Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Incidental irradiation of normal tissues is unavoidable during radiotherapy. The primary determinants of injury are the radiation dose (total dose and dose per fraction) and the volume of normal tissue irradiated. Additional treatment factors that influence risk include dose rate, overall treatment time, treatment energy, the use of concurrent chemotherapy, radiation protectors or other biological modifiers, and the interval between radiation courses in patients undergoing a second course of radiation. Host-related factors include comorbid conditions (e.g., diabetes and collagen vascular disease), inherent radiation sensitivity (e.g., underlying genetics), and patient age. Organ-related variables include preradiation organ compromise or loss, development of severe acute toxicity (resulting in consequential late effects), regional variation of radiosensitivity within an organ, and hierarchical organization of the organ (i.e., whether damage to a portion of the organ affects only that portion or has a more widespread effect). Furthermore, an organ may have more than one type of late toxicity that may or may not have different tolerance doses. Tumors can infiltrate into normal tissues, either at presentation or after treatment (i.e., local failure), compromising organ function and leading to late sequelae.
Different organs have different dose/volume thresholds for the development of radiation-associated injury. The critical question arising from this observation is as follows: What are the dose-sensitive targets in normal tissues resulting in late toxicity? Damage to either the functional (parenchymal) or stromal cells of the organ, or the fine vasculature, have been implicated. Differences in radiation susceptibility of different organs, therefore, may be due to different sensitivities of these functional cells, regional variation in the susceptibilities of small vessels due to the stroma or microenvironment, different capacities for neovascularization, and/or differences in the redundancy of the blood flow (i.e., those tissues relying on fewer vessels may be more susceptible to radiation damage) or functional reserve.
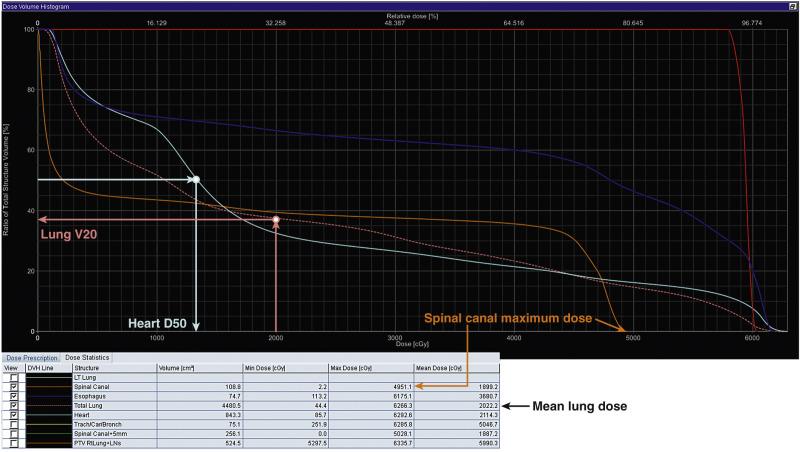
The focus of this chapter will be on the review of credible data associating radiation dose/volume parameters with the risk of normal tissue injury. Three-dimensional (3D) planning has become standard practice, allowing the radiation oncologist to quantitate doses to normal tissues in the region of interest. 3D dose/volume data can be difficult for clinicians to easily comprehend since the distributions are 2D. Visualizing isodose distributions is challenging and comparing competing distributions is largely subjective. Therefore, dose-volume histograms (DVHs; essentially, 2D representations of the 3D data) have been embraced as a rapid way to summarize the dose distribution. A DVH is generated by tallying the doses delivered to each (or a representative sample of) voxel of tissue and representing that information as a cumulative histogram of dose ( x -axis) and volume ( y -axis). Each point along the histogram represents the volume of that organ receiving more than or equal to that dose (e.g., V20 is the volume of an organ receiving at least 20 Gy). A DVH can be readily visualized and provides a quick and easy way to describe the dose/volume characteristics of the 3D dose distribution. However, a DVH achieves this by discarding all spatial information and the DVH does not account for variations in fraction size. Functional and structural complexities, and spatial variations in function/sensitivity, are thus not considered in DVHs. Possible interactions between organs is also not considered with this construct.
Beyond the marked data reduction in going from a 3D plan to a DVH, DVHs also remain challenging for clinicians to consider and compare owing to the previously mentioned issues relating to functional heterogeneity and the incomplete knowledge about radiation sensitivity of tissues. Therefore, it has become attractive to further reduce data and extract “figures of merit” from the DVH. The critical metrics that will considered in this review are the mean organ dose and discrete points on the DVH. These include ( Fig. 18.1 ):
Vx reflects the volume of tissue (generally a percentage) receiving ≥ X Gy. This is probably the most commonly used metric for parallel-type organs such as the lung and kidney, but also others such as the heart. For these, as discussed earlier, the portions of the organ exposed to a “regionally injuring” dose of radiation will become dysfunctional. Thus, the percentage of the organ exposed to that dose is a useful parameter.
Dx reflects the minimum dose to the hottest x% (generally percentage of total volume) of tissue. This parameter is not widely used clinically. It might be most useful for parallel-type organs in which the percentage of an organ's function that can be lost is known (e.g., say, 30%). Then, if the D30 is less than the locally injuring dose, global organ function should remain. Similarly, for organs in which an injury might be clinically manifest if there is a hot spot of a particular size, the Dx, where x is equal to that critical size, might be a useful parameter to predict outcomes.
Dmax is the maximum dose delivered to an organ and is most useful for series organs. Dmax is analogous to Dx, as the volume x decreases toward zero.
Mean dose is the simple arithmetic average of the dose to an organ. For parallel organs in which there is a gradual dose-response function for radiation-induced regional injury, the mean dose might reasonably correlate with outcomes.
More complex modeling has also been widely used to extract factors that better reflect the entire DVH rather than a single point (e.g., Dmax, Dx, Vx). These models will “sum up” the risk associated with each component of a DVH and apply different methods of summing depending on the type (or architecture/structure) of the organ. For example, for a series-structured organ, the high-dose region of the DVH might be most weighted more heavily in the “summing” while this is less strongly considered in a parallel-structured organ. Early work in this area led to the Lyman Kutcher Burman (LKB) model and more recently the equivalent uniform dose (EUD) model, both of which reduce a DVH to a single normal tissue complication probability (NTCP). These models and their relationships are summarized elsewhere.
More conformal radiation planning and delivery tools—such as intensity-modulated radiation therapy (IMRT), image-guided radiotherapy (IGRT), stereotactic body radiotherapy (SBRT), and charged particle irradiation—give the physician increased flexibility in determining how to deliver the desired target dose while minimizing and/or redistributing the dose exposure to normal tissue. The clinical application of these new technologies requires that the physician and dosimetrists/physicists have an in-depth knowledge of the dose/volume/outcome relationships for critical normal tissues. However, these new technologies have altered the relationship between the target doses and the doses to surrounding normal tissues that might impact on the applicability of historical data for our modern era. With conventional beams (often opposed beam pairs treated sequentially), the normal tissues were exposed to fraction sizes similar to that of the tumor. With these newer approaches, the fractional radiation doses delivered to the normal tissues adjacent to the target are typically lower than that received by the target. Further, there is a movement toward the use of shorter hypofractionated regimens. Ironically, the use of increasing fraction sizes, along with an increasing number of beams, leaves (at least some of) the surrounding normal tissue receiving a daily fraction size close to what is typically seen with conventional approaches. However, with the more modern techniques, the heterogeneity of dose within the normal tissues is increased. Despite these caveats, much of the published data regarding radiation-associated normal tissue injury remains applicable in the modern era. Continued study of this important topic is clearly needed.
Normal tissues can be functionally defined as “serial,” “parallel,” or a combination of both, analogous to the terminology used for electrical circuits ( Fig. 18.2 ). In parallel functioning organs, the functional subunits function independently (i.e., functional redundancy exists). Thus, when some functional subunits of a parallel organ are damaged, the surrounding functional subunits continue to function. Examples of parallel functioning organs include the lung, liver, and kidney. Small to moderate, and perhaps even large, volumes of parallel organs can be damaged without causing global dysfunction since there is enough reserve in the undamaged portion of the organ and/or there is a capacity to regenerate (e.g., the liver). In “serial” organs, the functional subunits are arranged in a linear or branching fashion; hence, there is interdependence. Damage to the subunits of a serial organ can result in compromise or incapacity of the entire organ. Examples of series organs include the spinal cord, portions of the central nervous system, peripheral and cranial nerves, the gastrointestinal tract, and the tracheal-bronchial tree. While the concept of serial and parallel functioning organs is useful in assessing risk to tissues, it should be appreciated that this is only a model. The function of many organs requires the integrity of both serial and parallel components (e.g., the lung requires the “parallel” alveoli as well as the “series” conducting airways).
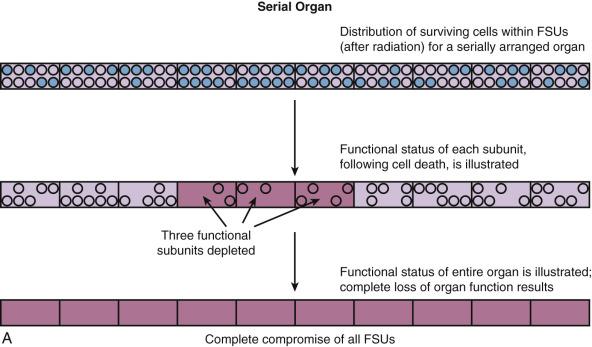
Further complicating this issue is the fact that, in both serial and parallel organs, there are often regional heterogeneities in function (e.g., gray matter and white matter tracts of the “parallel” spinal cord). These anatomic subregions may have different functions and different susceptiblities to treatment-related damage.
This chapter will summarize and expand on several reviews published as a special issue in the International Journal of Radiation Oncology Biology Physics (Volume 76, Issue 3, Supplement), all of which were written as part of the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) initiative. As the QUANTEC reviews were published in 2010, this chapter also summarizes relevant studies published after QUANTEC.
QUANTEC arose from a proposal from the Science Council of the American Association of Physicists in Medicine (AAPM) to revise and update guidelines published by Emani in 1991. QUANTEC's goals were (1) to provide a critical review of the current literature on quantitative dose-response and dose-volume relationships for clinically relevant normal-tissue endpoints, (2) to produce practical guidelines to allow reasonable toxicity risks based on dose-volume parameters, and (3) to identify future research initiatives. Using the QUANTEC reviews as a backbone, this chapter will focus on recently published data relevant to late toxicity from radiation, with an emphasis on relevant dose-volume metrics. Key summary points of the QUANTEC reviews are briefly summarized at the end of each section, and the QUANTEC reviews are referenced after each subheading. Table 18.1 summarizes the dose-volume metrics that are supported by the literature; this table was modified from the one published in the QUANTEC issue.
| Organ | Volume Segmented: Irradiation Type | Endpoint | Dose-Volume Parameters | Rate | Comments |
|---|---|---|---|---|---|
| Brain | Whole organ: 3DRT | Symptomatic necrosis | Dmax < 60 Gy | < 3% | Data at 72 and 90 Gy, extrapolated from BED models |
| Whole organ: 3DRT | Symptomatic necrosis | Dmax < 72 Gy | 5% | ||
| Whole organ: 3DRT | Symptomatic necrosis | Dmax < 90 Gy | 10% | ||
| Brain stem | Whole organ: whole brainstem |
Permanent cranial neuropathy or necrosis | Dmax < 54 Gy | < 5% | |
| Whole organ: 3DRT | Permanent cranial neuropathy or necrosis | D1-10 mL ≤ 59 Gy | < 5% | ||
| Whole organ: 3DRT | Permanent cranial neuropathy or necrosis | Dmax < 64 Gy | < 5% | Point dose < 1 mL | |
| Optic nerve/chiasm | Whole organ: 3DRT | Optic neuropathy | Dmax < 55 Gy | < 3% | Given the small size, 3DCRT is often whole organ. |
| Whole organ: 3DRT | Optic neuropathy | Dmax 55-60 Gy | 3%-7% | ||
| Whole organ: 3DRT | Optic neuropathy | Dmax 60 Gy | 7%-20% | ||
| Spinal cord | Partial organ: 3DRT | Myelopathy | Dmax 50 Gy | 0.2% | Including full-cord cross-section |
| Partial organ: 3DRT | Myelopathy | Dmax 60 Gy | 6% | ||
| Partial organ: 3DRT | Myelopathy | Dmax 69 Gy | 50% | ||
| Cochlea | Whole organ: 3DRT | Sensory neural hearing loss | Mean dose ≤ 45 Gy | < 30% | Mean dose to cochlea, hearing at 4 kHz |
| Parotid | Bilateral whole parotids: 3DRT | Long-term parotid salivary function reduced to < 25% of pre-RT level | Mean dose < 25 Gy | < 20% | For combined parotid glands |
| Unilateral whole parotid: 3DRT | Long-term parotid salivary function reduced to < 25% of pre-RT level | Mean dose < 20 Gy | < 20% | At least one parotid gland spared to < 20 Gy | |
| Bilateral whole parotids: 3DRT | Long-term parotid salivary function reduced to < 25% of pre-RT level | Mean dose < 39 Gy | < 50% | For combined parotid glands | |
| Pharynx | Pharyngeal constrictors: 3DRT | Symptomatic dysphagia and aspiration | Mean dose < 50 Gy | < 20% | |
| Larynx | Whole organ: 3DRT | Vocal dysfunction | Dmax < 66 Gy | < 20% | With chemotherapy |
| Whole organ: 3DRT | Aspiration | Mean dose < 50 Gy | < 30% | With chemotherapy | |
| Whole organ: 3DRT | Edema | Mean dose < 44 Gy V50 < 27% |
< 20% | Without chemotherapy | |
| Lung | Whole organ: 3DRT | Symptomatic pneumonitis | Mean dose 7 Gy | 5% | Excludes purposeful whole lung irradiation |
| Whole organ: 3DRT | Symptomatic pneumonitis | Mean dose 13 Gy | 10% | ||
| Whole organ: 3DRT | Symptomatic pneumonitis | Mean dose 20 Gy | 20% | ||
| Whole organ: 3DRT | Symptomatic pneumonitis | Mean dose 24 Gy | 30% | ||
| Whole organ: 3DRT | Symptomatic pneumonitis | Mean dose 27 Gy | 40% | ||
| Esophagus | Whole organ: 3DRT | Grade ≥ 3 acute esophagitis | Mean dose < 34 Gy | A variety of alternate threshold doses have been implicated. Appears to be a dose/volume response Similar constraints are applicable to late toxicity. |
|
| Whole organ: 3DRT | Grade ≥ 2 acute esophagitis | V35 < 50% | |||
| Whole organ: 3DRT | Grade ≥ 2 acute esophagitis | V50 < 40% | |||
| Whole organ: 3DRT | Grade ≥ 2 acute esophagitis | V70 < 20% | |||
| Heart | Pericardium: 3DRT | Pericarditis | Mean dose < 26 Gy | ||
| Pericardium: 3DRT | Pericarditis | V30 < 46% | |||
| Whole organ: 3DRT | Long-term cardiac mortality | V35 < 10% | Overly safe risk estimate based on model predictions | ||
| Liver | Whole liver – GTV: Whole liver or 3DRT |
Classic RILD | Mean dose < 30-32 Gy | < 5% | Excluding patients with preexisting liver disease or hepatocellular carcinoma |
| Whole liver – GTV:3DRT | Classic RILD | Mean dose < 42 Gy | < 50% | ||
| Whole liver – GTV: Whole liver or 3DRT |
Classic RILD | Mean dose < 28 Gy | < 5% | In patients with Child-Pugh A preexisting liver disease or hepatocellular carcinoma, excluding hepatitis B reactivation | |
| Whole liver – GTV:3DRT | Classic RILD | Mean dose < 36 Gy | < 50% | ||
| Kidney | Bilateral kidneys (not TBI): Bilateral kidneys or 3DRT |
Clinically relevant renal dysfunction | mean dose < 15-18 Gy | < 5% | |
| Bilateral kidneys (not TBI): Bilateral kidneys |
Clinically relevant renal dysfunction | Mean dose < 15-18 Gy | < 50% | ||
| Bilateral kidneys (not TBI): 3DRT (combined kidney) |
Clinically relevant renal dysfunction | V12 < 55% V20 < 32% V23 < 30% V28 < 20% |
< 5% | ||
| Stomach | Whole organ: Whole stomach |
Ulceration | D100 < 45 Gy | < 7% | |
| Small bowel | Individual small-bowel loops: 3DRT | Grade ≥ 3 toxicity (acute) | V15 < 120 mL | < 10% | Dose-volume data on late toxicity is lacking; data for acute toxicity may be a reasonably good surrogate. |
| Peritoneal cavity: 3DRT | Grade ≥ 3 toxicity (acute) | V45 < 195 mL | < 10% | ||
| Rectum | Whole organ: 3DRT | Grade ≥ 2 toxicity Grade ≥ 3 toxicity |
V50 < 50% | < 15% < 10% |
Data derived mostly from prostate cancer treatment |
| Whole organ: 3DRT | Grade ≥ 2 toxicity Grade ≥ 3 toxicity |
V60 < 35% | < 15% < 10% |
||
| Whole organ: 3DRT | Grade ≥ 2 toxicity Grade ≥ 3 toxicity |
V65 < 25% | < 15% < 10% |
||
| Whole organ: 3DRT | Grade ≥ 2 toxicity Grade ≥ 3 toxicity |
V70 < 20% | < 15% < 10% |
||
| Whole organ: 3DRT | Grade ≥ 2 toxicity Grade ≥ 3 toxicity |
V75 < 15% | < 15% < 10% |
||
| Bladder | Whole organ: 3DRT | RTOG Grade ≥ 3 late toxicity | Dmax < 65 Gy | < 6% | Based on bladder cancer treatment. Variations in bladder size/shape/location during RT hamper ability to generate accurate data. |
| Whole organ: 3DRT | RTOG Grade ≥ 3 late toxicity | V65 < 50% V70 < 35% V75 < 25% V80 < 15% |
< 6% | ||
| Penile bulb | Whole organ: 3DRT | Severe erectile dysfunction | Mean dose to 95% of gland < 50 Gy | < 35% | |
| Whole organ: 3DRT | Severe erectile dysfunction | D90 < 50 Gy | < 35% | ||
| Whole organ: 3DRT | Severe erectile dysfunction | D60-70 < 70 Gy | < 35% |
Although the QUANTEC initiative and associated reviews addressed a broad range of issues relating to normal tissue damage, this chapter will focus on late toxicity after an initial course (i.e., excluding reirradiation) of conventional radiation (i.e., excluding hypofractionated regimens). Many of the individual QUANTEC reviews discussed brachytherapy, reirradiation, and/or hypofractionated radiation, particularly in the context of hypofractionated stereotactic body radiation and stereotactic radiosurgery. The AAPM initiatives for hypofractionated tumor and tissue effects in the clinic (HYTEC) will focus on hypofractionated stereotactic radiation, a topic that is also reviewed elsewhere. A separate initiative is underway addressing quantitative dose-volume relationships for pediatric cancer survivors (PENTEC). This chapter will primarily focus on studies relating 3D dose-volume metrics to clinical outcomes in adults treated with conventionally fractionated radiation.
The structural and functional complexity of the brain puts this organ at risk for a spectrum of radiation-associated toxicities. Some specific functions of the brain can be correlated with discrete location(s) within the brain, whereas others are spread throughout the brain. Primary toxicity endpoints include frank brain necrosis with associated symptoms or signs and neurocognitive decline.
Prospective studies in adults have shown that partial (and limited) brain irradiation in the dose range of 50 to 60 Gy causes minimal to no discernible effect on memory and cognition. However, another study has suggested that patients undergoing partial brain radiation for low-grade glioma versus patients that do not undergo radiation are at greater risk for neurocogntive deficits, particularly attention, executive functioning, and information processing. More detailed studies correlating neurocognition with susceptible regions within the brain are needed. The volume of irradiated brain seemingly affects the degree of radiation-associated neurocognitive decline (with whole-brain radiation faring worse). The degree to which brain radiation (versus other factors, such as surgery, a history of hydrocephalus, other chronic diseases and comorbid illnesses, chemotherapy exposure, and tumor progression) impacts neurocognitive function is not clear. Chemotherapy-induced cognitive impairment (colloquially referred to as “chemo-brain”) is becoming better characterized.
There has been growing interest in the hypothesis that minimizing radiation dose to the hippocampal and/or subventricular zone stem cell niches, which are involved in neurogenesis, can reduce the risk of neurocognitive deficits. However, it is not known if there are particularly critical individual avoidance structures (or regions) or a combination of avoidance structures, nor whether there are well-defined dose-volume thresholds and what these dose-volume limits might be. Identifying correlates of dosimetric exposure to putative neuroanatomic structures to cognitive outcomes remains an active area of investigation. In the RTOG (Radiation Therapy Oncology Group) 0933 Phase II study of hippocampal sparing (100% dose and maximum dose to not exceed 10 Gy and 17 Gy, respectively) in 113 patients with brain metastases, memory preservation at 4 and 6 months (measured by the Hopkins Verbal Learning Test Delayed Recall) was significantly better than historical controls. In a study of 75 patients with pituitary adenoma, 30 of whom had undergone 3- to 5-field pituitary irradiation to a dose of 45 Gy, neither hippocampal dose nor prefrontal cortex dose correlated with cognitive outcomes. The NRG studies, randomizing patients to receive hippocampal sparing or standard brain radiation, are accruing patients undergoing prophylactic brain radiation for small-cell lung cancer (NCT02635009) and patients undergoing therapeutic whole-brain radiation for brain metastases (NCT02360215).
Radiation necrosis can occur in any part of the brain. While there may be regional variations of susceptibility within the brain related to differences in vascularity, glial cell population, and so on, this data is sparse. Thus, it is generally believed that location does not generally affect susceptibility to necrosis. However, certain regions, such as the brainstem, are more likely to cause symptoms. There is a paucity of data correlating dose-volume parameters with the risk of radiation necrosis with conventional fractions, though many studies have documented an association of fraction size with the risk of necrosis.
In a study from Queen Elizabeth Hospital in China, 1008 patients with nasopharyngeal cancer treated prior to 1985 received 45.6 to 53.2 Gy in 3.8 Gy fractions, 50.4 Gy in 4.2 Gy fractions or 60 Gy in 2.5 Gy fractions. The 10-year risk of temporal lobe necrosis was 18.6% in those treated with 4.2 Gy fractions versus less than 5% for the other dose schemes ( p < 0.001). A multi-institutional Chinese study examined 1032 patients with nasopharyngeal cancer treated after 1990 with one of several fractionation schemes (mostly 2.0-3.5 Gy fractions, though one scheme used a 1.6-Gy twice-daily component). The 5-year actuarial incidence of necrosis ranged from 0% (after 66 Gy in 2-Gy fractions) to 14% (after 2.5 Gy × 8 followed by 1.6 Gy twice daily to 71.2 Gy). In both of the studies mentioned earlier, the product of total dose and dose per fraction significantly impacted risk; shorter overall treatment time and twice-daily fractionation also increased risk. A Chinese study comparing the 71.2 Gy in 1.6-Gy twice-daily fractions versus 60 Gy in 2.5-Gy fractions was terminated early owing to excessive neurological toxicity, including temporal lobe toxicity, in both arms. The risk of toxicity was greater and the interval to developing toxicity was shorter with the twice-daily regimen. In another Chinese study, 27% of patients receiving an accelerated hyperfractionated regimen (64 Gy in 1.6-Gy twice-daily fractions) versus 0% receiving hyperfractionated radiation (70.8 Gy in 1.2-Gy twice-daily fractions) developed symptomatic radiation necrosis.
Radiation necrosis has also been studied in patients with primary brain tumors. The risk is dose dependent, with doses of less than 50 Gy rarely causing necrosis. In patients treated for brain metastases, there was a low (< 2%) risk of necrosis developing after 1.6 Gy twice daily to the whole brain (32 Gy) followed by a boost to 54.4 to 74.4 Gy, and no necrosis was seen after a boost to 48 Gy.
A high level of evidence to quantify the risks of radiation-induced brain injury is lacking. For brain necrosis, the brain appears to be especially sensitive to fraction size in excess of 2 Gy and to twice-daily fractionated treatment. Symptomatic necrosis is uncommon with doses less than 60 Gy with conventional (1.8-2 Gy) fractionation, though the risk increases with increasing dose (see Table 18.1 ). More detailed studies correlating neurocognition with susceptible regions within the brain are needed. Long-term (> 5 years) follow-up is necessary to best assess neurological/cognitive decline. For children, younger age and higher whole-brain dose strongly correlate with cognitive decline. Future studies should provide a clear definition of toxicity and report actuarial (as opposed to crude) rates that can be correlated with detailed normal brain dose-volume metrics.
The brainstem serves as a conduit from the brain to the cranial nerves and spinal cord. As a result, the brainstem is involved with motor, sensory, and special sensory function, as well as regulation of temperature, cardiac function, respiratory function, and consciousness. It is well accepted that the entire brainstem may be treated with 54 Gy using conventional fractionation with minimal risk of late brainstem toxicity. Small volumes of brainstem may tolerate higher doses. Similar to the brain, the brainstem is a very heterogeneous organ, and it is not clearly known which regions are most susceptible to radiation-induced damage. Complicating this matter are uncertainties about the radiobiological differences (relative biological effectiveness [RBE]) between photons and protons where the linear energy transfer (LET) differs within the distal segments of the spread out Bragg peak.
Several institutions have published their dose-volume constraints for the brainstem, most of which have not reported any brainstem toxicity. These constraints for patients undergoing external beam radiation therapy for head and neck cancer include V60 < 5 mL, V65 < 3 mL, V55 < 0.1 mL, D1 < 54 Gy, and maximum < 50 Gy ; for patients undergoing proton or combined proton/photon therapy for base of the skull lesions, the constraints include < 63-64 Gy CGE to the brainstem surface and 53-54 CGE to the brainstem center.
Some patients in these studies received a maximum brainstem dose of 66 to 68 CGE (greater than the recommended constraints) in order to adequately treat the tumor. In one study, the dose constraint of 63 CGE to the brainstem surface and 54 CGE to the brainstem center was “relaxed” in 38% and 17% of patients, respectively, with no reported neurological toxicity. In these patients, the volume receiving more than the threshold dose was 0.2 mL and 1.2 mL for the brainstem surface and center, respectively. In another study, 2 of 4 patients developing neurological toxicity received brainstem maximal doses in excess of 64 Gy CGE to the surface and 53 CGE to the center.
In an analysis of 367 patients from Massachusetts General Hospital, an increased risk of late toxicity was associated with the maximal delivered dose (> 64 CGE), V50 (> 5.9 mL), V55 (> 2.7 mL), V60 (> 0.9 mL), history of diabetes, hypertension, and ≥ 2 surgical procedures of the base of skull on univariate analysis. On multivariate analysis, only V60, history of diabetes, and ≥ 2 surgical procedures remained significant. A V60 < 0.9 mL versus > 0.9 mL resulted in toxicity-free survival of 96% versus 79% ( p = 0.0001), and on multivariate analysis resulted in an 11.4 risk ratio ( p = 0.001). In a recent study of 216 pediatric patients with posterior fossa tumors treated with proton therapy, 5 developed brainstem injury; the authors predict that a Dmax < 55.8 Gy RBE and V55 < 6.0% would result in < 2% risks.
In a study of 40 patients undergoing IMRT for meningioma, one patient developed fatal brainstem necrosis after receiving a maximum dose to the brainstem of 55.6 Gy, with 4.74 mL exceeding 54 Gy. This demonstrates that other poorly understood factors likely increase the risk of brainstem toxicity, as this dose constraint would be considered acceptable in most of the studies referenced earlier.
Investigating radiation-induced brainstem injury is challenging because of the low incidence of toxicity with conventional doses, the short survival of patients, and the challenges of distinguishing between tumor progression and toxicity. Whole brainstem doses < 54 Gy appear to be safe. Small volumes of brainstem appear to tolerate doses in excess of 55 to 60 Gy. The risk of brainstem necrosis is low if the volume receiving > 60 Gy is < 0.9 mL. One proton therapy study predicts a 5-year rate of radiation brainstem injury < 2% with Dmax and V55 < 55.8 Gy RBE and ≤ 6.0%, respectively.
The spinal cord consists of the motor and sensory tracts, communicating information between the peripheral nerves and the brain. Radiation-induced spinal cord injury can result in pain, paresthesias, sensory deficits, paralysis, Brown-Séquard syndrome, and bowel/bladder incontinence.
It is well accepted that the spinal cord can well tolerate 45 to 50 Gy with conventional fractionation, though the TD5 is most likely much higher. The spinal cord is generally limited to 45 to 50 Gy, since the anticipated risk of cord injury must be very low to be clinically acceptable. In an analysis of several studies, Schultheiss calculated the probability of cervical cord myelopathy after full cross-sectional irradiation as 0.03% after 45 Gy, 0.2% after 50 Gy, and 5% after 59.3 Gy. The thoracic spinal cord was calculated to be less sensitive than the cervical spinal cord (though, because of the dispersion of data, a good fit could not be obtained). This model does not incorporate spinal cord volume, which might be acceptable given the “series” nature of the cord. Nevertheless, Schultheiss cautions that long lengths of cord, concomitant chemotherapy, and other factors may increase risk.
Little data exists exploring the dose-volume tolerance of the spinal cord. No spinal cord toxicity was reported in a study in which the V50 was < 0.1 mL nor in another study in which the D1 was < 45 Gy. Massachusetts General Hospital studied 85 patients undergoing cervical spinal cord treatment in the range of 45 to 59.4 Gy (1.5 Gy equivalent fractions) EUD, 42 to 57.5 Gy maximal dose to cord center, and 57 to 74 Gy maximal dose to cord surface. Of these patients, 15% experienced Lhermitte syndrome (self-limited symptoms of electric shock–like sensation most notable with neck flexion, attributable to focal demyelination), and 5% developed objective neurological findings at or below the cord level treated. Toxicity was not significantly correlated with cord length, cord volume, maximal dose to cord center, maximal dose to cord surface, or effective uniform dose. The authors conclude that an EUD to the cervical cord of 60 Gy in 1.5-Gy fractions or 52.5 Gy in 2-Gy fractions is safe. In a study of 437 patients with laryngeal or oropharyngeal carcinoma (with maximum spinal cord dose of 22-69 Gy), none developed myelopathy (at a median follow-up 27 months), while 17 developed the Lhermitte sign; the average spinal cord V45 of these 17 patients was 14 mL versus 8 mL for those without the Lhermitte sign. It has been postulated that the dose to the spinothalamic tract is most clinically significant for the occurrence of the Lhermitte's sign.
There is not yet a consensus on the best approach to delineating the spinal cord, with options including delineating the entire thecal sac, spinal canal, spinal cord (as seen on magnetic resonance imaging [MRI]), or spinal cord plus a several-millimeter margin. Though rare, radiation-induced spinal cord injury can be clinically devastating. With conventional fractionation of 1.8 to 2.0 Gy per fraction to the full thickness of the spinal cord, the estimated risk of spinal cord myelopathy is < 1%, < 10%, and 50% at 54 Gy, 61 Gy, and 69 Gy, respectively. While there is limited data on high dose per fraction using conventional radiation techniques, the estimated α/β ratio of 0.87 suggests a strong dependence of spinal cord toxicity on dose/fraction. Small volumes of the spinal cord can likely receive doses in excess of 55 to 60 Gy (with the high doses limited to the surface) with low risk of toxicity, though long-term data are lacking to derive a dose-volume relationship for myelopathy risk. Thus, recommendations for safe dose-volume metrics above 55 to 60 Gy are lacking.
The optic nerve and chiasm serve as the neural conduit connecting the retinal fibers to the optic tracts, which, via the lateral geniculate body, terminate in the visual cortex. It is well accepted that the entire optic nerves and chiasm may be treated with 54 Gy using conventional fractionation with minimal risk of late visual toxicity, though lower doses can result in other ophthalmological toxicity. A classic study showed that among patients treated with the same dose for pituitary adenomas or craniopharyngiomas, those who developed optic neuropathy (5-34 months after radiation) had received ≥ 2.5 Gy per fraction. In a study from the M. D. Anderson Cancer Center (MDACC), in which 219 patients were treated in the pre-3D radiation era, 10-year actuarial rates of optic neuropathy were 0%, 3%, and 34% for 43 to 49 Gy at ~ 1.9 Gy/fraction, 50 to 60 Gy at ~ 2.1 Gy/fraction, and 61 to 76 Gy at ~ 2.2 Gy/fraction, respectively. Chiasm damage was similar, with rates of 0%, 8%, and 24% for 15 to 49 Gy at ~ 1.5 Gy/fraction, 50 to 60 Gy at ~ 2.0 Gy/fraction, and 61 to 76 Gy at ~ 2.1 Gy/fraction. Greater total dose as well as larger fraction size impact risk. With 3D planning, small volumes of optic nerve and chiasm may tolerate higher doses.
Several institutions have published their dose-volume constraints for the optic nerves and chiasm, most of which have not reported any neurological visual toxicity. These constraints for patients undergoing external beam radiation therapy for head and neck cancer include V55 < 0.1 mL of optic nerves/chiasm, D1 < 54 Gy for optic nerves and D1 < 45 Gy for optic chiasm and maximum < 54 Gy to the optic nerves and 52 Gy for the chiasm. These dose constraints are more conservative compared with what has been reported with proton therapy for base of skull tumors; base of skull tumors may be in close proximity to the optic apparatus, and tumors such as chordomas and chondrosarcomas are prescribed relatively high doses. For patients undergoing proton or combined proton/photon therapy for base of skull lesions, published dose contraints include < 55 to 56 GGE or < 60 Gy CGE to optic nerves and optic chiasm.
Several studies have reported ophthalmological toxicity after radiation. In a study from the University of Florida, the optic nerve dose (defined as the minimum dose delivered to one-third of the optic nerve) in patients who developed optic neuropathy was 50.4 to 79 Gy (median 68 Gy). In a University of Michigan study, 7 patients developed ophthalmological toxicity: 1 patient received a chiasm maximum of 59.5 Gy; 6 patients received an optic nerve maximum of 47.5 to 75.5 Gy (average 63 Gy). Moderate to severe optic nerve complications (4 patients) were associated with doses > 64 Gy. In one study, 4 patients developed ophthalmological toxicity, of which 3 received a maximal dose of 56 to 62 CGE to the optic chiasm/nerves and 1 received a maximum dose of > 62 CGE. In two studies, bilateral visual loss occurred, with no evidence of tumor progression, ~ 8 months after conventional radiation. In one study, the prescribed target dose was 49.3 Gy (maximum dose, 56.1 Gy; the chiasm maximum was not reported, but < 1 mL received >45 Gy) ; in the other, the chiasm maximum was < 58 CGE.
Some patients appear to tolerate a maximum optic nerve or chiasm dose of > 60 Gy or > 63 to 69 CGE. This demonstrates that other poorly understood factors likely increase the risk of optic nerve and chiasm toxicity. Optic nerve maxima > 80 Gy and chiasm maxima > 70 Gy were tolerated in some patients in the University of Michigan study discussed earlier. In another study, the dose constraint of 56 CGE to the optic nerve and chiasm was “relaxed” in 28% and 48% of patients, respectively, with no reported visual toxicity. In these patients, the volume receiving above the threshold dose was 0.11 mL and 0.12 mL for the optic nerves and chiasm, respectively; the volume receiving > 105% of the threshold dose was 0.05 mL and 0.01 mL.
Data clearly show that the total dose and fraction size are the most important treatment-related risk factors for optic nerve/chiasm injury. There is scarce data to suggest a dose-volume effect on the optic nerve and chiasm. The risk of visual problems is < 3% with < 55 Gy, 3% to 7% for 55 to 60 Gy, and > 7% for > 60 Gy. In the 55- to 60-Gy experience, almost all of the reported cases of optic nerve injury received doses in the 59- to 60-Gy range (i.e., the very high edge of that dose range).
The cochlea and acoustic nerve are the essential auditory structures that are susceptible to radiation injury and consequential sensory neural hearing loss. These are small structures; therefore, dose-volume measures are less determinable and clinically relevant. Other dose-dependent susceptible parts of the auditory system include the external ear and ear canal, tympanic membrane, ossicles, and eustachian tube. Platinum-based chemotherapy also is a well-established cause of sensory-neural hearing loss. Other factors such as baseline function and patient age are also relevant.
Several studies suggest that the dose to the cochlea correlates with the rate of sensory-neural hearing loss. A study from the University of Florida showed that incidence of sensory-neural hearing loss increased consistently with dose to the cochlea; the 10-year actuarial risk of sensory neural hearing loss was 3% at doses < 60.5 Gy versus 37% at doses > 60.5 Gy.
Several other studies have shown hearing loss to be directly related to the inner-ear dose, with sensory neural hearing loss becoming more apparent at doses > 45 to 50 Gy. A German study examined hearing loss during and after radiotherapy for head and neck cancer patients; for bone and air conduction after radiation, a 15-dB reduction in 50% of patients over a range of frequencies was in the 20- to 30-Gy range of doses to the inner ear (which ranged from 1.7 to 64.3 Gy). Cisplatin dose is also relevant to the threshold for auditory impairment. In one study of head and neck cancer patients, in those treated with radiation alone, hearing loss developed with cochlear doses > 40 Gy; however, among those who received cisplatin (100 or 40 mg/m 2 ) and radiation, hearing loss developed with cochlear doses > 10 Gy, although the risk is likely low below doses of 30 Gy. The sequence of chemoradiotherapy also appears to influence risk. Risk and severity of ototoxicity are greater when cisplatin is administered after cranial radiation.
In a study of patients with base of skull tumors treated with radiation (median, 50.4 Gy), radiographic opacification of the middle ear and/or mastoid, which correlates with subacute/chronic otitis media with effusion, occurred in 40 of 61 patients (with median follow-up of 21 months). This resolved in 17 of 40 patients 2 to 45 months (mean, 17 months) after radiation. Dose-volume analyses were performed for the eustachian canal, middle ear, mastoid air cells, vestibular apparatus, cochlea, internal auditory canal, lateral and posterior nasopharynx, and temporal lobes. Multivariate analysis showed that a mastoid dose > 30 Gy (odds ratio [OR], 28.0; 95% confidence interval [CI], 5.6-140.8; p < 0.001), and a posterior nasopharynx dose > 30 Gy (OR, 4.9; 95% CI, 1.5-16.3; p = 0.009) were associated with grade 2 to grade 3 middle ear effusions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here