaired liver function. This has proven exceedingly difficult, and such a test is not available for a number of reasons.
First, as outlined above, the liver has a remarkable capacity to regenerate very rapidly, which emphasises that there is tremendous overcapacity of several liver functions. In this context, it is known that it is entirely safe to resect 50% of an otherwise healthy liver, because the residual half liver will simply take over all vital liver functions such as clearing bacteria, urea synthesis and synthesis of crucial proteins. It has been estimated that a crucial liver function, such as urea synthesis, has an overcapacity of 300%, which implies that a static preoperative liver function test will be unable to assess this particular function. An alternative and innovative strategy would be to give a challenge to the liver and measure the ability of the liver to respond or cope—a dynamic test.
![]() The critical minimum residual liver volume for healthy liver parenchyma has been estimated to be approximately 25% after resection.
The critical minimum residual liver volume for healthy liver parenchyma has been estimated to be approximately 25% after resection.
The second crucial problem has been that there is only a poor correlation between volume and function. However, it is still unclear why some patients with smaller hepatic remnants do not develop liver failure whilst some with greater residual volumes do. These observations suggest, however, that peri- and intraoperative events superimposed on the innate hepatic capacity to withstand injury play a role. Hepatic insufficiency in this situation may arise either if not enough liver volume is left after partial hepatectomy or if the residual volume does not function properly. A functional limitation may arise, for example, in patients who have received chemotherapy in order to reduce the number and size of metastases prior to surgical treatment by liver resection. One of the factors contributing to defective defence may be preoperative fasting, but equally, prior chemotherapy and pre-existent steatosis may play a role.
A third important aspect is that during liver surgery, deliberate hypotension and temporary hepatic blood inflow occlusion (Pringle manoeuvre) are used by many surgeons to reduce blood loss during hepatic surgery (15 minutes ischaemia, 5 minutes reperfusion [15/5 Pringle]). Other surgeons do not use this manoeuvre, assuming that it causes oxidative stress and ischaemia/reperfusion (I/R) injury. , There is little doubt that this procedure does cause oxidative stress and I/R injury; however, the consequence of this is variable. In a situation where defence mechanisms against oxidative stress are deficient, it may adversely affect liver function. In this situation, hepatic steatosis may constitute an additional predisposing factor to damage by I/R.
![]() Ischaemia/reperfusion is the basis of ischaemic preconditioning, a process in which temporary clamping and release of the liver blood flow has been shown to be beneficial in terms of increasing resistance to subsequent injury.
Ischaemia/reperfusion is the basis of ischaemic preconditioning, a process in which temporary clamping and release of the liver blood flow has been shown to be beneficial in terms of increasing resistance to subsequent injury.
In this situation, it is assumed that defence mechanisms against oxidative stress are adequate and are indeed enhanced by short-term I/R injury.
The above three factors explain why it has been exceedingly difficult to design a proper liver function test that reliably singles out those patients at risk of liver failure following liver resection. The term ‘liver function’ is a rather crude denominator for a range of functions that includes ammonia detoxification, urea synthesis, protein synthesis and breakdown, bile synthesis and secretion, gluconeogenesis and detoxification of drugs, bacteria and bacterial toxins.
The clinical signs of chronic liver failure are often insidious and can also be related to the type of disease. Cirrhosis is associated with a failure of hepatic function and the consequences of increased hepatic vascular resistance. Metabolic impairment is manifest by jaundice, coagulopathy, impaired ammonia clearance and encephalopathy, hypoalbuminaemia and oedema. The presence of increased vascular resistance is associated with the development of splenomegaly, ascites and gastro-oesophageal or abdominal wall varices. The slow progression of many chronic liver diseases, over years, implies a gradual, almost incremental, loss of liver cell mass or function. There are many causes of liver failure, including hepatitis B and C virus, autoimmune diseases such as primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis, alcoholic liver disease, Wilson’s disease, α 1 -antitrypsin deficiency and others. All are associated with chronic or repeated cell injury and attempts at repair. The fibrosis and scarring associated with this regeneration and repair lead to the clinical condition termed cirrhosis, with a typically small shrunken irregular liver and an increased risk of cancer.
The Child–Pugh score for chronic liver disease has served as a useful means of categorising patients based on the severity of their liver disease. It employs five clinical measures of liver disease and each measure is scored 1–3, with 3 indicating the most severe derangement ( Table 2.1 ). In the setting of liver transplantation, the Model for End-stage Liver Disease (MELD) or MELD-Na (MELD including sodium) score or in the United Kingdom UKELD score has partly replaced Child–Pugh scoring in the assessment of severity of liver disease.
| Measure | 1 point | 2 points | 3 points | Units |
|---|---|---|---|---|
| Bilirubin (total) | < 34 (< 2) | 34–50 (2,3) | > 50 (> 3) | μmol/L (mg/dL) |
| Serum albumin | > 35 | 28–35 | < 28 | g/L |
| INR | < 1.7 | 1.71–2.20 | > 2.20 | No unit |
| Ascites | None | Suppressed with medication | Refractory | No unit |
| Hepatic encephalopathy | None | Grade I–II (or suppressed with medication) | Grade III–IV (or refractory) | No unit |
The liver plays a central role in fat, carbohydrate and protein metabolism, as well as in acid–base homeostasis. In the context of liver failure, disturbances of fat metabolism are probably not crucially important. With respect to carbohydrate metabolism, it is well known that the liver plays a central role in the conversion of lactate to glucose. Part of this lactate is formed due to anaerobic metabolism of, amongst others, glucose in skeletal muscle. This metabolic route of glucose to lactate (muscle) and then back to glucose (liver) is very important for glycaemic homeostasis and is called the Cori cycle. Liver failure will be manifested by lactic acidosis and hypoglycaemia.
Next to its role in carbohydrate metabolism, the liver plays a central function in nitrogen homeostasis. Hepatic synthesis and breakdown of proteins and amino acids, and detoxification and clearance of the nitrogenous waste products from other organs are of central importance. For example, the gut uses the amino acid glutamine as a fuel for enterocytes, which results in the production of waste end-products of intestinal metabolism, such as ammonia. This ammonia is then transported via the portal vein to the liver, where it is detoxified with the formation of urea.
The failing liver can trigger a range of events resulting in multi-organ failure, sepsis and death. When three or more organs are involved, the chance of death approaches 80%. Bacterial infection and hepatic encephalopathy are the leading causes of death in this group who experience progressive systemic failure.
Risk of bacterial infection is increased considerably following partial hepatectomy. Immune function is compromised as the liver’s phagocytic and synthetic capacity is impaired by its reduced size. The shear force generated by increased portal venous blood flow per unit area, as well as ischaemic injury at the time of surgery, can further impair immune capacity. Management of sepsis in these patients necessitates intensive care, multi-organ support and broad-spectrum antibiotics subsequently guided by culture results.
Hepatic encephalopathy is a reversible neuropsychiatric syndrome, with a multifactorial cause. It is characterised by cerebral oedema, raised intracranial pressure, with risk of brain herniation and death. A range of factors may contribute to this phenomenon, including rising concentration of ammonia, glutamine and lactate. Ammonia has a range of effects on brain function, affecting neurotransmission as well as impairing mitochondrial function and key cellular transport systems. There is a direct correlation between arterial ammonia concentration and the presence of brain herniation. Brain glutamine concentration is elevated in acute liver failure, which may influence the development of hepatic encephalopathy through toxic metabolites, modulation of hepatic blood flow or by amplifying the toxic effects of ammonia. Lactate can result in significant swelling of astrocytes in culture and raised brain lactate concentration is seen in a wide range of experimental models of acute liver failure.
Therapeutic approaches in hepatic encephalopathy aim to address these areas with strategies to lower ammonia levels, protect systems by inducing mild hypothermia, reduce blood–brain ammonia transfer, decrease brain lactate synthesis and reduce inflammation. However, in the face of overwhelming liver failure, attempts to modulate these mechanisms of hepatic encephalopathy have been shown at best to prolong life by hours to a few days. In some selected patients, this may provide a ‘bridge to liver transplantation’; however, patients undergoing surgery for metastatic disease are ineligible for transplantation and therefore their only hope lies in the intrinsic ability of the liver to regenerate.
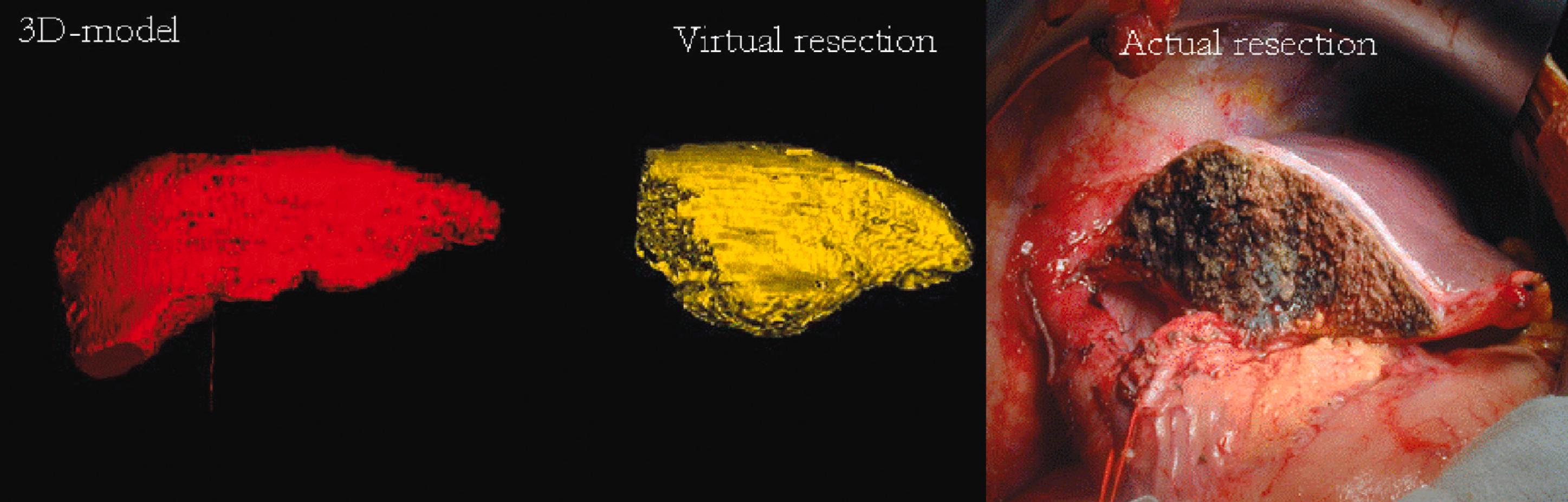
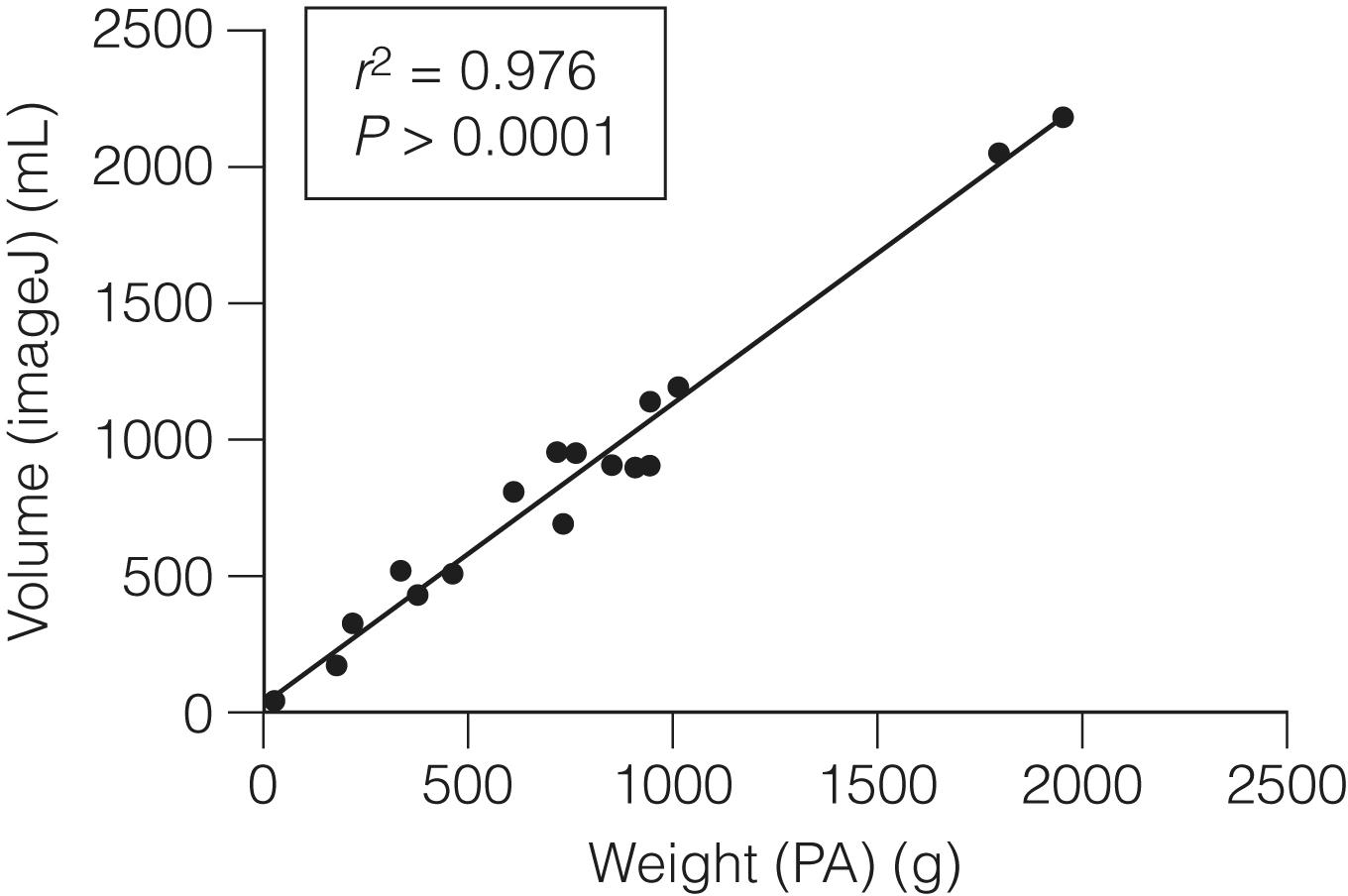
Advances in imaging techniques have permitted the development of in vivo imaging of the liver. Three-dimensional models of the liver can be constructed from computed tomography (CT) or other cross-sectional imaging modalities, such as magnetic resonance imaging (MRI). The volume of the liver can then be calculated based on known separation of image slices combined with planar mapping of cross-sectional areas. In addition, such three-dimensional computer models can be simulated to map the effects of surgery by performing virtual hepatic resection, and studies have demonstrated that there is a good correlation between computer modelling and actual resection weight of surgical liver specimens ( Figs. 2.1–2.3 ). , Some centres use 3D printers to create a replica of the patient’s liver. This enables the relationship between the tumour and the vascular/biliary anatomy of the liver to be better understood, aiding complex liver resections.
Liver function tests refer to the transaminases, alkaline phosphatase, γ-glutamyl transferase and bilirubin. They are not truly measures of function but do give an indication of processes going on within the liver. Aspartate aminotransferase and alanine aminotransferase are hepatocyte enzymes that are released in conditions in which hepatocytes are damaged or killed, such as ischaemic injury, hepatitis, severe sepsis and in response to cancer. Liver-specific alkaline phosphatase is expressed predominantly in the biliary epithelium and is elevated in conditions such as cholangitis or biliary obstruction. γ-Glutamyl transferase is expressed by both hepatocytes and biliary epithelium; it is often elevated in patients with cancer but can also be induced by high alcohol consumption.
Biochemical markers of true liver function vary depending on whether acute or chronic liver failure or injury is being considered ( Table 2.2 ).
| Acute | Chronic | |
|---|---|---|
| Albumin | – | +++ |
| Prothrombin time | +++ | +++ |
| Bilirubin | + | +++ |
| Lactate | ++ | – |
| Glucose requirement | ++ | – |
| Ammonia | + | + |
The ability to accurately predict postoperative outcome based on preoperative liver function would be a valuable addition to preoperative assessment. The tests currently in common use include the indocyanine green (ICG) clearance test, hepatobiliary scintigraphy with radioisotope clearance, lidocaine clearance test, aminopyrine breath test and the galactose elimination test. These tests aim to provide an indicator of dynamic liver function, in that they can provide real-time assessment of liver function in response to a challenge. However, none of these tests challenge the liver to demonstrate its full functional capacity. Serum bilirubin and clotting factors provide a static indirect estimation of liver metabolism and synthetic function, but are influenced by a range of other factors that limit their relevance and suitability to predict postoperative outcome. The most commonly used test for liver function prior to liver resections is the ICG clearance test.
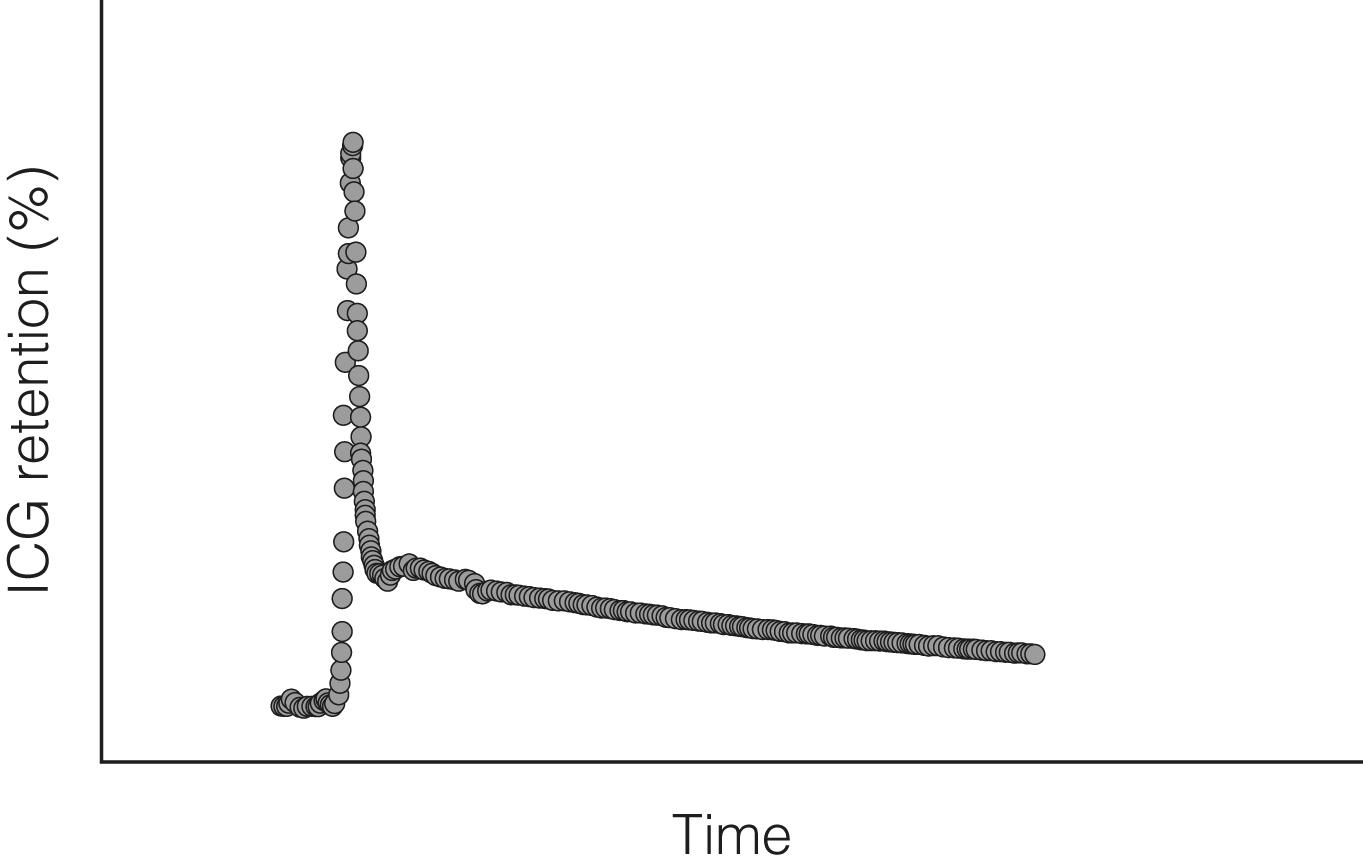
ICG is a compound that is used widely to measure liver function. It is rapidly cleared from blood, specifically by hepatocytes, and is excreted into bile without enterohepatic circulation. Hepatocytes are highly effective at clearing ICG such that hepatic blood flow is the limiting factor in patients with otherwise normal liver parenchyma. In more severe liver disease, both hepatic blood flow and hepatocyte function may be compromised, thereby impairing the clearance of ICG. ICG clearance can be measured as ‘disappearance’ from the blood or can also be measured as accumulation in bile. Liver dysfunction is suggested by a slower rate of clearance from the blood and is usually expressed as percentage retention at 5 or 15 minutes after injection. Continuous measurement of ICG clearance can also be performed, offering potentially improved accuracy, by measurement of the area under the clearance curve ( Fig. 2.4 ). In some centres, ICG clearance is routinely performed during preoperative work-up, with cut-off values set for which patients are ‘safe’ to proceed to resection. However, there is no evidence to suggest that outcomes are improved in centres that use this test compared with centres that do not. In chronic liver disease, the discriminative ability of ICG clearance is greatest in those with intermediate to severe liver failure. Addition of this test to the MELD score can improve prognostic accuracy for patients with intermediate to severe liver dysfunction. However, given the relationship with hepatic blood flow, caution should be exercised when interpreting ICG clearance in the context of abnormally high cardiac output.
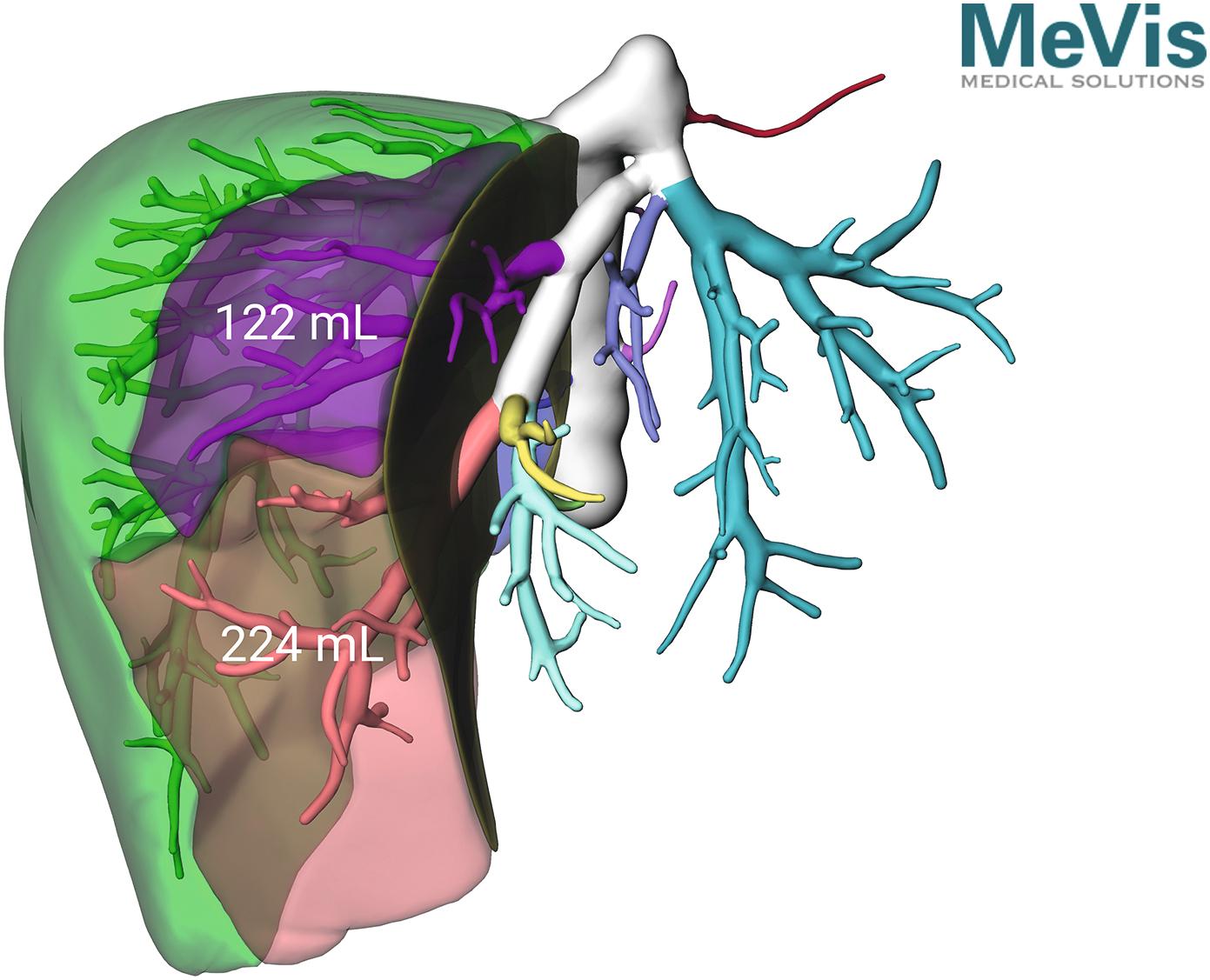
Using a radiolabelled tracer that is eliminated exclusively by the liver, such as [ 99m Tc]mebrofenin (technetium is a gamma-emitting radioisotope), blood clearance and hepatic uptake can be measured using a gamma camera to provide an indication of hepatic function ( Fig. 2.5 ). Hepatobiliary scintigraphy may improve predictive value compared to future liver remnant volume, especially in patients with uncertain quality of liver parenchyma. Combining nuclear medicine techniques with CT (single-photon emission computed tomography [SPECT]) enables the generation of a 3D image of liver function which can be related to liver volume. Using this technique, segmental liver function and liver functional volume can be calculated.
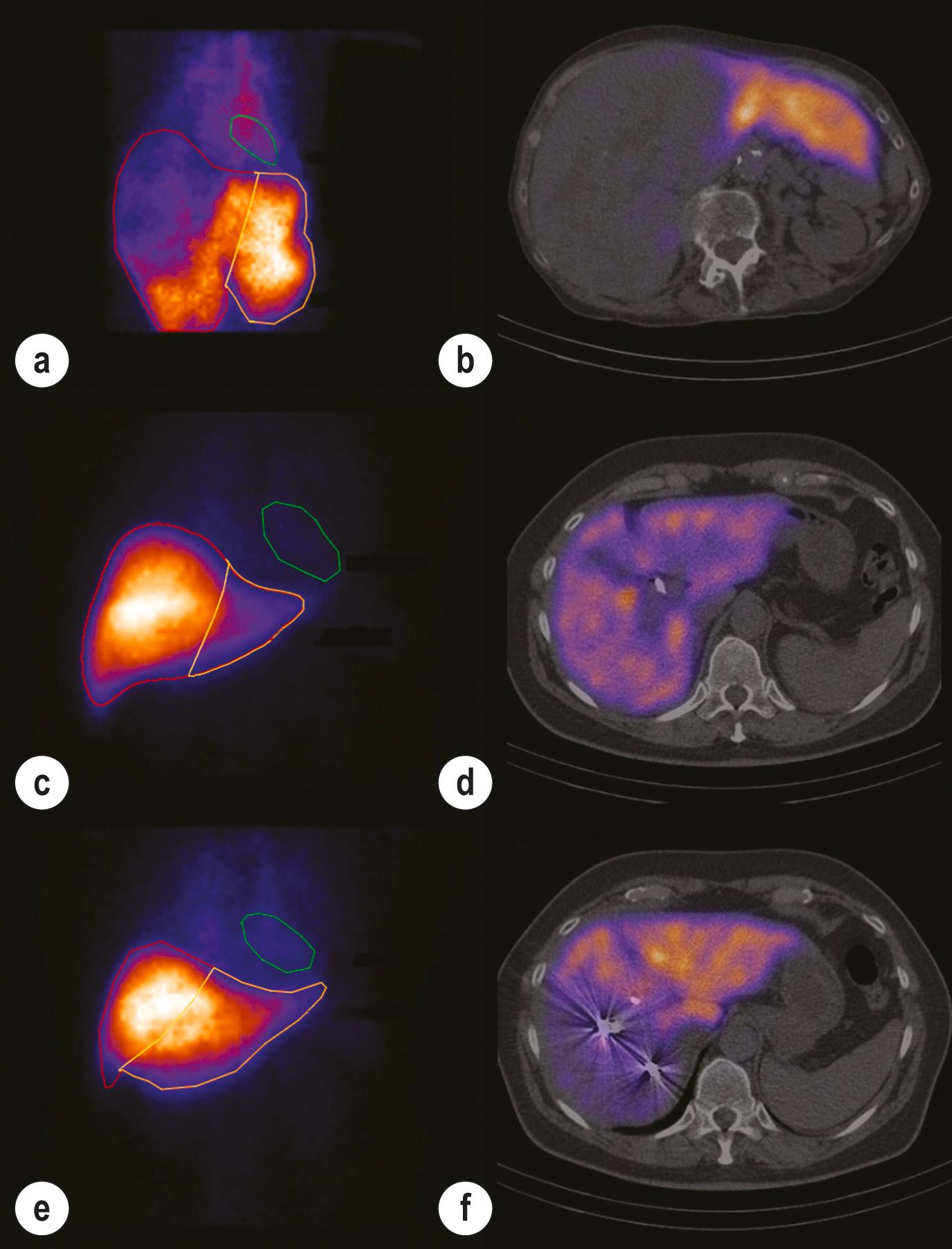
In 2010, de Graaf et al. demonstrated that by combining CT with [ 99m Tc]mebrofenin SPECT, the function of the proposed future liver remnant can be accurately obtained. This group, based at the Amsterdam Medical Centre, subsequently demonstrated that routine implementation of this technique for patients requiring major liver resection significantly reduced postoperative liver failure and failure-related mortality. The authors reported that a better understanding of preoperative liver function improved patient selection and led to an increased use of portal vein embolisation (PVE) to optimise the future liver remnant.
![]() Combining CT with nuclear medicine techniques enables regional liver function to be calculated and can be used to assess preoperative function. ,
Combining CT with nuclear medicine techniques enables regional liver function to be calculated and can be used to assess preoperative function. ,
Lidocaine, also known as monoethylglycinexylidide (MEG-X), is a local anaesthetic that is taken up by the liver and undergoes biotransformation by a cytochrome P450 enzyme, CYP1A2. The rate of disappearance of lidocaine from plasma correlates with liver function; however, measurement of lidocaine is more complex than that of ICG.
The aminopyrine breath test was the first breath test proposed for the assessment of liver function in patients with liver disease. The test uses 13 C 2 -aminopyrine, which is a stable, non-radioactive, isotopically labelled compound eliminated almost exclusively by the liver. Following oral intake, the compound is taken up by the gut and then transported to the liver, where it is metabolised by microsomal cytochrome P450 function. This metabolism liberates 13CO 2 , which can be measured non-invasively in exhaled air. This test is not readily available at the bedside and requires fairly sophisticated apparatus to measure stable isotopic enrichment in the exhaled air. Induction of microsomal metabolism by various drugs may constitute a problem.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here