Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Circulation in the liver is unique because of a dual afferent blood supply, derived from the hepatic artery (HA) and the portal vein (PV; see Chapter 2 ). The oxygen-rich arterial blood and the nutrient-rich PV blood merge in the hepatic parenchymal microcirculation to sustain the complex functions of the liver before returning to the heart through the hepatic veins (HVs). This chapter outlines how liver blood flow (LBF) is controlled to maintain the hepatic perfusion within an acceptable physiologic range, describes techniques used for LBF measurement, and explores clinical situations in which blood flow is altered.
The peculiar double afferent blood supply to the liver (see Chapter 2 ) results in 75% to 80% of the entering blood being partially deoxygenated PV blood draining the stomach, intestine, spleen, and pancreas. The remainder is well-oxygenated blood from the aorta, carried by the HA. Mixing of arterial and portal blood occurs in terminal branches in the sinusoidal microcirculation around hepatocytes arranged into roughly polyhedral-shaped lobules, from their periphery toward their centrilobular venule ( Fig. 5.1 ). The centrilobular venules drain into the HVs and into the inferior vena cava (IVC). Although the liver mass constitutes approximately 2.5% of the total body weight, the liver receives nearly 25% of the cardiac output. The total LBF ranges between 800 to 1200 mL/min, which is equivalent to approximately 100 mL/min per 100 g liver wet weight. The liver blood volume is estimated to range from 25 to 30 mL per 100 g of liver wet weight, which accounts for 10% to 15% of the total body blood volume. The sinusoids hold 60% of the blood volume, whereas the remaining 40% lies in large vessels (HA, PV, and HV). , Of note is the high compliance of the liver, calculated as the change in its blood volume per unit change in venous pressure. The liver thus acts as an important blood reservoir because its blood volume can expand considerably in cardiac failure or, in case of bleeding episodes, can compensate as much as 25% of the hemorrhage by rapid expulsion of blood into the circulation. ,
The HA normally supplies approximately 25% of the total blood flow to the liver (25 to 30 mL/min per 100 g of liver tissue) in a high-pressure/high-resistance system. The mean pressure in the HA is similar to that in the aorta. The HA provides as much as 50% of the liver’s oxygen requirement because of the greater oxygen content found in arterial blood. In addition, the HA provides the exclusive blood supply to the intrahepatic bile ducts through the peribiliary plexus ( Fig. 5.2 ). In the hepatic lobules, the hepatic arterioles empty directly or via peribiliary plexi into the sinusoids. Direct artery-to-HV connections do not usually exist but may arise in liver disease.
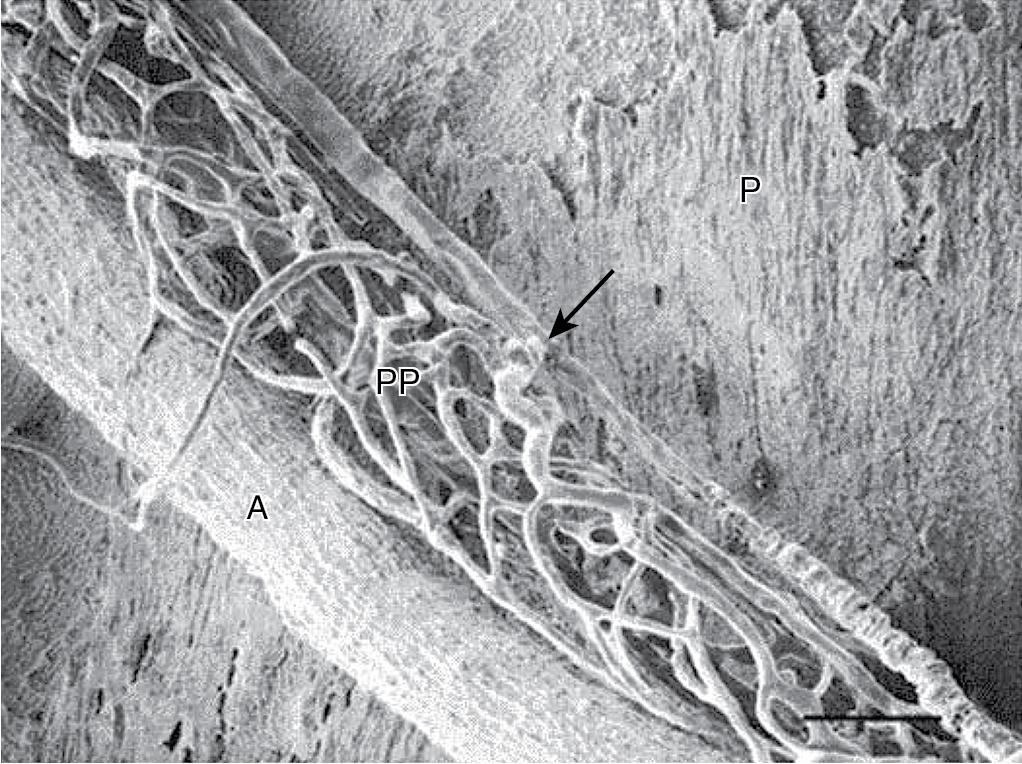
Within the liver parenchyma, the pressure in the arterial system is reduced toward that existing in the portal circulation and sinusoids. This is suggested to be achieved mainly by (1) the presinusoidal arteriolar resistance in the peribiliary plexus and (2) the intermittent closure of the arterioles, which protect the portal bloodstream from arterial pressure. In the event of HA occlusion, numerous intrahepatic collaterals can provide a source of arterial blood. Additionally, extrahepatic collateral supply can develop after HA ligation and depends on the site of occlusion. If the common HA is interrupted, revascularization occurs through extrahepatic collaterals arising from (1) the inferior phrenic arteries and (2) from the gastroduodenal arteries, which derive blood flow from the superior mesenteric artery. Ligation of the proper HAs leads to revascularization mainly via a hypertrophied inferior phrenic circulation, which can develop connections with HAs within the liver. If only the right or left HA is interrupted, intrahepatic translobar anastomoses reestablish arterial flow in the ligated system. Thus complete long-term dearterialization of the liver by any form of arterial vascular occlusion is difficult to achieve.
The PV normally carries approximately 75% of the total blood flow to the liver (90 mL/min per 100 g of liver weight) in a valveless, low-pressure/low-resistance venous system. The inferior and superior mesenteric veins join with the splenic vein to form the PV, and jointly they collect the venous outflow from the entire prehepatic splanchnic vascular bed (the intestinal tract from the lower esophagus to the rectum plus the pancreas and spleen). The PV pressure ranges between 6 and 10 mm Hg in humans when measured by direct cannulation. PV pressure depends primarily on the degree of constriction of mesenteric and splanchnic arterioles, coupled with the intrahepatic vascular resistance. Because portal blood is derived from postcapillary beds, it is partly deoxygenated. However, because of its large volume flow rate, it may supply 50% to 70% of the liver’s normal oxygen requirement. During fasting states, the oxygen saturation in the portal blood approaches 85%, which is greater than other systemic veins. Hepatic oxygen supply is diminished if portal blood flow is significantly reduced, but the effect is minimized by an increase in oxygen extraction from the HA blood and not by increasing flow.
The HV system is the systemic drainage tract of the entire splanchnic circulation. A total LBF of 1.5 L/min is considered the normal value in the average male, but the range can be quite wide (1–2 L/min). In normal conditions, the free pressure in the HVs and IVC is 1 to 2 mm Hg and is 1 to 5 mm Hg lower than the pressure measured in the sinusoids and PV. The portal pressure gradient, defined as the difference in pressure between the PV and the IVC, has been a useful clinical indicator of the perfusion pressure of the liver with portal blood. The pressure gradient in the liver is thus extremely low, in the range of 5 mm Hg compared with all other organs, where it is in the range of 115 mm Hg. Hepatic venous blood is normally approximately two-thirds saturated with oxygen, but this may be markedly reduced during periods of low delivery of oxygen to the liver, when oxygen is extracted by hepatocytes. In resting states, the liver accounts for approximately 20% of the total oxygen consumption of the body.
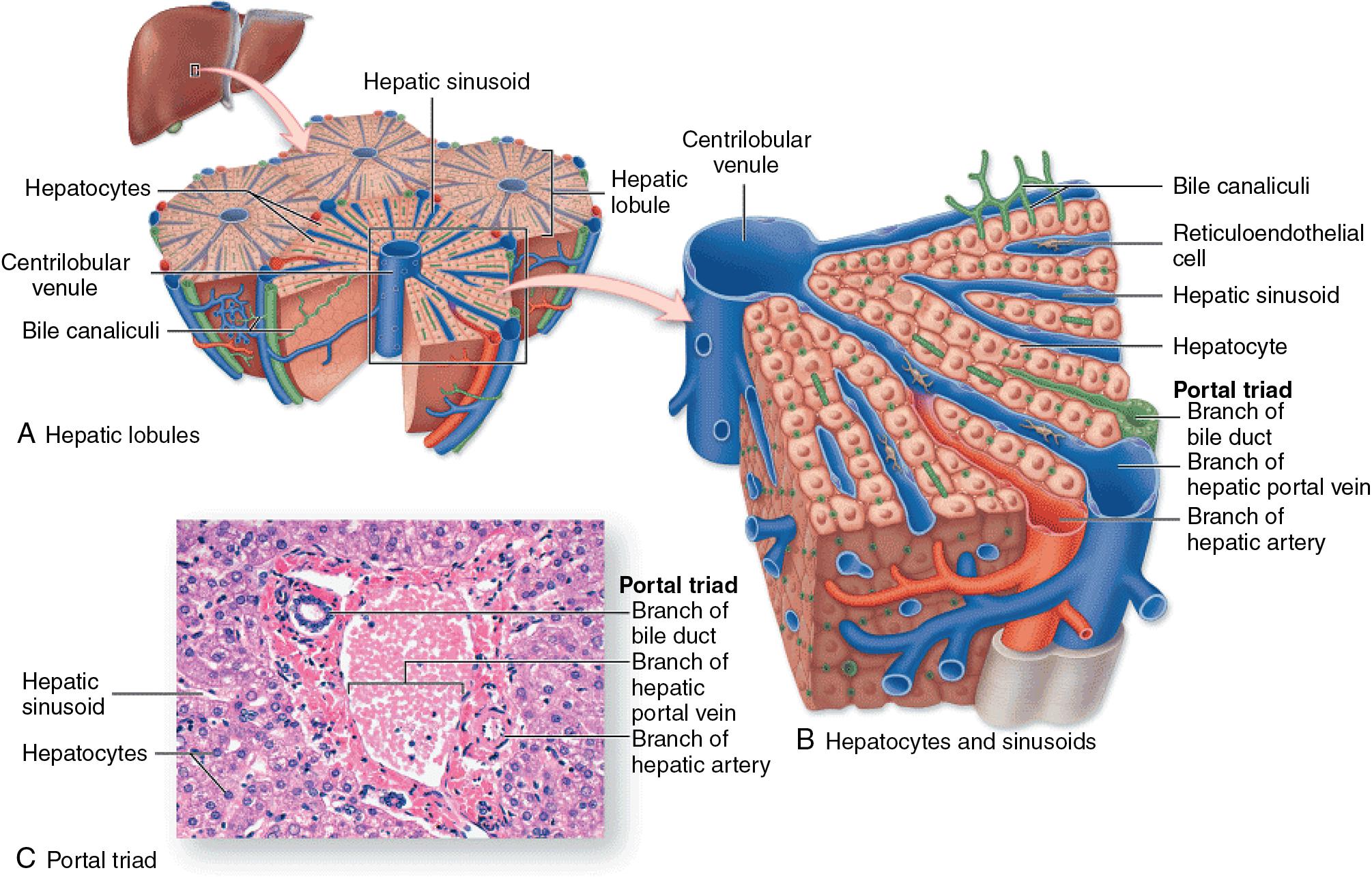
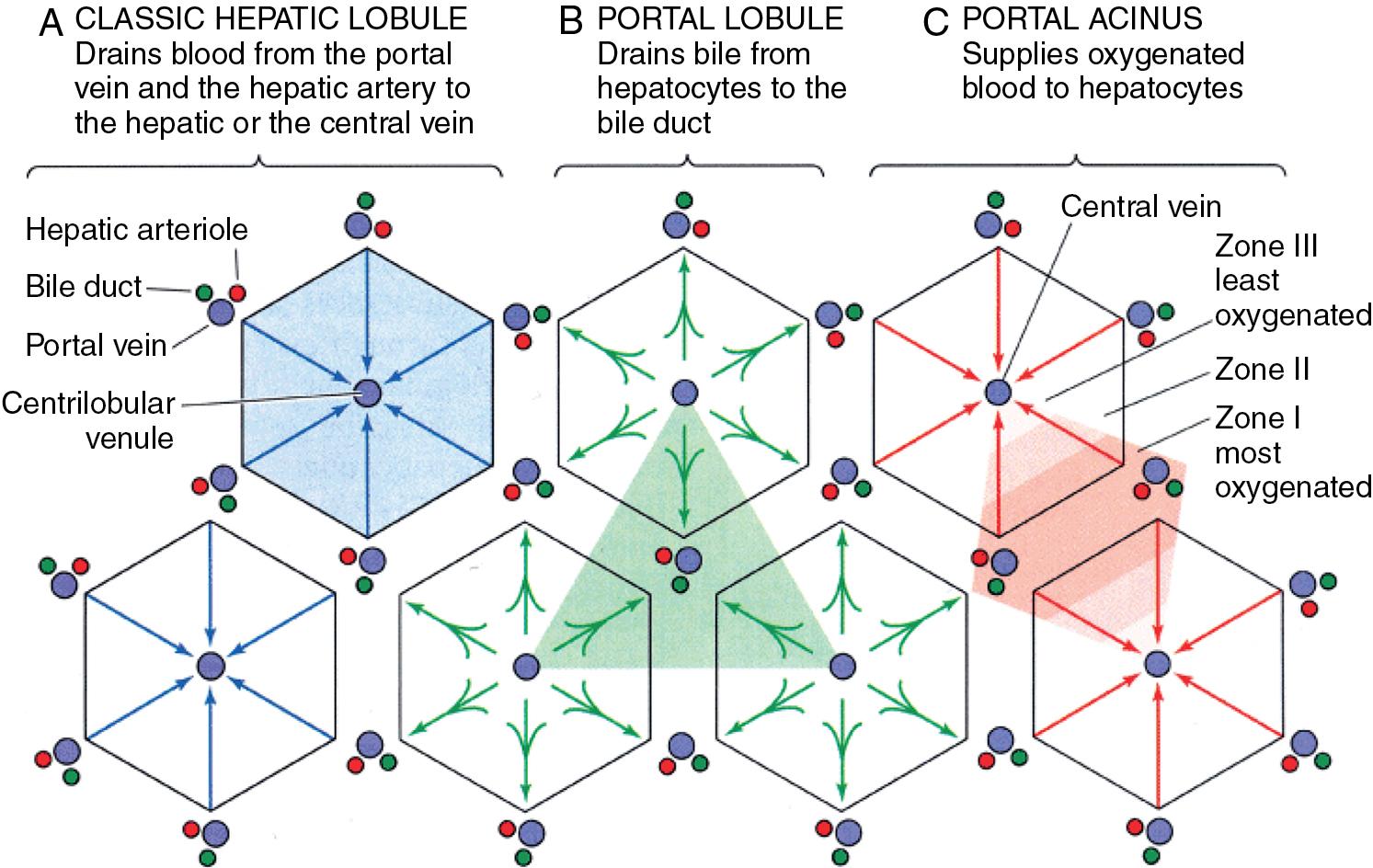
Although the organization of the liver into morphologic and functional units has been a matter of debate, the hexagonal polyhedral-shaped hepatic lobule, encompassing hepatic microvascular subunits consisting of a portal triad of terminal branches of the HA, PV, and bile duct; a network of sinusoids; and an efferent centrilobular venule is a widely accepted framework , (see Fig. 5.1 ). The portal triads, surrounded by lymphatics and autonomous nerves, all travel together in parallel in the space of Mall, through the liver parenchyma, and form portal tracts. Lymphatics transport proteins and other macromolecules that are trapped extravascularly because of hindrance of hepatocellular uptake, as in the case of cirrhosis, which will, in turn, contribute to ascites formation (see Chapters 74 and 79 ). The hepatic sinusoids correspond to the capillary bed of the liver and represent the segment of the microcirculation in which supply of nutrients and removal of metabolic products by hepatocytes takes place. Bile canaliculi closely assemble around hepatocytes and collect bile flowing in an opposite direction from blood in the sinusoids. As depicted in Figure 5.3 , histologic and physiologic studies of the liver have given rise to three related ways to view the liver’s microcirculation, emphasizing different functional aspects useful for the classification of various pathologic processes.
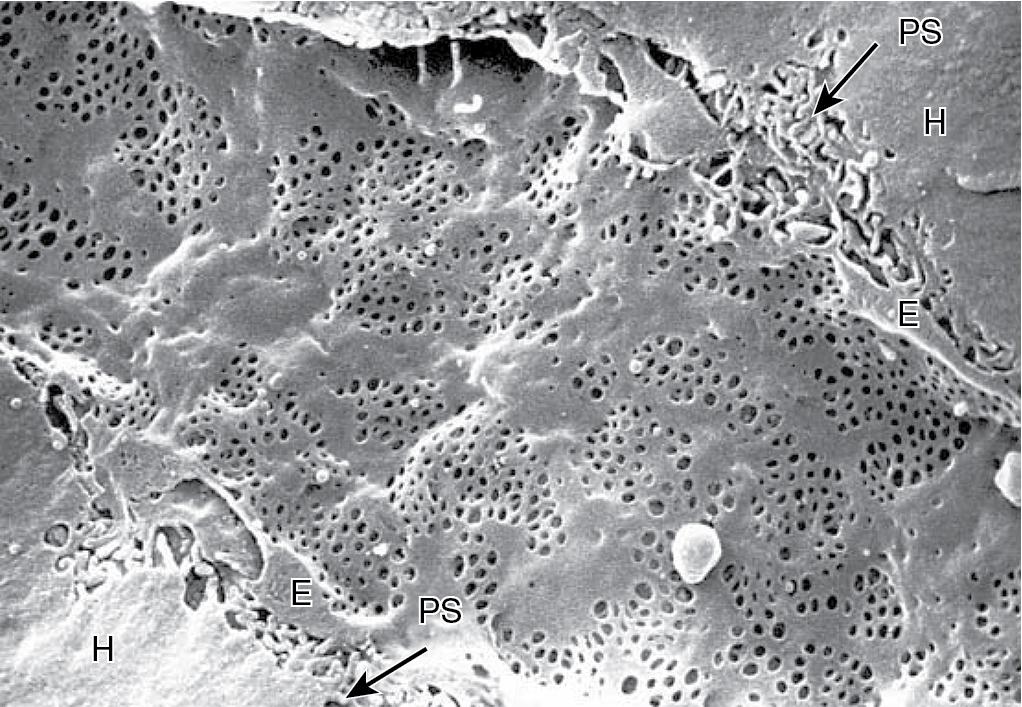
Apart from the absence of a basement membrane, the structural peculiarity of hepatic sinusoids is their unique lining, consisting of endothelial cells with flattened processes perforated by small fenestrae. These open fenestrations are arranged in clusters of 10 to 50 pores, forming so-called “sieve plates” with a diameter of 150 to 175 nm ( Fig. 5.4 ). The sieve plates occupy as much as 8% of the endothelial surface and are not uniform in size or distribution throughout the length of the sinusoids. There is a decrease in diameter but an increase of frequency from periportal to centrilobular zones, which results in higher centrilobular porosity. , The fenestrae are dynamic structures that contract and dilate in response to alterations of sinusoidal blood flow and perfusion pressure. Red blood cells (RBCs) remain restricted within the sinusoids, whereas molecules as large as albumin can pass through the fenestrations and enter the small space of Disse before making contact with the microvilli of the hepatocytes.
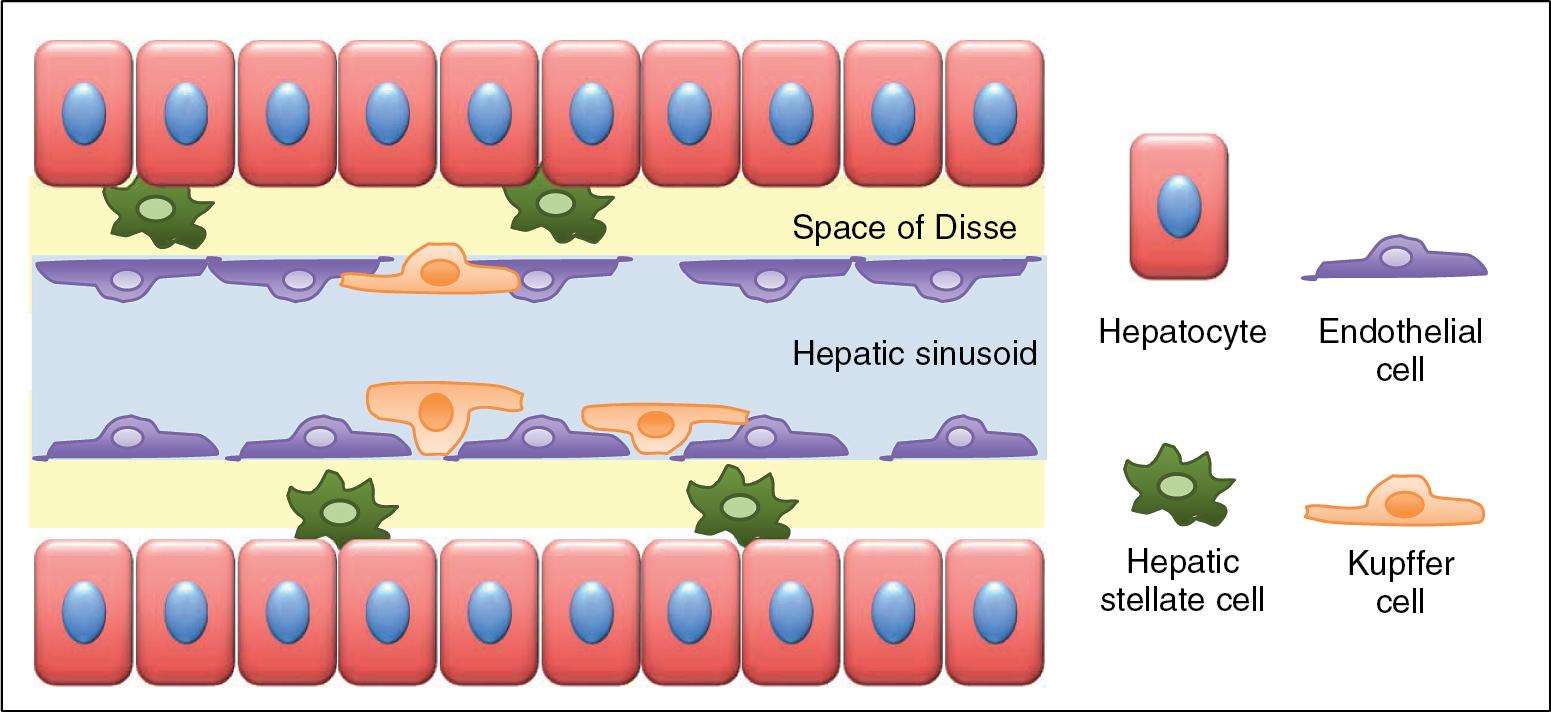
As represented in Figure 5.5 , other unique cellular components, such as the hepatic stellate cell (HSC) and the Kupffer cell (KC), are found in the hepatic sinusoids and may regulate the sinusoidal microcirculation in response to various mediators. External to the endothelium cell lining, HSCs (also known as fat-storing cells, Ito cells, or hepatic perisinusoidal lipocytes) are contractile cells distributed homogeneously around the exterior of the endothelial cells in the space of Disse, which is the space between the basal microvilli-rich surfaces of the hepatocytes and the sinusoidal lining cells. In addition to their well-known importance in retinol metabolism and as key actors in the hepatic fibrogenic response to injury (see Chapters 7 and 74 ), HSCs are capable of compressing the sinusoidal diameter by squeezing the endothelial cells and therefore play a central role in the regulation of blood flow through hepatic sinusoids. , KCs are liver-specific macrophages, and, in contrast to HSCs, are anchored to the luminal side of the sinusoids. They account for approximately 15% of the liver-cell population and constitute approximately 80% of the total population of macrophages in the body. By their large bodies, with cytoplasmic process that sometimes reach the opposite wall of a sinusoid, KCs represent a flow hindrance and can secrete large amounts of the vasodilator nitric oxide, but their direct regulation role of the sinusoidal microcirculation is debated. ,
The hepatic blood flow required to meet the physiologic function of the liver is mainly controlled by intrinsic physiologic mechanisms that are independent of extrinsic innervation and vasoactive agents. Instead, the interrelationship of arterial and portal inflow circuits is the major contributor to hepatic perfusion.
Adequate and homogeneous blood flow to the liver is necessary to sustain hepatic functions and clearance of metabolites. Because the liver does not control portal blood flow, which is simply the outflow of the extrahepatic splanchnic organs, the main mechanism by which hepatic blood flow can remain constant relies on modulation of the hepatic arterial flow ( Fig. 5.6 ). Although this phenomenon was observed in the late 19th and early 20th centuries, , it was characterized and coined as the hepatic arterial buffer response (HABR) by Lautt in 1981.
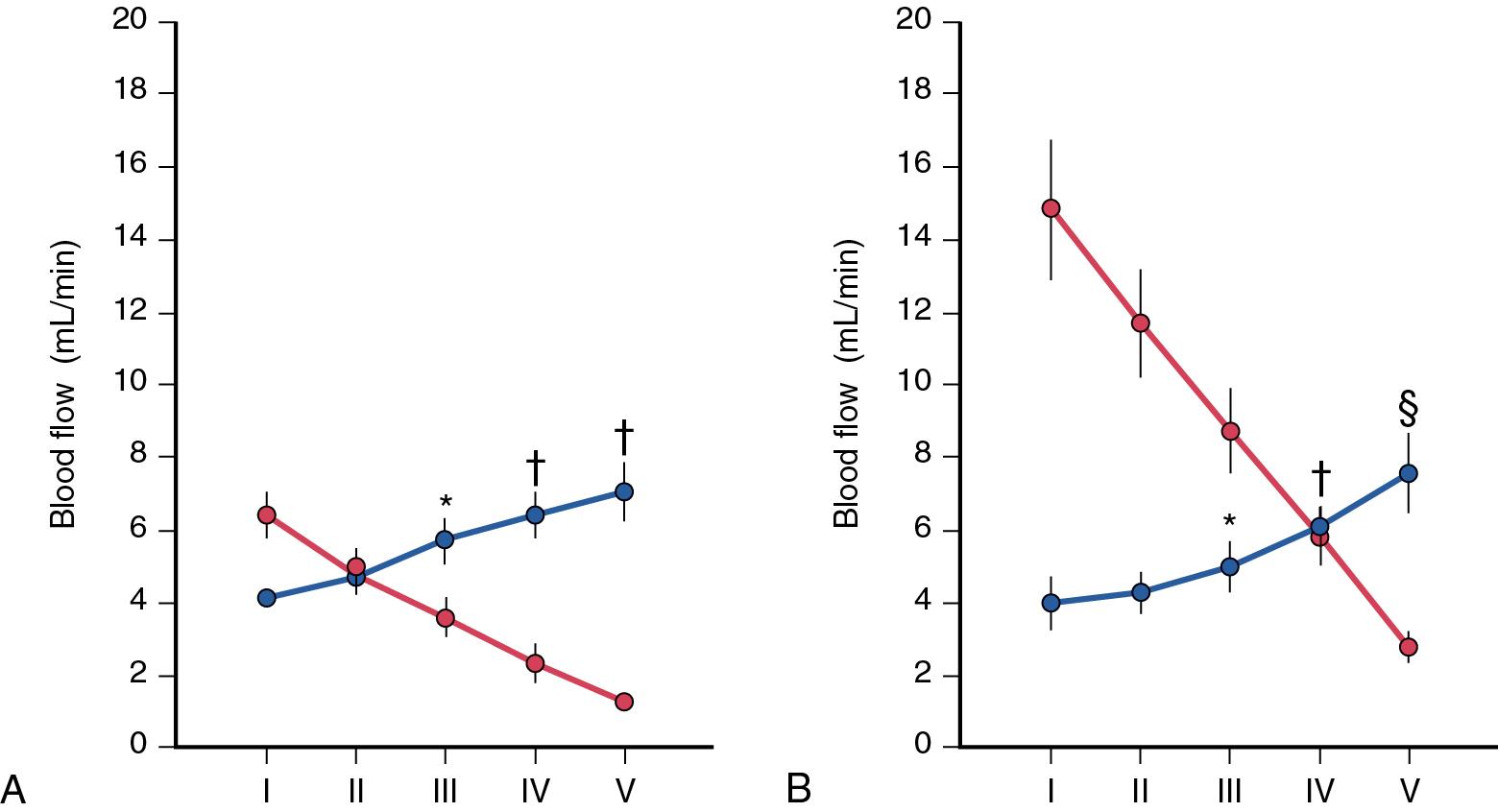
HABR represents the ability of the HA to produce compensatory flow changes at the presinusoidal level in response to changes in PV flow (see Fig. 5.6 ): If portal blood flow is reduced, the HA dilates and increases its flow into the sinusoids, and the HA constricts when the portal flow is increased. , In patients undergoing abdominal surgery, a temporary occlusion of the PV resulted in a sharp increase in HA flow of about 30%, whereas temporary occlusion of the HA did not have significant effect on PV flow. The HABR seems to operate under various physiologic and pathologic conditions and has even been suggested to operate prenatally. The HABR appears mainly regulated by the washout of adenosine, a potent vasodilator. Although adenosine is produced at a constant rate and secreted into the space of Mall (see Fig. 5.1 ), its concentration depends on the rate of washout from the space of Mall into the sinusoids. When portal blood flow decreases, less adenosine is washed away, and the elevated adenosine concentration leads to dilation of the HA. Of importance, the source of the extracellular adenosine found in the space of Mall remains to be elucidated. If portal blood flow is severely reduced, the buffer response results in the HA dilating maximally, as demonstrated by the inability to produce additional dilation in response to intraarterial infusion of adenosine. Conversely, when portal flow is doubled, the HA constricts to a maximal extent, as demonstrated by the inability of intraarterial norepinephrine to produce further constriction. Although the HABR is sufficiently powerful to regulate the vascular tone in the HA over the full range from maximal vasodilation to maximal vasoconstriction, this mechanism is capable of buffering 25% to 60% of the decreased portal flow.
Although adenosine appears to be the main mediator of the HABR, a possible contribution of the afferent sensory nerves and neuropeptides is suggested by studies in fully denervated animal livers and in transplanted human livers, , in which the HABR upon partial PV occlusion is partially impaired. , It has also been shown that hydrogen sulfide (H 2 S), a vasoactive gaseous mediator produced within the liver, appears to almost double the HABR by increasing the HA conductance, and, in turn, its inhibition has the opposite effect.
Until the mid-1970s, the HA flow was believed to be under metabolic control of the liver. As for most organs, the liver metabolism, estimated by its oxygen requirements, was postulated to participate in vascular inflow control. It has, however, been established that the liver normally receives more oxygen than it requires and can extract more oxygen to compensate for reduced delivery. , , Additionally, the unique one-way sinusoidal flow arrangement precludes substances diffusing back from the hepatic parenchyma or venous blood into the HA resistance vessels. Among other studies, this concept has been exemplified by isovolemic hemodilution or upregulation of hepatic enzymes, leading to oxygen deprivation to the liver parenchyma, which does not result in HA dilation. , 38 Therefore the hepatic metabolic demands do not control the HA flow, even if the liver parenchymal cells can release large quantities of potent vasoactive molecules during metabolic stress.
The term autoregulation refers to the tendency for local arterial blood flow to remain constant in the face of pressure changes in the arteries that perfuse a given organ. Overall, the degree of autoregulation is considered small in the liver, and mixed results have been reported in animal models. In fact, the adenosine washout may well account for HA autoregulation because endogenous adenosine produced by the HA tributaries can contribute to high presinusoidal adenosine concentration in situations where hepatic clearance is impaired by a reduction in the portal flow, leading to HA vasodilation.
Sinusoidal blood pressure and vascular resistance are so low that a pressure gradient across the liver from the PV inflow to the HV outflow is only approximately 5 mm Hg. The low-pressure gradient is remarkable, considering that 30% of the inflow to the liver sinusoids is provided by the HA under arterial pressure. How the hepatic pressure gradient is maintained has been studied in multiple animal models. About 60 years ago, Knisley suggested the presence of sphincter-like structures at the entrance and exit of sinusoids that maintain the PV pressure gradients, but these proved to be species dependent. , , In humans, although smooth muscle cells are found throughout all segments of the hepatic microvascular subunits, no such sphincter-like structures have been described for controlling intrahepatic blood flow.
In contrast, there is a growing body of evidence that contracting cells associated with the sinusoids, such as the HSCs and the sinusoidal endothelial cells, through a complex interplay with vasoactive mediators, may dynamically regulate the hepatic microvascular blood flow. With such a low sinusoidal perfusion pressure, local regulators at a single-cell level may actively control the flow within the sinusoids toward the HVs. It seems plausible that the HSCs could dilate to pull outward on the endothelial cells and enlarge the sinusoidal space (see Fig. 5.5 ); however, this does not seem to have been shown. Many endothelial mediators known to control vascular tone by acting on HSC contractility have been described ( Table 5.1 ), notably: (1) the endothelium-derived relaxing factor nitric oxide (NO), (2) the endothelium-constricting factor endothelin-1 (ET-1), , and (3) two vasodilatory gaseous molecules: carbon monoxide (CO) , and H 2 S. , With the exception of H 2 S, a direct contribution of these vasoactive agents to the HABR is not well established.
| VASOACTIVE AGENT | FUNCTION | ENZYME SYSTEM | CELLULAR SOURCE AND DISTRIBUTION | TARGET CELL | PATHWAYS |
|---|---|---|---|---|---|
| Thromboxane A 2 | Vasoconstriction, platelet activation and aggregation, leukocyte adhesion | COX-1, COX-2 | SEC, KC | SEC, platelet, leukocyte | TxA 2 R |
| Prostaglandin I 2 | Vasodilation, inhibition of platelet aggregation | COX-1, COX-2 | SEC | SEC, HSC | PGI 2 R |
| Angiotensin II | Vasoconstriction | ACE | HSC | HSC | AT 1 |
| Nitric oxide | Vasodilation | eNOS | SEC | VSMC, HSC | sGC |
| Nitric oxide | Vasodilation | iNOS | SEC, KC, VSMC, HSC, HC | VSMC, HSC | sGC |
| Endothelin-1 | Vasoconstriction | SEC, HSC, KC | VSMC, HSC, SEC, KC | ET A R, ET B2 R | |
| Endothelin-1 | Vasodilation | SEC, HSC, KC | SEC | ET B1 R | |
| Carbon monoxide | Vasodilation | HO-1 | SEC, KC, VSMC, HSC, HC | VSMC, HSC | sGC |
| Vasodilation | HO-2 | HC | VSMC, HSC | sGC | |
| Hydrogen sulfide | Vasodilation | CSE (CBS) | HSC, HC | VSMC | K ATP channels |
Hypercarbia (partial pressure of carbon dioxide in arterial blood [PaCO 2 ] > 70 mm Hg) increases PV flow and decreases HA flow in dogs, whereas hypocarbia (PaCO 2 < 30 mm Hg) decreases both. Systemic hypoxia (partial pressure of oxygen in arterial blood [PaO 2 ] < 70 mm Hg) causes a decrease in arterial flow but has no effect on the contribution from the PV. The response to metabolic acidosis is similar to that induced by hypercarbia, whereas metabolic alkalosis has essentially no significant effect. The sympathetic nervous system is thought to be responsible for the HA vasoconstriction observed in hypercarbia and hypoxia.
The liver is a significant blood reservoir, and 50% of its blood volume may be mobilized by nerve stimulation. Denervation experiments have shown that the sympathetic nervous system is not involved in basal arterial tone in the liver. Hepatic sympathetic nervous stimulation causes HA vasoconstriction and reduced blood flow, which appears secondary to an autoregulatory response. Sensory denervated rats and pigs have a diminished arterial buffer response on partial occlusion of the PV. Portal pressure increases as a result of an increase in PV resistance, but portal flow does not decrease unless there is a decrease in intestinal or splenic blood flow caused by simultaneous sympathetic stimulation of these vascular beds. Although the HA contains both α-adrenergic and β-adrenergic receptors, the PV system is believed to contain only α-receptors. At low doses, epinephrine causes hepatic and mesenteric arterial vasodilation, whereas at high doses, vasoconstriction occurs in the HA and PV vascular beds and in the mesenteric circulation. ,
Intraportal administration of exogenous vasoactive agents affects HA resistance. The mechanisms underlying this intrahepatic transvascular effect are not understood, but it is likely that the close anatomic association between arterioles and venules could permit this and may be a means by which HA blood flow is finely controlled by endogenous agents, such as gut hormones. Gastrin, secretin, cholecystokinin, and vasoactive intestinal peptide cause vasodilation of the HA. Hepatic blood flow is profoundly increased by glucagon as a consequence of its strong vasodilatory action on the mesenteric vasculature, but insulin has little hemodynamic effect on the hepatic circulation. In addition, antagonists of calcitonin gene–related peptide and neurokinin significantly reduce HA blood flow, suggesting the presence of their receptors on the arterial vasculature. Histamine causes HA dilation and, in the dog only, HV constriction. Bradykinin is a potent HA vasodilator that has little effect on the PV system. The HA vascular bed is dilated by most prostaglandins; however, prostacyclin does not affect HA flow but increases portal blood flow through a vasodilator effect on the prehepatic vascular bed. Vasopressin decreases portal flow and pressure by mesenteric arterial vasoconstriction but has variable effects on the HA. Serotonin is believed to mediate vasoconstriction of portal radicles.
Liver-extrinsic NO causes vasodilation in the HA and mesenteric vascular beds. Endothelin molecules can exert a powerful and prolonged generalized systemic constriction , that also has a direct effect on the hepatic blood flow. Endothelins reduce hepatic perfusion, increase portal pressure, and reduce sinusoidal diameter. , , Angiotensin decreases HA and portal blood flow and is one of the few substances to produce a significant vasoconstrictor effect on the HA. In contrast, H 2 S, either endogenously or exogenously, can reverse the norepinephrine-induced vasoconstriction in an NO-independent fashion.
Liver size and blood flow decrease with age in humans. Similarly, apparent liver blood flow per unit volume of liver (liver perfusion) falls with age. Age does not seem to affect sinusoidal dimensions or sinusoidal density, but rather the geometry and the complexity of the sinusoidal network changes. These small age-related changes in the architecture of the liver sinusoidal network may influence hepatic function and reflect broader aging changes in the microcirculation. The reduced elimination of both capacity-limited and flow-limited drugs, which is seen in the elderly, predisposed to adverse drug reactions, is likely because of these morphologic combined with physiologic changes. Reduction in the activity of drug-metabolizing enzymes are probably of less significance in the healthy aged population.
Postprandial hemodynamic changes have been studied extensively in animals and humans. A meal induces a marked vasodilatation of the mesenteric artery with a consequent increase in PV flow, and compensatory vasoconstriction of the HA. The intrahepatic hemodynamic changes are greater in the right lobe of the liver, with a more significant increase of portal flow velocities on that side, in association with a bilateral reciprocal HA response that may be maximal on both sides. The meal composition did not influence the magnitude of the hemodynamic changes but rather the timing of the response. ,
The effect of anesthetic agents on the LBF has been mainly studied in animal models some 30 years ago. HA and PV blood flow decreases passively in parallel with cardiac output during halothane inhalation, with little change in vascular resistance. , Enflurane has been found to have similar effects as those of halothane, although there is a decrease in HA vascular resistance as part of a generalized decrease in peripheral vascular resistance. NO concentrations of 30% to 70% reduce HA and PV flow, possibly as a result of a generalized stimulatory action on α-adrenergic receptors. Isoflurane seems to have minimal effects on HA and PV flows, and the intravenous (IV) agent fentanyl may have little effect on prehepatic splanchnic blood flow. Thiopentone in low doses vasoconstricts the HA and mesenteric vascular beds.
The effect of regional anesthesia on the LBF is poorly characterized. Sympathetic block might logically improve flow by reducing splanchnic vascular resistance, whereas reductions in systemic vascular resistance and cardiac output might offset this beneficial effect. Thoracic epidural anesthesia caused a reduction in blood flow in mesenteric arteries that was associated with a decrease in systemic mean arterial pressure.
Intermittent positive pressure ventilation predictably reduced splanchnic perfusion via a reduction in preload with a fall in cardiac output. The splanchnic circulation is also susceptible to more direct effects of positive pressure ventilation. The use of very large tidal volumes, high levels of positive end expiratory pressure (PEEP), and high inspiratory pressures have been shown to reduce splanchnic perfusion. These effects appear to be because of increased HV pressures and mesenteric vascular resistance, with reduced portal blood flow.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here