Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In patients with significant valve disease, symptom onset, morbidity, and mortality after fixing the valve lesion are influenced by ventricular and vascular factors.
The structure and function of the left ventricle adapt differently over time to pressure overload (e.g., aortic stenosis) and volume overload (e.g., mitral regurgitation).
Changes to ventricular structure and function can be both adaptive and maladaptive and reverse to various degrees after significant valvular abnormalities are fixed.
The pattern, extent, and timing of hypertrophic ventricular remodeling are influenced by numerous factors beyond the specific valve abnormality.
Pulmonary hypertension is common in patients with valve disease and associated with symptom onset, symptom severity, and lower survival.
In patients with left-sided valve disease, pulmonary venous pressures are commonly elevated, but reactive changes in the pulmonary vasculature can also cause concomitant increases in pulmonary vascular resistance.
The systemic vasculature contributes to the afterload on the left ventricle in patients with valve disease, including aortic stenosis.
From a pathophysiologic and clinical perspective, it is helpful to think of the pulmonary vasculature, left atrium and ventricle, mitral and aortic valves, and systemic vasculature as an integrated unit in which the components have bidirectional influences on one another.
The adaptations to and consequences of left-sided valve lesions underlie the morbidity and mortality that accompany them. Commonly, the adaptive response is conceptualized in terms of how it affects the size, remodeling, and function of the left ventricle (LV). Overgeneralizing, mitral regurgitation (MR) is a volume overload lesion that over time produces LV dilation and eccentric hypertrophy, whereas aortic stenosis (AS) is a pressure overload lesion that initially induces a smaller LV cavity and concentric hypertrophy. These changes in LV size and remodeling are accompanied by changes in diastolic and systolic function that become more abnormal if the valve lesion is not fixed.
While not diminishing the importance of these changes in LV structure and function in response to valve lesions, it is increasingly clear that the pulmonary and systemic vasculature changes in response to valve lesions plays an important role in the manifestations of valve disease and their clinical consequences. It is likely best to take a more integrative perspective of the relationships of the pulmonary vasculature, left atrium (LA), mitral and aortic valves, LV, and systemic vasculature. The relationships within the valvular-ventricular-vascular unit are not linear or unidirectional, but bidirectional ( Fig. 5.1 ). These complex interactions are modified by genetics, environment, sex, metabolic health, age, coronary artery disease, and other factors. Dynamic changes brought on by activity or exercise also influence these interactions and the consequences of left-sided valve lesions.
Although some of the structural and functional changes in the LV and vasculature may not manifest overtly (e.g., reduced LV ejection fraction [EF]) before valve treatment, they may be maladaptive and only partially reversible, making the patient more vulnerable to heart failure or other adverse consequences after valve interventions. To optimize long-term patient-centered outcomes, it is important to elucidate the pathophysiology of these ventricular and vascular changes and understand their short-term and long-term consequences to more appropriately time interventions to fix valve pathology and to identify novel targets for adjunctive medical therapy in the vasculature and the LV.
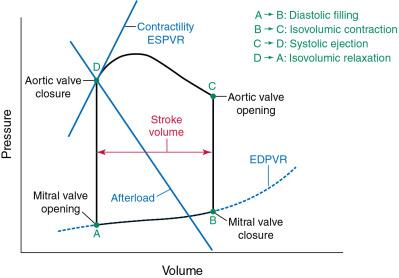
Familiarity with pressure-volume (PV) loops is foundational for understanding the LV response to stenosis or regurgitation of the mitral or aortic valves. Core concepts include preload, afterload, and contractility. Fig. 5.2 shows a generic PV loop, with labels describing each part of the loop and important lines.
Preload is the stretch of the sarcomeres in the myocardium at end-diastole before contraction. Preload is proportional to the volume of blood in the ventricle at end-diastole, but it is also influenced by the pressure required to achieve that volume. Preload is increased by increased blood volume or increased vasomotor tone in the venous system that increases blood return to the heart. An increase in preload, specifically an increase in end-diastolic volume, is associated with a rise in stroke volume if afterload and contractility remain constant ( Fig. 5.3 ).
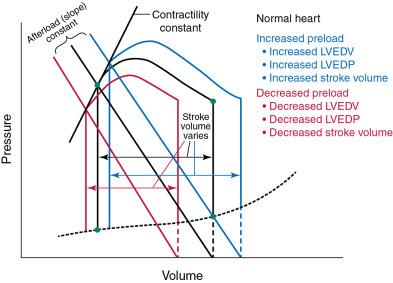
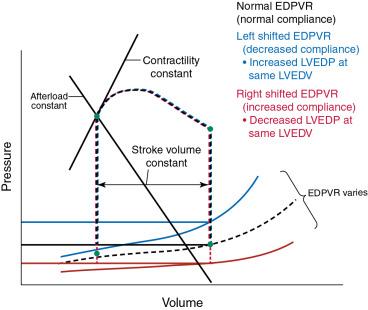
In contrast, a decrease in preload is associated with a decrease in stroke volume (see Fig. 5.3 ). Pertinent to this chapter, valve disease that alters the normal flow of blood through and out of the heart may also increase LV end-diastolic volume (e.g., MR, aortic regurgitation [AR]). Moreover, changes in the diastolic properties of the heart may increase preload, even at the same end-diastolic volume. Hypertrophic remodeling that decreases the compliance of the heart shifts the end-diastolic PV curve, or compliance curve, upward and to the left, and for any given LV end-diastolic volume, the end-diastolic pressure is higher ( Fig. 5.4 ).
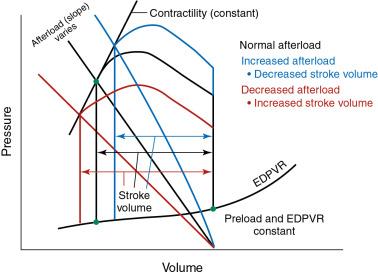
Afterload is the resistance to muscle fiber shortening during the systolic, or ejection, phase of the cardiac cycle. Muscle fiber tension, or wall stress, is quantified by the law of Laplace, which states that wall stress equals the intraventricular pressure multiplied by the radius of the LV cavity, divided by twice the thickness of the LV wall. Usually, the intraventricular pressure is essentially equal to aortic pressure. Increased pressure in the aorta (e.g., hypertension) and increased LV chamber dilation increase afterload, whereas an increase in wall thickness reduces wall stress and afterload.
On a PV loop, afterload, or arterial elastance, is represented by the line between the maximum LV end-diastolic point on the x-axis and the point of aortic valve closure at end-systole ( Fig. 5.5 ). An increase in the slope of this line reflects an increase in afterload, which is accompanied by a decrease in stroke volume for a given preload and contractility (see Fig. 5.5 ). In contrast, a decrease in the slope of the line, perhaps related to administration of a vasodilator, reflects a decrease in afterload, which is accompanied by an increase in stroke volume for a given preload and contractility (see Fig. 5.5 ). An LV outflow tract obstruction, such as AS, adds resistance to LV emptying. The pressure gradient across the obstructed valve yields a much higher intraventricular pressure than aortic pressure, contributing to higher wall stress that is magnified as the severity of valve stenosis progresses.
Contractility is the calcium-dependent ability of muscle to contract, or generate force, at a given fiber length. In terms of PV loops, the contractility of the ventricle as a whole is considered, and it may be altered by multiple factors, including inotropes, acidosis, and exercise. However, myocardial contractility may differ across the heart due to infarction, nonmyocyte infiltration, variation in electrical activation, and other regional differences. On a PV loop, contractility is represented by the line connecting the intersection of the x-axis and y-axis and the point representing end-systole (i.e., end-systolic PV line) ( Fig. 5.6 ). This is also referred to as end-systolic elastance .
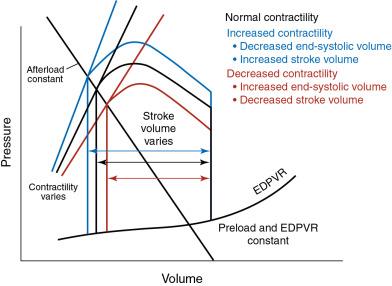
For a given preload and afterload, an increase in contractility leads to a decrease in the LV end-systolic volume and a resulting increase in stroke volume (see Fig. 5.6 ). In contrast, a decrease in contractility is associated with an increase in end-systolic volume and decrease in stroke volume (see Fig. 5.6 ). For patients with valve disease, compensatory mechanisms in place that alter contractility to preserve hemodynamics in the face of increased afterload or preload may begin to fail over time, which may precede or accompany the clinical decline of the patient.
The heart can undergo hypertrophic remodeling in response to a variety of stimuli. Depending on the stimulus, this hypertrophic growth may be partially or completely reversible. Hypertrophic growth can be adaptive, as in the case of exercise, or pathologic, as in the case of hypertrophic cardiomyopathy.
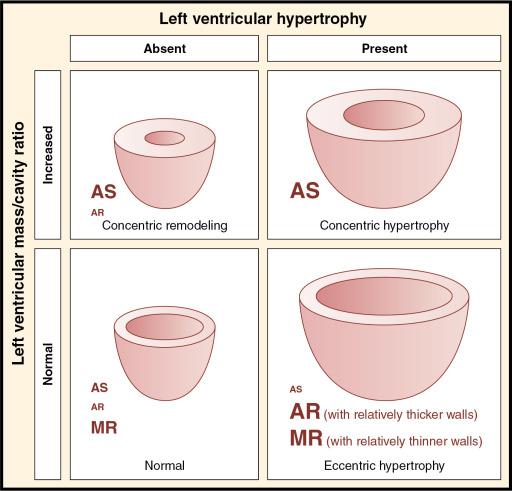
The four principal categories of hypertrophic remodeling are (1) normal geometry; (2) concentric remodeling; (3) concentric hypertrophy; and (4) eccentric hypertrophy ( Fig. 5.7 ). The patterns of hypertrophic remodeling associated with each left-sided valve lesion are shown in Fig. 5.7 . Numerous factors influence the type and degree of remodeling that may occur in a given individual, including age, sex, genetics, metabolic factors, coronary disease, and blood pressure. Pressure or volume overload, or both, from heart valve disease can have an important effect on hypertrophic remodeling of the LV.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here