Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In memory of Professor Marisa Di Donato.
In response to the increasing health, economic, and social impacts of heart failure, clinicians are investigating new surgical therapies that do not involve heart transplantation. Heart failure affects about 5 million patients in the United States, and more than 250,000 die annually. Nearly 70% of patients with heart failure have coronary artery disease, and nearly all have had a myocardial infarction (MI). In part, the increase in the prevalence and incidence of heart failure results from improved diagnosis and treatment of cardiac disease (especially ischemic disease), but another factor is the aging of the population. Despite improvements in medical treatment since the 1960s and the introduction of potent new drugs, the prognosis for patients affected by heart failure remains extremely poor. However, with recent advances in surgical therapy for cardiac disease, new surgical options can be offered to some of these patients as an alternative to medical therapy. Some conventional drug therapies have not shown any significant improvement in the survival of patients with heart failure.
After a diagnosis of heart failure, the 5-year mortality rate is still 60% for men and 45% for women, despite improvements in its medical management. Currently, improvements in surgery technology and in surgeons' skills have broadened the indications for cardiac surgery. Historically, patients with severe congestive heart failure (CHF) were listed for heart transplantation, and although this is a very effective therapy for end-stage heart failure, the limited number of donor organs remains a crucial problem. Indeed, the Registry of the International Society for Heart and Lung Transplantation shows that the number of heart transplant centers worldwide has dropped from 248 in 1995 to 201 in 2005.
Until recently, surgical management was directed toward the underlying pathology of coronary disease and the secondary mitral insufficiency that evolves as the heart dilates, but surgeons do not systematically approach the ventricle. Surgical ventricular restoration (SVR), the topic of this chapter, was launched by Dor and colleagues and represents a relatively novel surgical approach that aims to restore (i.e., bring back to normal) the dilated, distorted left ventricular (LV) cavity to improve function. It requires knowledge of the remodeling infrastructure, of the structural changes that lead to geometric abnormalities, and of the role of compensatory, remote muscle and stretching mechanisms that lead to electrical instabilities.
SVR is more than a single procedure, because it includes coronary grafting and mitral repair when needed, and thus it has the potential to treat the three components of the disease: the ventricle, the vessels, and the valve (“triple V” as defined by Buckberg ).
In dilated cardiomyopathy (a common cause of heart failure), a primary determinant of the disease process is LV remodeling. The underlying causes of dilated cardiomyopathy are diverse, and the origin may be nonischemic or ischemic.
LV remodeling is a complex, dynamic, and time-dependent phenomenon that involves molecular, cellular, interstitial, and genome-expression changes that manifest clinically as changes in the size, shape, and function of the heart after cardiac injury. The process can evolve slowly or rapidly after the myocardial injury, and it contributes importantly in the progression to end-stage CHF. Box 100-1 summarizes the principal changes that occur in LV remodeling. The extracellular matrix participates in the altered ventricular geometry after MI. Cardiologists and cardiac surgeons traditionally viewed the extracellular matrix as an inert collection of structural macromolecules that serve as a scaffold for cells. However, a large body of evidence supports a central role for the extracellular matrix in the control of numerous cellular functions and supports the idea that the extent of LV remodeling is a critical determinant of clinical outcome after MI.
Current working models for heart failure include the cardiorenal model (excessive salt and water retention), the hemodynamic model (pump failure and excessive vasoconstriction), and the neurohormonal model (overexpression of biologically active molecules capable of exerting unfavorable effects on the heart and circulation). Each of these may be necessary but not entirely sufficient to explain all the causes of disease progression in the failing heart. After the myocardial insult and the initial decline in pumping capacity, a variety of compensatory mechanisms are evoked to restore cardiovascular function and normal homeostasis. Adrenergic, renin-angiotensin, and cytokine systems are activated systemically and locally in the myocardium in response to reduced cardiac output. A variety of circulating and tissue proteins and peptides (e.g., norepinephrine, angiotensin II, endothelin, aldosterone, tumor necrosis factor, interleukins) are generated in an initial adapting response. However, chronic overexpression of these biologically active molecules may fundamentally alter gene expression, changing protein synthesis in both myocytes and fibroblasts.
The biomechanical model for heart failure described by Mann and Bristow helps to explain the progression of heart failure independent of the neurohormonal status of the patient. In fact, current medical therapy, acting against neurohormonal activation, tends to slow progression but fails to arrest the process of remodeling. In addition, many types of neurohormonal inhibition proved to be ineffective or even harmful in patients with heart failure.
To explain the failure of neurohormonal antagonism, Mann and Bristow focus on LV size and geometric abnormalities as being responsible for progression of the disease. Geometric changes lead to structural abnormalities of the myocytes and of the myocardium, which worsen cardiac function and increase neurohormonal activation; this may make the cardiovascular system less responsive to normal homeostatic control mechanisms.
The determinants of LV geometry are shape, volume, and cardiac mass, and these three components may be altered by cardiac disease, which can be primarily degenerative, valvular, or ischemic in origin. Ischemic cardiomyopathy leads to a sequence of structural changes to compensate for the increased load produced from the nonfunctional akinetic or dyskinetic regions.
In anterior myocardial infarction, the LV apex is primarily involved, so regional changes affect the anterior, septal, and inferoseptal ventricular components. Globally, the elongation and widening of the ventricle occur proportionally to maintain a constant ratio (i.e., a constant sphericity index , or short ratio to long axis). However, when patients develop secondary mitral insufficiency, the sphericity index is abnormal and the ventricle is more spherical. In the absence of mitral regurgitation (MR), therefore, the sphericity index fails to detect shape abnormalities in anterior postinfarction cardiomyopathy. The conicity index has been proposed to assess the conical shape of the apex and its changes after MI ( Fig. 100-1 ). Another important shape change that occurs after an anterior MI is the displacement of the papillary muscles laterally and toward the apex, which produces tethering of the posterior mitral leaflet and mitral restriction.
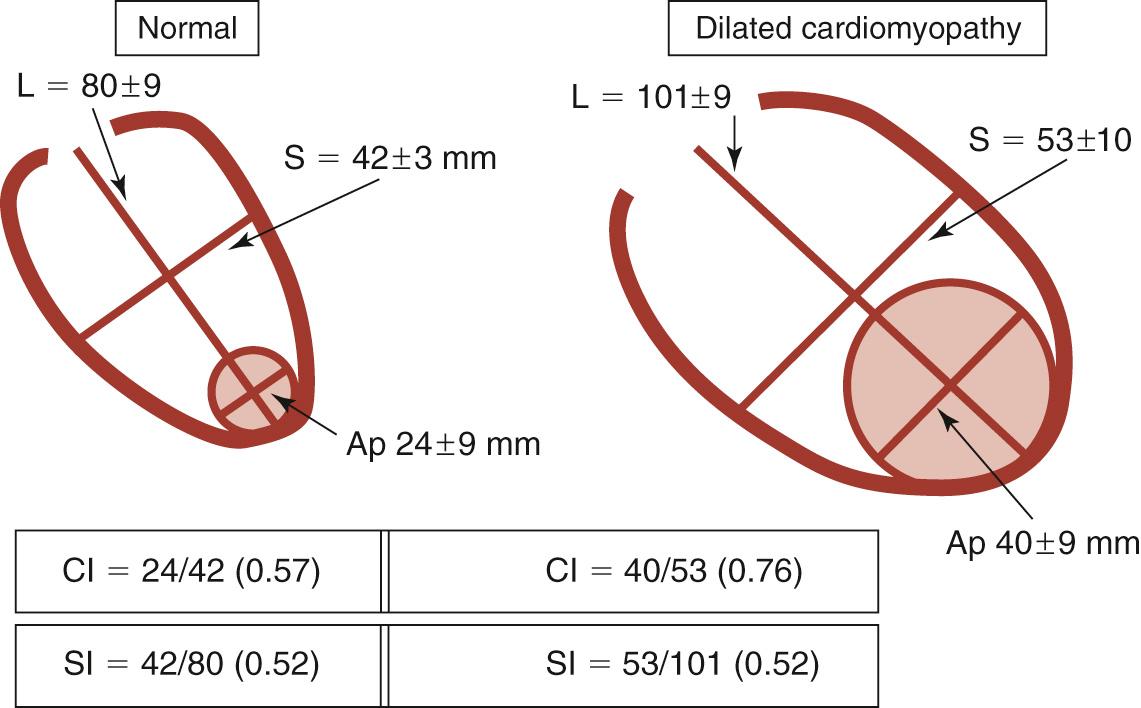
Alternatively, shape changes after an inferior MI are different : the short axis is widened more than the long axis is elongated, which leads to an increase in the sphericity index and accounts for the more frequent occurrence and the more severe degree of MR. Table 100-1 compares the geometric abnormalities that occur in anterior and inferior myocardial infarctions.
| Inferior | Anterior | P value | |
|---|---|---|---|
| Diastolic diameter (mm) | 69 ± 10 | 63 ± 9 | 0.009 |
| Systolic diameter (mm) | 57 ± 12 | 50 ± 11 | 0.009 |
| Septum thickness in diastole (mm) | 12.8 ± 3 | 10.5 ± 3 | 0.001 |
| Posterior thickening (%) | 16 ± 15 | 32 ± 23 | 0.008 |
| Left ventricular mass index (gm/m 2 ) | 217 ± 51 | 165 ± 39 | 0.000 |
| Left atrium size (mm) | 48 ± 8 | 44 ± 7 | 0.008 |
| Mitral regurgitation present (%) | 87 | 69 | 0.04 |
| Mitral regurgitation grade | 2.9 ± 1.1 | 2.0 ± 1.1 | 0.0003 |
Nonischemic dilated cardiomyopathy exhibits a more spherical shape than ischemic cardiomyopathy: the short axis is enlarged, MR is more frequently involved, and wall motion is severely and diffusely hypokinetic. Pump function is markedly reduced and there are apparently no regional differences in contractility. However, biopsy studies show nonhomogeneous disease in many patients, with the amount of scarring and fibrosis ranging from 4% to 60% between the free wall and the septum, which may account for the failure of some types of surgery, such as the Batista operation, to reduce the ventricular cavity in patients with nonischemic dilated cardiomyopathy.
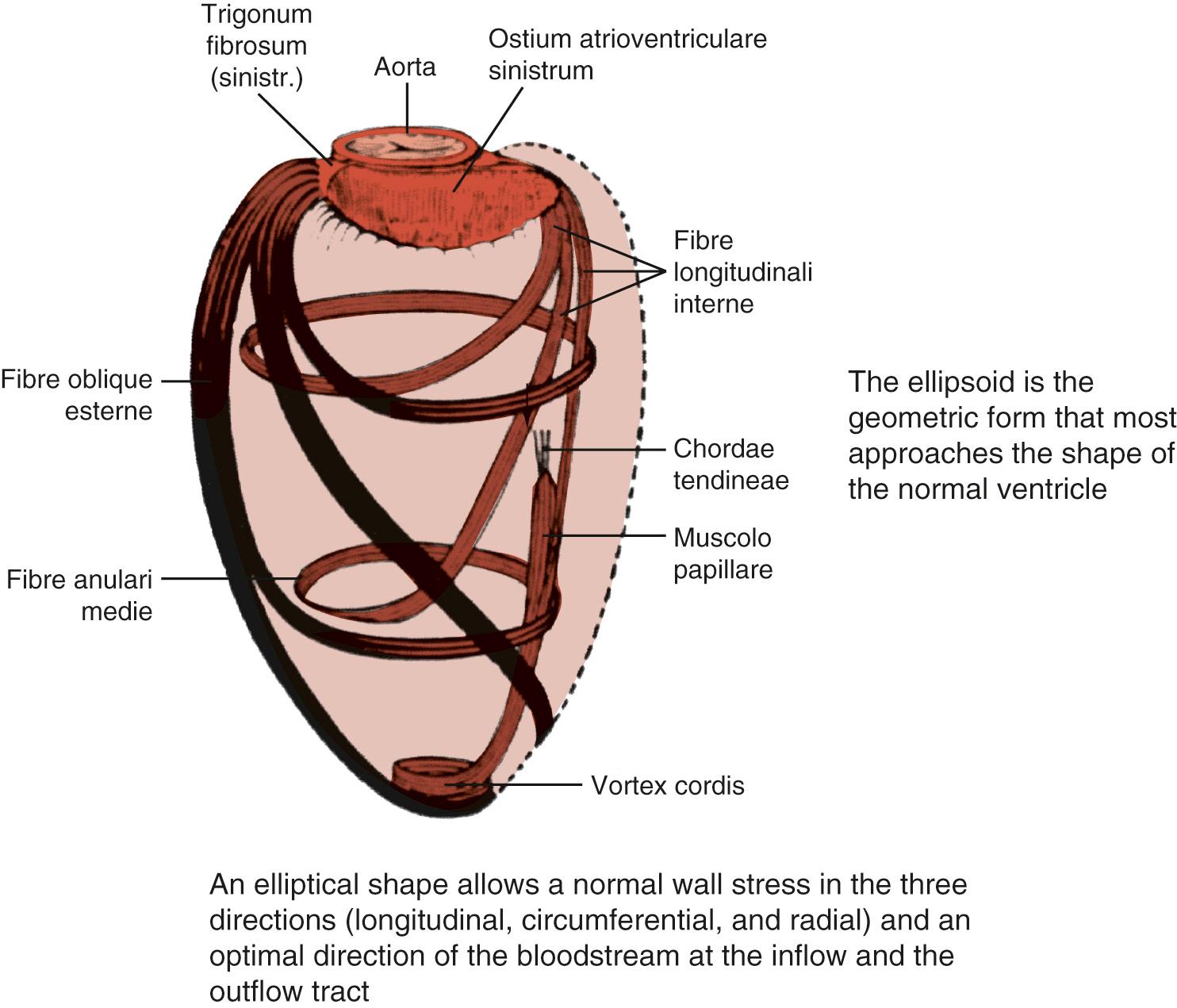
Figure 100-2 shows the spiral arrangement of myocardial fibers as reported in a very old anatomy atlas. Studies established that fiber orientation was a function of transmural location, with fiber direction being predominantly longitudinal in the endocardial region, transitioning into a circumferential direction in the midwall, and becoming longitudinal again over the epicardial surface. Such an alignment of fibers in double layers (left- and right-handed from the base to the apex) forms a double helix. These layers are not aligned parallel to one another; rather, the myofiber sheets diverge within the LV wall, creating angulations with respect to the plane of the epicardial surface. These fiber angulations serve to resist deformation and to maintain the distribution of tension within normal limits at the three levels: longitudinal, radial, and circumferential. Moreover, this architecture gives to the heart a form resembling a geometric ellipsoid, which favors the direction of flow toward the aorta. When angulations are deformed by fiber disruption, fibrosis, or scarring of myocardial tissue, the shape of the ventricle loses its characteristics and its functionality (following the strict relationship between form and function). For a given fiber contractile status, the ejection fraction (EF) changes according to the shape of the ventricle, being low in a spherical ventricle (sphericity index, toward 1) and high in an elliptical shape (sphericity index, toward 0). In fact, macroscopic anatomic alterations can affect wall tension, which, according to the Laplace law, is directly proportional to LV pressure and chamber radius and inversely proportional to wall thickness. In animal models of myocardial damage or after MI in humans, ventricular remodeling alters the major determinants of wall stress, and functional impairment has been reported in the remote zones of dilated LV with geometric abnormalities. When the ischemic systolic dysfunction is superimposed on the use of preload to improve cardiac output during exercise, the ventricle can be further dilated because of its inability to eject blood, starting a cycle that further deteriorates LV function. In addition, LV end-diastolic pressure can increase in the presence of reduced myocardial compliance (resulting from tissue stiffening), which tends to resist deformation, whereas increased stiffness leads to decreased systolic function.
A further increase in end-diastolic and end-systolic volumes (ESVs) leads to an adverse prognosis. As part of the GUSTO (Global Utilization of Streptokinase and t-PA for Occluded Arteries) trial, Migrino and associates evaluated ESV at 90 to 180 minutes into reperfusion during acute MI. They demonstrated that in successfully reperfused patients after MI, an increase in ESV beyond 40 mL/m 2 worsens prognosis in terms of both development of heart failure and mortality. A post-MI ESV index equal to or greater than 60 mL/m 2 carries a 1-year mortality rate of up to 30%. In the SAVE (Survival and Ventricular Enlargement) trial, left ventricular size was a strong independent risk factor for mortality after 2 years.
Even after coronary artery bypass surgery, patients with large ventricles have a worse prognosis. Yamaguchi and coworkers identified LV ESV index greater than 100 mL/m 2 as an independent risk factor for the development of CHF in ischemic cardiomyopathy. These reports helped cardiac surgeons focus on the importance of reconstruction of the left ventricle in ischemic cardiomyopathy.
Left ventricular aneurysm was first described in the eighteenth century. In 1816, Cruveilhier attributed ventricular aneurysm to myocardial fibrosis, although its association with coronary thrombosis was not generally appreciated until a century later. It was not until Tennant and Wiggers showed paradoxical motion in acutely ischemic myocardium that the physiologic implications of ventricular injury became apparent. Subsequently, Murray described systolic paradoxical expansion of acutely infarcted myocardium and correlated this with diminished cardiac output and falling blood pressure. In 1967, Klein and coworkers published a hemodynamic study on LV aneurysm that became a milestone in the field of mechanics and energetics of the left ventricle after an ischemic injury. Gorlin and coauthors were the first to state that when approximately 20% to 25% of left ventricular area is inactivated by any pathologic process, the degree that the myofiber must shorten to maintain stroke volume exceeds physiologic limits, and cardiac enlargement (Starling mechanism) must ensue to maintain adequate ejection of blood. With this concept, they anticipated the concept of LV remodeling and described the way MI relates to the genesis of aneurysm. Furthermore, they described that the aneurysm can be either dyskinetic (i.e., paradoxical expansion resulting during systole) or akinetic (i.e., myocardial fibrosis, calcification within the scar, thickened overlying pericardium, mural thrombosis, and endocardial thickening may rigidify the aneurysm wall and prevent its expansion).
Gorlin offered the following definition:
An aneurysm is identified by a left ventricular angiogram as any akinetic or dyskinetic segment of myocardium. An akinetic segment is defined as a segment that appears to have no motion during systole, whereas a dyskinetic segment appears to bulge paradoxically during systole. Intraoperatively, an aneurysm is identified as a circumscribed area of scar, which is thin, often adherent to the pericardium and which may or may not bulge paradoxically during systole. The aneurysmal segment is easily outlined by looking for the area that puckers and collapses when the left ventricle is vented.
This cineangiographic and surgical definition of ventricular aneurysm is compatible with the pathologic definition: “A localized outpouching of the cavity of a cardiac chamber, with or without outward bulging of the external surface.”
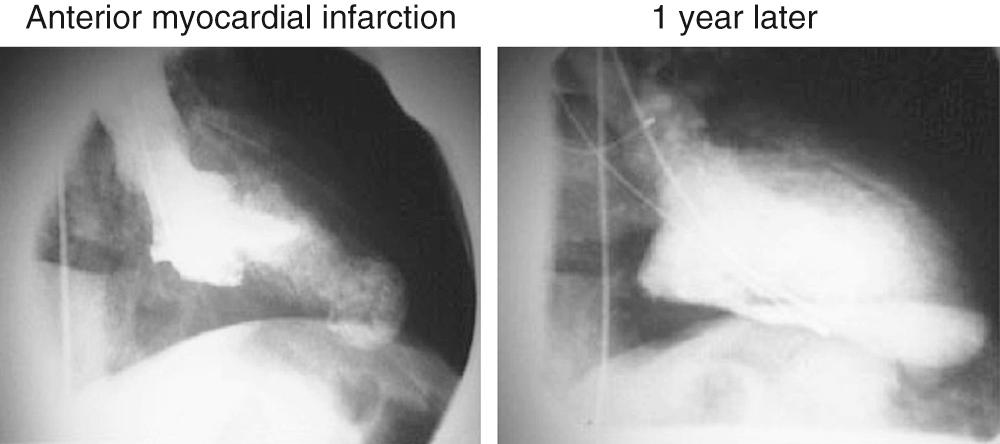
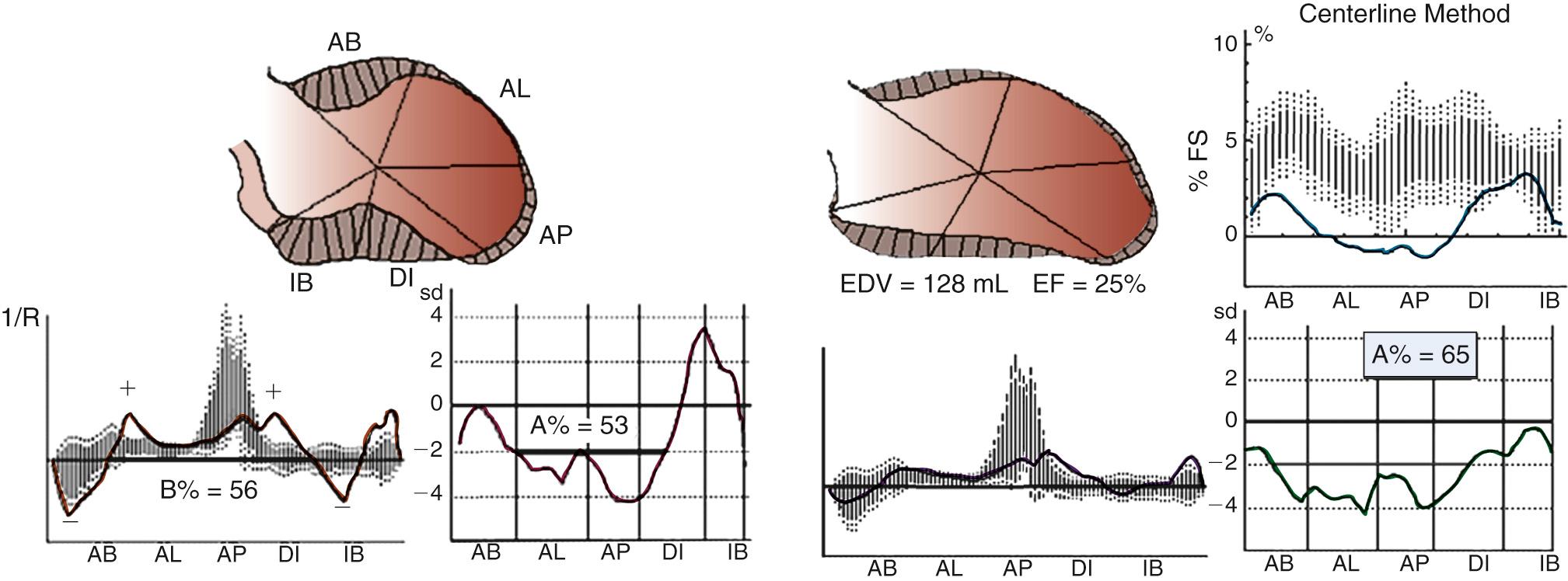
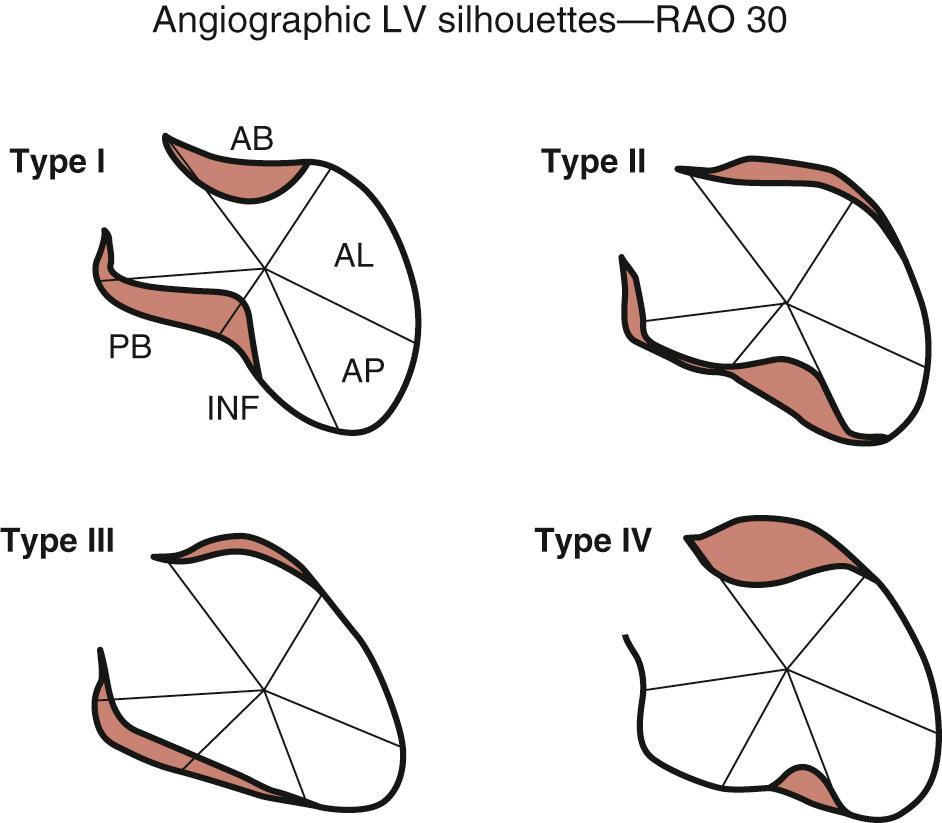
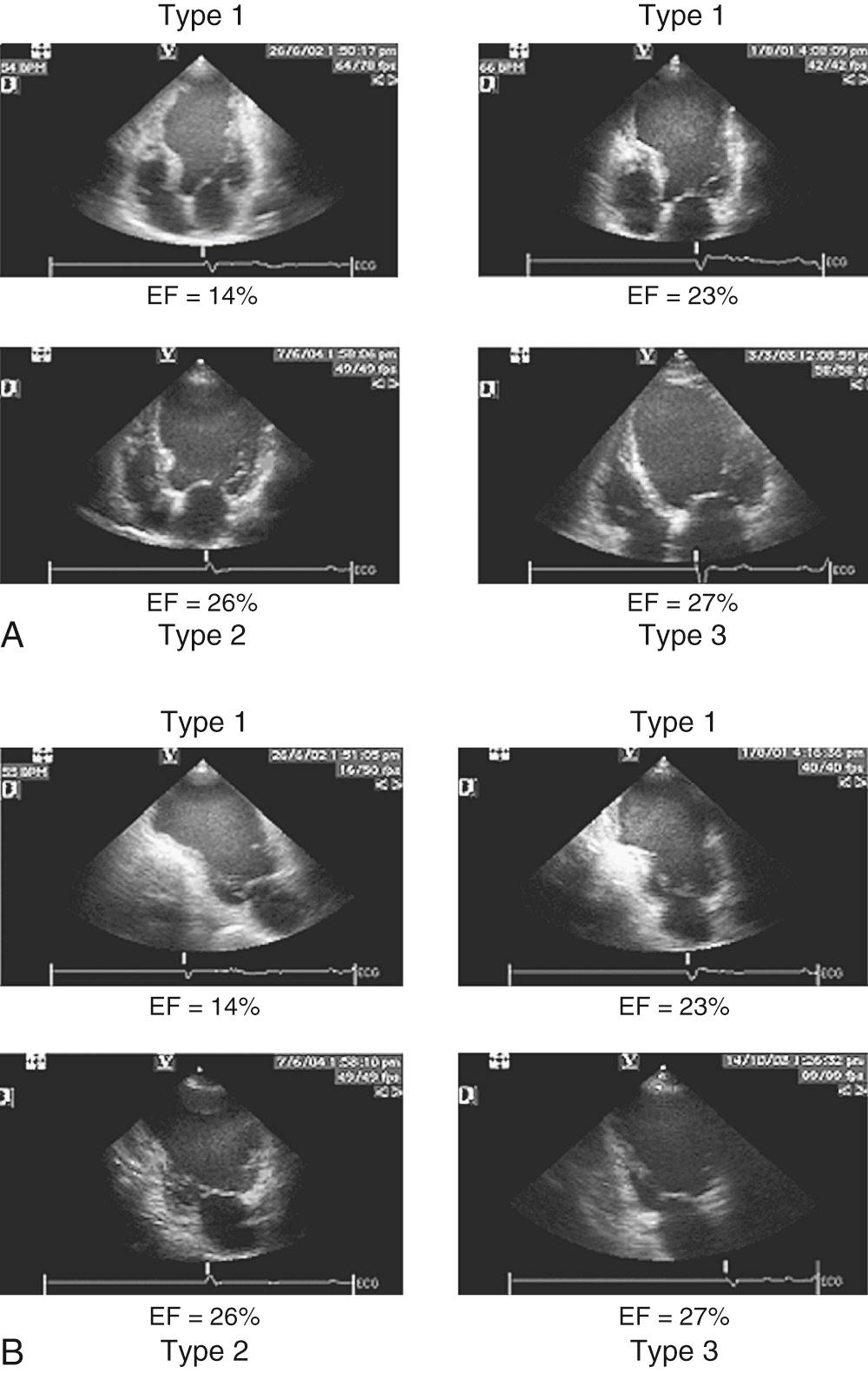
Early revascularization, with either thrombolysis or primary percutaneous transluminal coronary angioplasty, has beneficial effects on infarct size and LV function and therefore has profoundly changed the picture of MI and its sequelae. However, even with early mechanical relief of the coronary occlusion, unfavorable global LV remodeling may occur. Bolognese and coauthors demonstrated that almost 30% of patients with excellent infarct-related artery patency at 6 months continue to undergo LV remodeling. In patients with an anterior MI, this occurs more frequently when myocardial contrast echocardiography demonstrates a higher incidence of microvascular dysfunction. Figure 100-3 shows the progression of an anterior MI toward ischemic dilated cardiomyopathy in one of our patients. Figure 100-4 shows two of the shape abnormalities that may occur after successful early recanalization for anterior MI as analyzed by the centerline method. Thus, an acute MI, either anterior or inferior, may result in any of four types of shape abnormalities ( Fig. 100-5 ). This classification is incomplete because it does not take into account abnormalities occurring at the septum or at the lateral wall. However, it is useful for assessing treatment results, especially after volume reduction surgery, when the results after repair of a true aneurysm look profoundly different from the results after surgery for types 2, 3, or 4. Figure 100-6 shows an echocardiographic study from four patients with three types of shape abnormalities. Apical four-chamber and two-chamber views are shown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here