Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When appropriately ordered and interpreted, serum liver biochemical tests, the so-called “liver function tests” or “liver chemistries,” can be useful in the evaluation and management of patients with liver disorders. The term liver biochemical tests is preferable to liver function tests because the most commonly used tests—the aminotransferases and alkaline phosphatase—do not measure a known function of the liver. These tests have the potential to identify liver disease, distinguish among types of liver disorders, gauge the severity and progression of liver dysfunction, and monitor response to therapy. Understanding the shortcomings of these tests, however, is important. No test can accurately assess the liver’s total functional capacity; biochemical tests measure only a few of the thousands of biochemical functions performed by the liver. Furthermore, considered individually, these tests lack sensitivity and specificity for liver injury; a battery of tests must be used to evaluate the liver. The standard battery of tests that is most helpful in assessing liver disease includes total and direct bilirubin, albumin, prothrombin time, and the serum enzymes: ALT, AST, alkaline phosphatase (ALP), and occasionally GGTP and 5′ nucleotidase (5′NT). Interpretation of these results in concert with careful history taking and a physical examination may suggest a specific type of liver injury, thereby allowing a directed evaluation, risk assessment for surgical procedures, and estimation of prognosis. Other more specialized tests include quantitative tests of liver function and a growing number of options to assess the degree of hepatic fibrosis.
Bilirubin is a breakdown product of heme (ferroprotoporphyrin IX). About 4 mg/kg body weight of bilirubin is produced each day, nearly 80% from the breakdown of hemoglobin in senescent red blood cells and prematurely destroyed erythroid cells in the bone marrow and the remainder from the turnover of hemoproteins such as myoglobin and cytochromes distributed throughout the body. The initial steps of bilirubin metabolism occur in reticuloendothelial cells, predominantly in the spleen. Heme is converted to biliverdin by the microsomal enzyme heme oxygenase. Biliverdin is then converted to bilirubin by the cytosolic enzyme biliverdin reductase.
Bilirubin formed in reticuloendothelial cells is lipid soluble and virtually insoluble in water. In order to be transported in blood, unconjugated bilirubin must be solubilized. The process is initiated by reversible, noncovalent binding to albumin, which has both high-affinity and lower-affinity binding sites for unconjugated bilirubin. The unconjugated bilirubin-albumin complex passes readily through the fenestrations in the endothelium lining the hepatic sinusoids into the space of Disse, where the bilirubin dissociates from albumin and is taken up by hepatocytes via a protein-mediated, facilitated process, possibly mediated by a liver-specific organic anion transport protein.
After entering the hepatocyte, unconjugated bilirubin is bound in the cytosol to a number of proteins, including proteins in the glutathione S -transferase superfamily. These proteins serve to reduce efflux of bilirubin back into the serum and present the bilirubin for conjugation. The enzyme bilirubin uridine diphosphate glucuronyl transferase (B-UGT) found in the endoplasmic reticulum solubilizes bilirubin by conjugating it to glucuronic acid to produce bilirubin monoglucuronide and diglucuronide. The now hydrophilic bilirubin diffuses to the canalicular membrane for excretion into the bile canaliculi. Conjugated bilirubin is transported across the canalicular membrane by the multidrug resistance–associated protein 2 (MRP2) via an ATP-dependent process. This is the only energy-dependent step in bilirubin metabolism and explains why even patients with ALF have a predominantly conjugated hyperbilirubinemia. Once in the bile, conjugated bilirubin passes undisturbed until it reaches the distal ileum and colon, where bacteria containing β-glucuronidases hydrolyze conjugated bilirubin to unconjugated bilirubin, which is further reduced by bacteria to colorless urobilinogen. The urobilinogen is either excreted unchanged, oxidized and excreted as urobilin (which has an orange color), or absorbed passively by the intestine into the portal venous system. The majority of the absorbed urobilinogen is re-excreted by the liver. A small percentage filters across the renal glomerulus and is excreted in urine. Unconjugated bilirubin is never found in urine because in the serum it is bound to albumin and not filtered by the glomerulus. The presence of bilirubin in urine indicates conjugated hyperbilirubinemia and hepatobiliary disease.
The terms direct and indirect bilirubin , which correspond roughly to conjugated and unconjugated bilirubin, respectively, derive from the original van den Bergh reaction. Serum bilirubin is still measured in clinical laboratories by some modification of this method. In this assay, bilirubin is exposed to diazotized sulfanilic acid. The conjugated fraction of bilirubin reacts promptly, or “directly,” with the diazo reagent without the need for an accelerant and thereby allows measurement of the conjugated bilirubin fraction by photometric analysis within 30 to 60 seconds. The total bilirubin is measured 30 to 60 minutes after the addition of an accelerant such as alcohol or caffeine. The unconjugated, or indirect, fraction is then determined by subtracting the direct component from the total bilirubin.
Newer and more accurate methods of measuring bilirubin, such as high-performance liquid chromatography, have been developed but are generally not available because they are more difficult to perform and do not add additional information beyond that provided by the diazo method in most clinical situations. These newer methods allow the identification of delta bilirubin—conjugated bilirubin tightly linked to albumin through covalent binding. Delta bilirubin is found in cases of prolonged and severe elevation of serum conjugated bilirubin levels, and because of the strength of the covalent binding, the half-life of delta bilirubin is that of albumin, 14 to 21 days, which far exceeds the usual serum half-life of bilirubin of 4 hours. The identification of delta bilirubin explains why the decline in serum bilirubin in some patients with prolonged jaundice seems to lag behind clinical recovery and why some patients with conjugated hyperbilirubinemia do not have bilirubinuria.
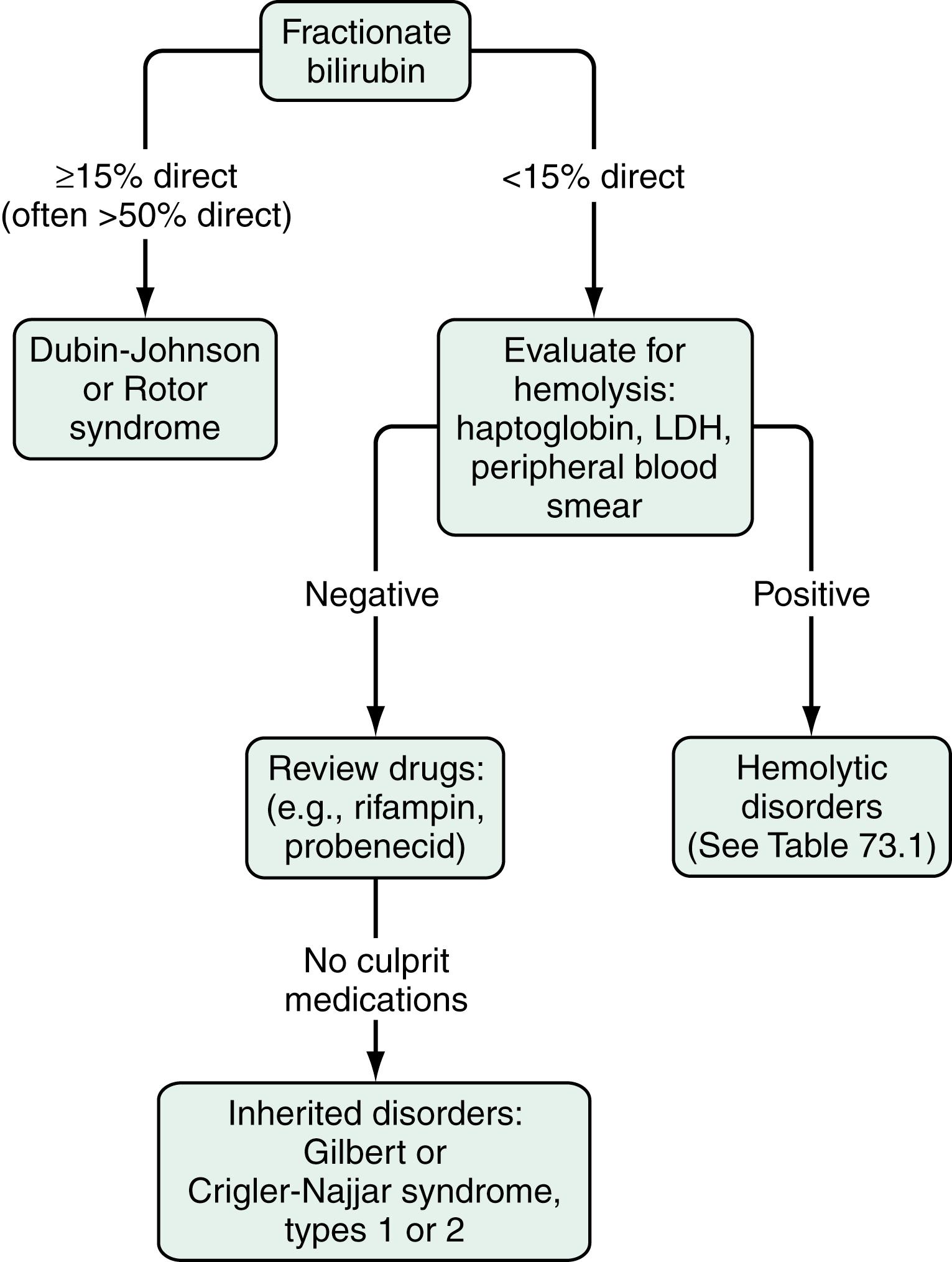
Using the diazo method, normal values of total serum bilirubin are between 1.0 and 1.5 mg/dL, with 95% of a normal population falling between 0.2 and 0.9 mg/dL. Normal values for the indirect component are between 0.8 and 1.2 mg/dL. The diazo method, however, tends to overestimate the amount of conjugated bilirubin, particularly within the normal range. As a result, “normal” ranges for conjugated bilirubin have crept upward over time. In general, if the direct acting fraction is less than 15% of the total, the bilirubin can be considered to be entirely indirect. The most frequently reported upper limit of normal for conjugated bilirubin is 0.3 mg/dL. The presence of even a mild increase in conjugated bilirubin in the serum should raise the possibility of liver injury. The measurement and fractionation of serum bilirubin in patients with jaundice does not allow differentiation between parenchymal (hepatocellular) and obstructive (cholestatic) jaundice.
The magnitude and duration of hyperbilirubinemia have not been critically assessed as prognostic markers. In general, the higher the serum bilirubin level in patients with viral hepatitis, the greater the hepatocellular damage and the longer the course of disease. Patients may die of ALF, however, with only a modest elevation of serum bilirubin. The total serum bilirubin level correlates with poor outcomes in alcoholic hepatitis and is a critical component of the MELD score, which is used to estimate survival of patients with end-stage liver disease (see later and Chapter 97 ). Pretreatment levels of serum bilirubin and bilirubin levels during treatment are associated with prognosis in patients with PBC.
Hyperbilirubinemia may be the result of overproduction of bilirubin through excessive breakdown of hemoglobin; impaired hepatocellular uptake, conjugation, or excretion of bilirubin; or regurgitation of unconjugated and conjugated bilirubin from damaged hepatocytes or bile ducts. The presence of conjunctival icterus suggests a total serum bilirubin level of at least 3.0 mg/dL but does not allow differentiation between conjugated and unconjugated hyperbilirubinemia. Tea- or cola-colored urine may indicate the presence of bilirubinuria and thus conjugated hyperbilirubinemia.
The evaluation of the patient with an isolated elevation of the serum bilirubin level is quite different from that of the patient with an elevated bilirubin associated with elevated liver enzyme levels; the latter suggests either a hepatocellular or cholestatic process, as discussed later. The first step in the evaluation of a patient with an isolated elevation of the serum bilirubin level is to fractionate the bilirubin to determine if it is conjugated or unconjugated ( Fig. 73.1 ). If less than 15% of the total is conjugated, one can be assured that virtually all the serum bilirubin is unconjugated. Overproduction of bilirubin as a result of excessive breakdown of hemoglobin can occur with any of a number of inherited or acquired disorders ( Table 73.1 ). The patient’s medication history should be reviewed for drugs that can cause impaired hepatocellular uptake of bilirubin. If no cause is identified, a genetic enzyme deficiency that results in impaired conjugation of bilirubin, the most common of which is Gilbert syndrome, is likely.
| Cause | Mechanism |
|---|---|
| Indirect Hyperbilirubinemia | |
| Hemolytic Disorders | Overproduction of bilirubin |
|
|
|
|
| Ineffective Erythropoiesis | Overproduction of bilirubin |
| Cobalamin deficiency Folate deficiency Profound iron deficiency Thalassemia |
|
| Drugs: Rifampin, Probenecid | Impaired hepatocellular uptake of bilirubin |
| Inherited Conditions | Impaired conjugation of bilirubin |
| Crigler-Najjar syndrome types I and II Gilbert syndrome |
|
| Other | |
| Hematoma and massive blood transfusion | Overproduction of bilirubin |
| Direct Hyperbilirubinemia | |
| Inherited Conditions | |
| Dubin-Johnson syndrome Rotor syndrome |
Impaired excretion of conjugated bilirubin |
As discussed in Chapter 21 , Gilbert syndrome is common, with a reported frequency of 6% to 12% (see Table 21.2). A mutation in the TATAA element in the 5′ promoter region of the B-UGT gene results in a reduction in enzyme activity to approximately one third of normal. The mildly elevated indirect serum hyperbilirubinemia seen in Gilbert syndrome is generally of no clinical consequence. This benign clinical course contrasts with those of much rarer conditions, Crigler-Najjar syndrome types I and II (see Table 21.2). The mutations in these conditions result in significantly reduced B-UGT activity: less than 10% in Crigler-Najjar type II and complete absence of enzyme activity in Crigler-Najjar type I, leading to much greater elevations of unconjugated serum bilirubin to levels that carry an increased risk of kernicterus.
When isolated hyperbilirubinemia is associated with a conjugated fraction of over 15%, and typically over 50%, the diagnosis is either the uncommon Dubin-Johnson syndrome or the even rarer Rotor syndrome (see Fig. 73.1 , Tables 21.2, and 64.5). The defect in Dubin-Johnson syndrome is in the gene that encodes MRP2. A 2012 study identified the defect in Rotor syndrome as co-existing deficiencies of the organic anion transporting polypeptides OATP1B1 and OATP1B3 (see Chapter 64 ). In both Dubin-Johnson and Rotor syndromes, excretion of conjugated bilirubin across the bile canalicular membrane is reduced, resulting in an increase in the conjugated serum bilirubin level. Neither syndrome is associated with adverse clinical outcomes. Additional genetic disorders of bile acid transport that may be associated with hyperbilirubinemia are discussed in Chapters 64 and 77
The serum aminotransferases (also called transaminases ), the most sensitive markers of acute hepatocellular injury, have been used to identify liver disease since the 1950s. ALT (formerly serum glutamic pyruvic transaminase, or SGPT) and AST (formerly serum glutamic oxaloacetic transaminase, or SGOT) catalyze the transfer of the α-amino groups of alanine and l -aspartic acid, respectively, to the α-keto group of ketoglutaric acid. AST, found in cytosol and mitochondria, is widely distributed throughout the body; it is found, in order of decreasing concentration, in liver, cardiac muscle, skeletal muscle, kidney, brain, pancreas, lung, leukocytes, and erythrocytes. ALT, a cytosolic enzyme also found in many organs, is present in greatest concentration by far in the liver and is, therefore, a more specific indicator than AST of liver injury. Increases in serum values of the aminotransferases reflect either damage to tissues rich in these enzymes or changes in cell membrane permeability that allow ALT and AST to leak into serum; hepatocyte necrosis is not required for the release of aminotransferases, and the degree of elevation of the aminotransferases in serum does not correlate with the extent of liver injury.
Aminotransferases have no function in serum and act like other serum proteins. They are distributed in plasma and interstitial fluid and have half-lives measured in days. The activity of ALT and AST at any moment reflects the relative rate at which they enter and leave the circulation. They are probably cleared by cells of the reticuloendothelial system, with AST cleared more rapidly than ALT.
Normal values for aminotransferases in serum vary widely among laboratories, but values gaining general acceptance are equal to or below 30 U/L for men and 19 U/L for women. The inter-laboratory variation in the normal range is the result of technical issues; no reference standards exist to establish the upper limits of normal for serum ALT and AST levels. Therefore, each reference laboratory is responsible for identifying a locally defined reference population or for using a normal range first established in the 1950s. The normal range is defined as the mean of the reference population plus 2 standard deviations; approximately 95% of a uniformly distributed population will fall within this “normal” range. Some investigators have recommended revisions of normal values for the aminotransferases with adjustments for sex and BMI, but others have raised concern about the potential costs and unclear benefits of implementing such a change. A longitudinal analysis observed that serum levels of ALT decrease with age, independent of sex, alcohol use, BMI, diabetes mellitus, serum TG levels, and other factors known to affect ALT levels, thereby prompting the investigators to suggest that clinicians consider a patient’s age, especially in older adults, when interpreting serum ALT levels. A serum aminotransferase level below the lower limit of normal is of no clinical importance; it has been reported in patients with chronic kidney disease on hemodialysis and is believed to be caused in part by vitamin B 6 deficiency.
Serum aminotransferase levels are typically elevated in all forms of liver injury; levels up to 300 U/L are nonspecific. In certain circumstances the degree and pattern of elevation of the aminotransferases, evaluated in the context of a patient’s characteristics, symptoms, and physical examination findings, can suggest particular diagnoses and direct the subsequent evaluation ( Box 73.1 ). The differential diagnosis of marked elevations of aminotransferase levels (>1000 U/L) includes viral hepatitis (A to E), toxin-induced liver injury, DILI, ischemic hepatitis, and less commonly, autoimmune hepatitis, acute Budd-Chiari syndrome, ALF caused by Wilson disease, and acute obstruction of the biliary tract.
a Virtually any liver disease can cause moderate aminotransferase elevations (5-15 × normal).
α 1 -Antitrypsin deficiency
Autoimmune hepatitis
Chronic viral hepatitis (B, C, and D)
Hemochromatosis
Medications and toxins
Steatosis and steatohepatitis
Wilson disease
Celiac disease
Hyperthyroidism
Acute bile duct obstruction
Acute Budd-Chiari syndrome
Acute viral hepatitis
Autoimmune hepatitis
Drugs and toxins
Hepatic artery ligation
Ischemic hepatitis
Wilson disease
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here