Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors thank Dr. Peter Dodds for editing and linguistic revision of the manuscript. Preparation of this chapter was carried out in part with grants from the Universidad San Pablo—CEU, the Fundación Ramón Areces (CIVP16A1835) of Spain, and the Spanish Ministry of Science and Innovation (SAF2012-39273).
In utero, the fetus receives a continuous supply of substrates for growth and oxidative metabolism, and produces large quantities of heat and carbon dioxide. , Based on experiments with rats and various other animal species, it is thought that the continuous placental transport of glucose and amino acids represents over 80% of the energy supply during late intrauterine life. At birth there is an abrupt termination of these major fetal fuels and a switch to intermittent feedings with breast milk, which contains a high proportion of lipids, meaning that about 50% of neonatal energy supply comes from the oxidation of fatty acids. , ,
However, several laboratories have reevaluated the role of mitochondrial fatty acid oxidation (FAO) in human placental and fetal metabolism. Using a variety of approaches it has been shown that mRNA expression and activity of FAO enzymes are both present in substantial proportions in several human fetal tissues and in placenta , leading to the conclusion that FAO is an important component of fetal and placental energy production.
Before breast-feeding is established, the newborn infant must produce glucose to meet the needs of the central nervous system. The human neonate is capable of producing sufficient glucose to meet cerebral energy needs, initially by glycogenolysis. However, glycogen stores last for only about 10 to 12 hours in a term infant, and gluconeogenesis becomes the principal source of hepatic glucose production soon after birth, but daily glucose production alone can barely satisfy the whole-body metabolic requirements in the first day of life. Therefore during early postnatal life, there is an absolute need for other oxidizable endogenous substrates to meet the infant’s energy demands. Near term, the human fetus has an increased accumulation of adipose tissue, , attaining fat stores of around 15% of body weight at birth. , Immediately after birth, there is an increase in adipose tissue lipolysis with an intense increase in plasma nonesterified fatty acids (NEFA). A fall in the respiratory quotient (RQ) takes place, indicating that mobilized fatty acids become the primary source of energy. Thus, FAO becomes an important supply of energy for the newborn infant. Ketone bodies are formed in liver from the end product of β-oxidation and become an alternative energy substrate for the neonatal brain. Lipolysis of adipose tissue triglycerides (TG, more formally known as triacylglycerols ) also releases glycerol into the circulation, which can be converted into glucose in the gluconeogenic pathway. , These metabolic interactions explain the decrease in plasma glucose concentration of the newborn infant immediately after birth, which lasts approximately two hours, followed by a rise to reach its steady state a few hours after birth. ,
In this chapter the importance of lipids as a fuel for oxidative metabolism in premature and full-term neonates is discussed.
During pregnancy, the fetus is continuously supplied through the placenta with a diet rich in carbohydrates and amino acids and poor in fat. In fact, from animal studies, the fetus is considered to be primarily dependent on glucose oxidation for energy production because (1) glucose is quantitatively the main substrate supplied by the mother to the fetus, (2) mRNA expression and activity of FAO enzymes in fetal heart and liver are low with a rapid rise after birth, and (3) the high utilization of glucose by the fetus results in the conversion of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase, and the resultant inhibition of the carnitine palmitoyl-CoA transferase 1 (CPT1), the key enzyme controlling the entry of long-chain fatty acids into the mitochondria and consequently their oxidation.
Based on the evidence above, one of the dogmas in fetal and perinatal medicine has been that the fetus depends upon the constant supply of glucose transported to and across the placenta from the maternal circulation to generate all placental and fetal energy needs, using glycolysis and the tricarboxylic acid (TCA) cycle, for essential functions. However, immediately after birth the newborn infant has to withstand a brief period of starvation before being fed at intervals with milk. In human mature milk, the lactose content is higher (7 g/100 g) than that of fat (4 g/100 g), although it depends on the stage after delivery, colostrum having a lower lactose and a higher fat content. Around 95% of that fat is in the form of TG and represents more than 60% of the energy intake in the neonate. Thus, the fetal-to-neonatal transition implies a very rapid switch from glucose to fat as the major source of energy.
Several reports have characterized recessively inherited disorders in the more than 20 genes of the mitochondrial FAO pathway that cause early morbidity and mortality. , The majority of the affected infants are premature and show phenotypes of growth restriction, fasting-induced hypoketotic hypoglycemia, and hepatic encephalopathy, and may progress to coma and death. , , Two pathogenic mechanisms for FAO disorders have been proposed: first, a lack of sufficient energy production, and second, the accumulation of fatty acid intermediates that enter the maternal circulation in toxic concentrations. Data to support both hypotheses exist, and it has been shown that FAO enzyme disorders in the affected fetus may cause significant maternal morbidity and mortality, including acute fatty liver of pregnancy (AFLP), the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, placental floor infarction, and pre-eclampsia. Furthermore, a higher frequency of prematurity, intrauterine growth retardation (IUGR), and intrauterine death have been described, in association with deficiencies in enzymes involved in the FAO pathway like long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) or the mitochondrial trifunctional protein (MTP) (see below), , , which is a protein with fatty acyl-CoA dehydrogenase activity. All these findings suggest that FAO plays an important role in the human fetal-placental unit, in contrast to the results obtained in animal studies and to the widely accepted view that embryologic development depends on glucose as the major source of metabolic energy.
Fig. 33.1 shows the mitochondrial long-chain FAO pathway. The process starts with the uptake of fatty acids and carnitine (3-hydroxyl-4-trimethylamino-butanoic acid) into the cell. Long-chain fatty acid transport is facilitated by membrane associated transporters such as specific fatty acid transport proteins (FATPs), fatty acid-binding protein (FABP pm ), and fatty acid translocase CD36/FAT, whereas carnitine uptake is mediated by its transporter OCTN2. Intracellular long-chain fatty acid is activated to fatty acyl-CoA by the action of fatty acyl-CoA synthase (FACS) in a reaction that consumes the equivalent of two adenosine triphosphates (ATPs). Activated fatty acyl-CoA are converted to carnitine esters by the action of CPT1, and then transferred by carnitine-acylcarnitine translocase (CACT) across the mitochondrial membranes, where fatty acyl-CoA is reconstituted by carnitine palmitoyl-CoA transferase 2 (CPT2). Once inside the mitochondria, the FAO process (usually called β-oxidation ) brings about the breakdown of a long-chain acyl-CoA molecule to a number of acetyl-CoA molecules, the number of which depends on the length of the fatty acid’s carbon chain. The process involves a variety of enzymes, the four main ones of which are acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase. In each β-oxidation cycle, an acetyl-CoA, an acyl-CoA two carbons shorter, one NADH, and one FADH 2 are formed. Acetyl-CoA may be used for steroidogenesis, enter the TCA cycle for oxidation and energy production, or become transformed into ketone bodies in the liver; the electrons derived from NADH and FADH 2 are used by the respiratory chain eventually leading to the production of energy as ATP. The β-oxidation enzymes have different isoforms with affinities for different fatty acid chain lengths (e.g., there is a very-long-chain acyl-CoA dehydrogenase [VLCAD], a medium-chain acyl-CoA dehydrogenase [MCAD], and a short-chain acyl dehydrogenase [SCAD]). The enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase isoforms specific for long-chain fatty acids form an enzyme complex in the inner mitochondrial membrane that is named the MTP. The activity of several FAO enzymes and different acylcarnitines have been found in human embryo, fetus, and placenta, showing that the mitochondrial FAO enzymes are metabolically active. ,
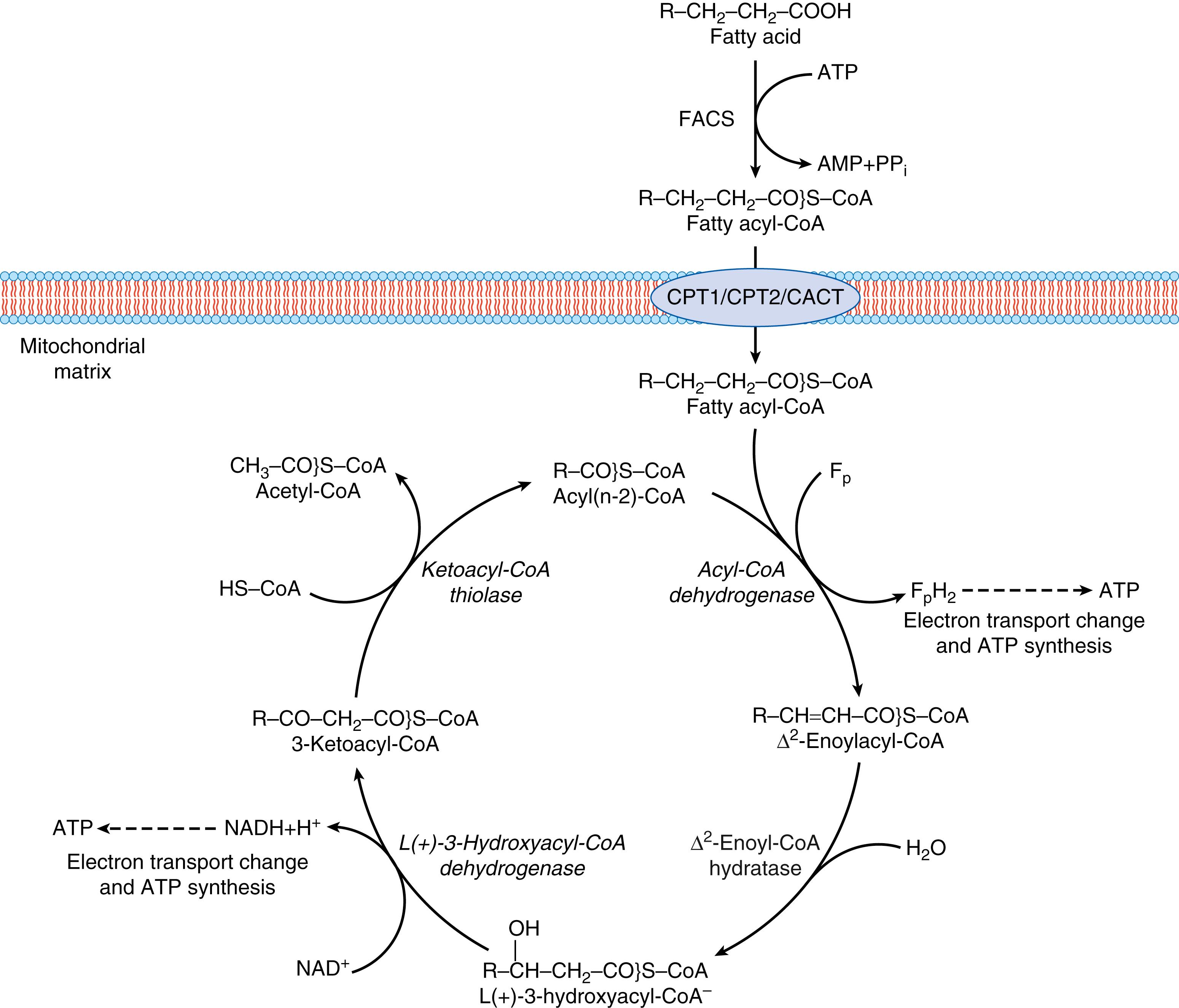
The activities of the enzymes of FAO are subject to feedback inhibition by the products of their own reaction, so that any buildup of acyl-CoA product will inhibit the specific β-oxidation enzyme that produced it. Moreover, the proteins involved in fatty acid β-oxidation are regulated by both transcriptional and post-transcriptional mechanisms. There are a number of transcriptional factors that regulate the expression of these proteins, the best known being the peroxisome proliferator-activated receptors (PPARs) and a transcription factor coactivator PGC-1α.
In animal studies, the ablation of genes encoding enzymes of the FAO pathway is associated with reduced fertility, fetal demise, and fetal growth restriction, , , and the ablation or inactivation of genes encoding for the transcription factors involved in the regulation of FAO like PPARs cause embryonic lethality as well as the failure of the syncytiotrophoblast to develop and sustain pregnancy.
It may be therefore concluded that FAO plays an essential role in the fetoplacental unit, where it is critical for placental function and fetal development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here