Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Hypertrophic pyloric stenosis (HPS) is one of the most common surgical diseases in infants. Most infants present between the ages of 2 and 8 weeks with nonbilious projectile vomiting. They are often dehydrated with a hypochloremic, hypokalemic metabolic alkalosis. The degree of dehydration and electrolyte imbalance is not as dramatic as in the past because of the trend toward earlier diagnosis with ultrasound (US). The disease is caused by progressive hypertrophy of the pyloric muscle, causing a luminal obstruction. Since Ramstedt described the first successful pyloromyotomy in 1912, treatment has been operative pyloromyotomy. In selected high-risk patients, atropine has been used with some success. The first laparoscopic pyloromyotomy was described by Alain in 1991. Over the past 20 years, this approach has become more widely used and is the preferred technique by most pediatric surgeons today.
Infants with HPS typically present with progressive nonbilious emesis. The emesis typically occurs just after feeding and is often projectile in nature. The infants may be hungry after the emesis. The symptoms can be very similar to those seen with gastroesophageal reflux disease (GERD). A thorough history and physical examination can help distinguish GERD from HPS. It is common for the infant to have had several formula changes prior to presentation. On physical examination, the infants often appear dehydrated. An experienced pediatric surgeon can palpate the hypertrophied pylorus the majority of the time; however, most patients present to the pediatric surgeon with an US demonstrating HPS. To palpate the olive, the stomach must be emptied with a nasogastric tube and the infant can be satiated with a pacifier dipped in sugar. Once the olive is palpated, no further diagnostic workup is necessary. Preoperative electrolytes should be checked to guide resuscitation. Objective measurements used to determine HPS on US are a thickness greater than 3.5 to 4 mm and a length greater than 14 mm. Also, there is a distinct characteristic to the pylorus on US. The diagnosis may also be made by an upper gastrointestinal series contrast study, but this is uncommon today.
Prior to operation, it is imperative to correct the dehydration and any electrolyte abnormalities. Preoperative orogastric decompression may be needed if the patient continues to have emesis. In our institution, resuscitation is started with D5 NS plus 20 mEq KCl/L at 1.5 times the maintenance infusion rate based of the weight of the child. The infant is given a bolus of 10 to 20 mL/kg normal saline as needed. Electrolytes are rechecked every 6 to 8 hours to monitor the electrolyte correction. The urine output is closely monitored. The endpoints of resuscitation are adequate urine output and normalization or near normalization of the electrolytes. Our protocol is to have the bicarbonate less than 30 and the chloride greater than 100 before operative intervention.
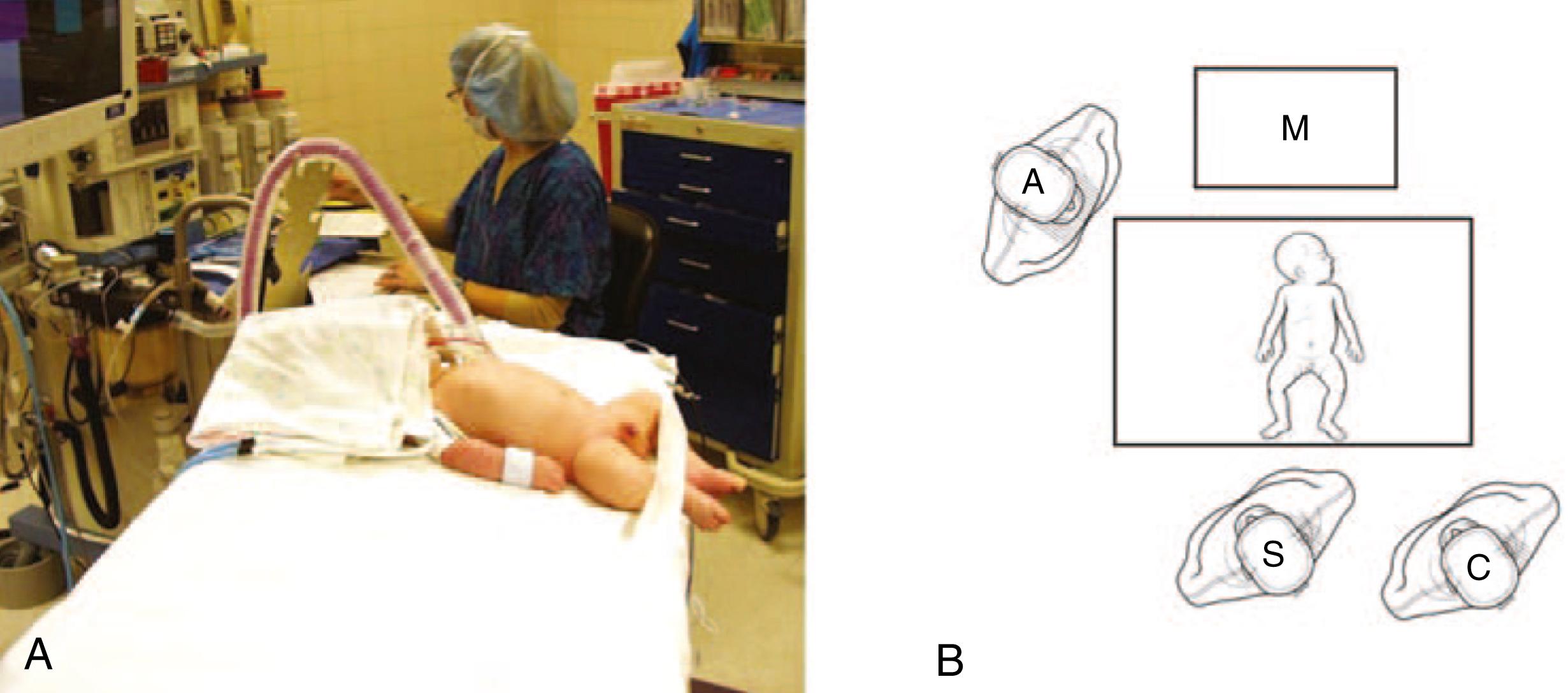
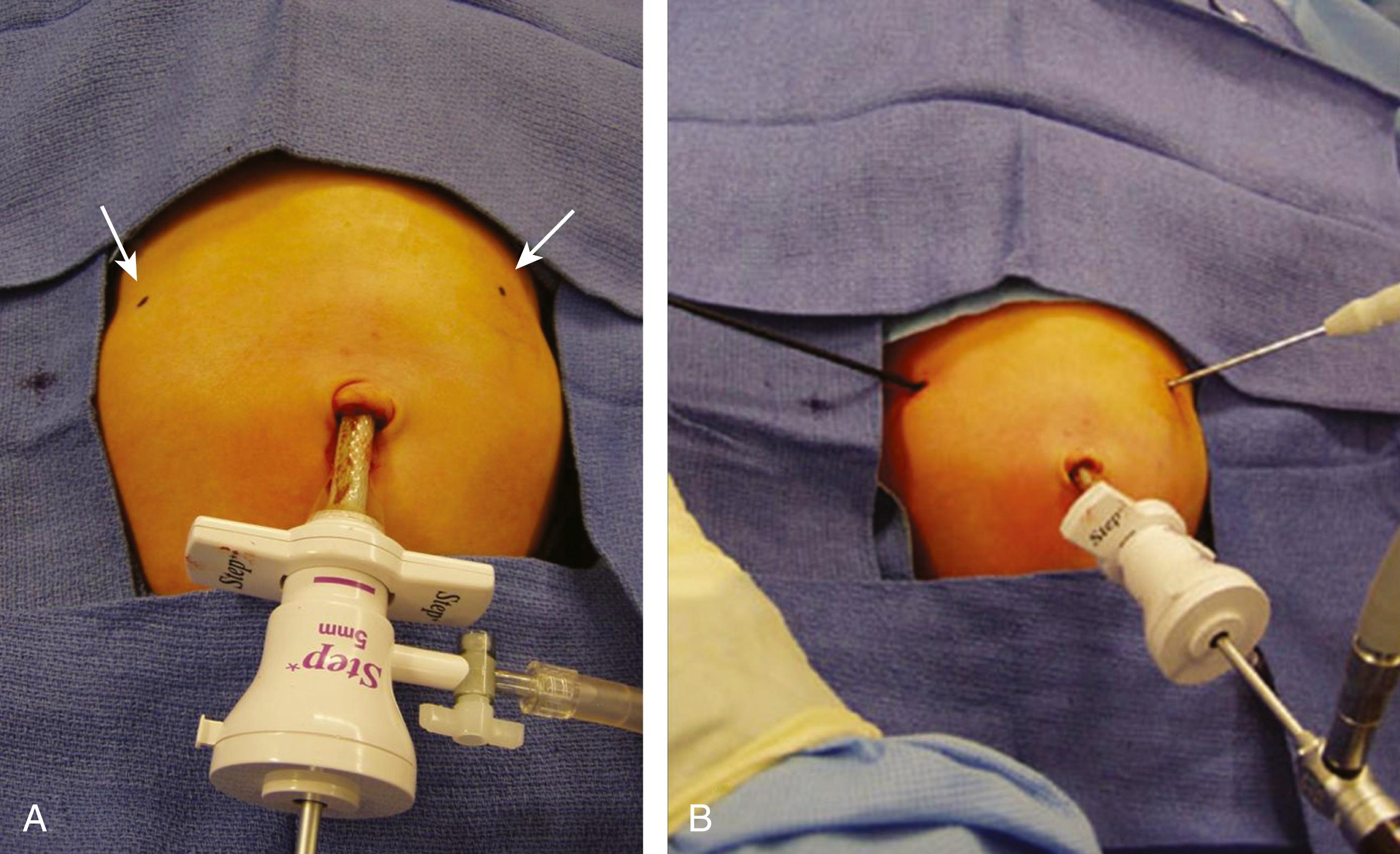
After induction of general anesthesia and endotracheal intubation, the patient is placed transversely on the operating room table ( Fig. 5-1A ). The monitor is positioned across the table from the surgeon and assistant, cephalad to the patient ( Fig. 5-1B ). The stomach is suctioned with an orogastric tube. The initial cannula is inserted using a modified Veress technique. There have been reports of umbilical vein cannulation and CO 2 embolus in this patient population. We now always utilize a transverse inferior umbilical incision. Care is taken to ensure that the Veress needle is inserted into the peritoneal cavity. The needle should not be directed cephalad, as it may inadvertently cannulate the umbilical vein. The needle should be directed laterally or into the pelvis. After insufflation, we place a 3.5-mm reusable cannula and introduce a 3-mm 30-degree laparoscope. Alternatively, a 5-mm port and a 5-mm laparoscope can be used as well ( Fig. 5-2A ). The abdomen is insufflated to 10 to 12 mm Hg. Stab incisions are then made with a #11 or #15 scalpel in the right and left epigastrium, followed by insertion of 3-mm instruments ( Fig. 5-2B ). A bowel grasper is used in the surgeon’s left hand, and an arthrotomy knife, pyloric knife, or blade cautery tip is used in the right hand.
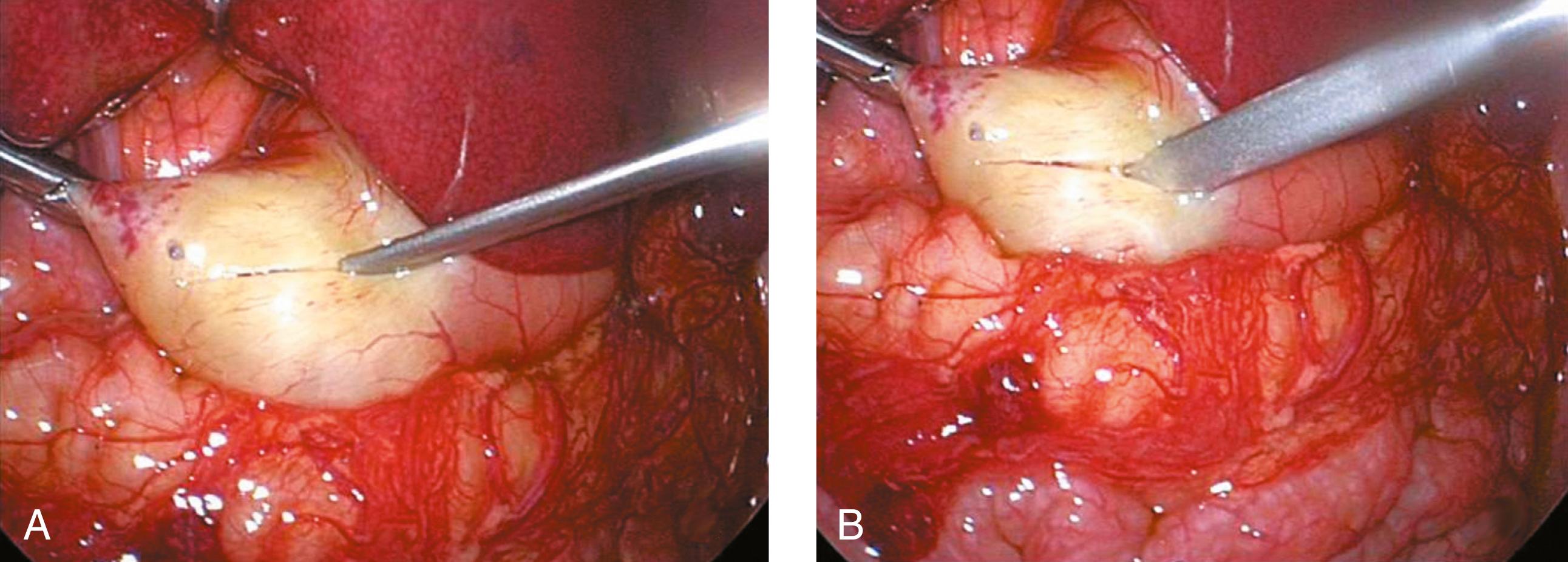
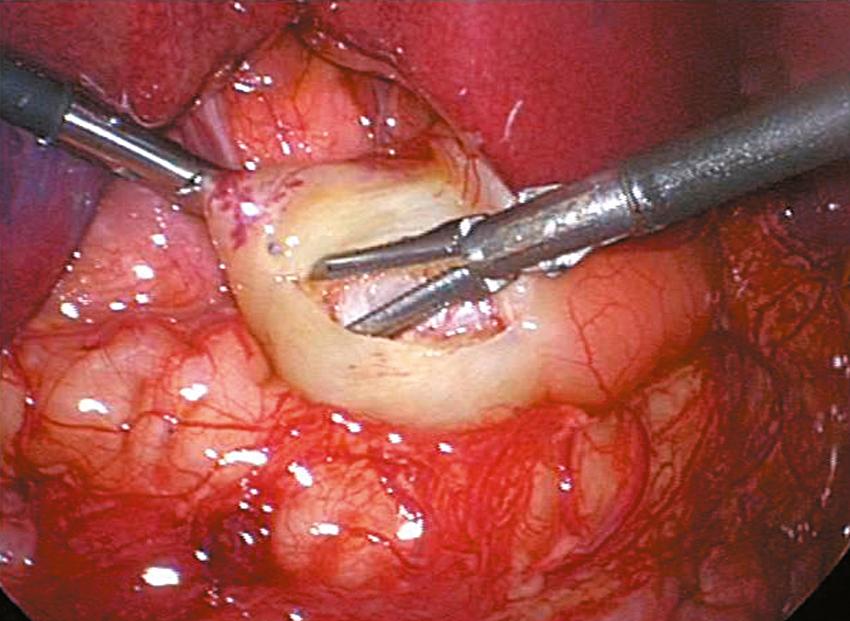
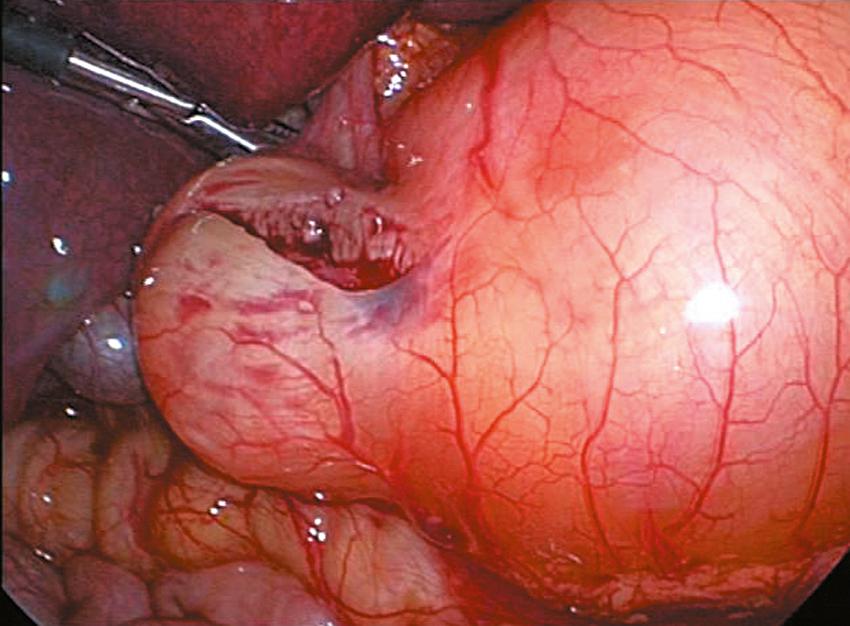
An incision is made on the anterior surface of the pylorus beginning just proximal to the junction with the duodenum (near the vein of Mayo) and is extended proximally onto the antrum of the stomach ( Fig. 5-3A ). If the cautery is used, it follows the same course and it is important to use the cut mode on the electrocautery. One of the pitfalls is making the incision too superficial. Remember, if the patient truly has hypertrophic pyloric stenosis, the muscular wall thickness should be at least 4 mm thick. The knife is then retracted into the sheath (if it has a retractable blade) and the sheath is slid into the seromuscular incision to begin the myotomy. The sheathed knife is then twisted 60 to 90 degrees to begin breaking the muscular wall ( Fig. 5-3B ). This maneuver deepens the seromuscular incision and makes it easier to insert the pyloric spreader ( Fig. 5-4 ). Alternatively, this maneuver can be performed with the blade cautery or carefully with the knife blade. The myotomy is extended distally to the junction of the stomach and the duodenum, and proximally onto the antrum of the stomach. Perforations usually occur distally, and inadequate myotomies are usually caused by incomplete muscular division proximally on the stomach. An adequate proximal extent of the myotomy is determined by visualization of the oblique muscular fibers seen in the antrum without seeing the mucosa. Care is taken distally not to enter the fold of duodenal mucosa at the gastroduodenal junction. The inferior and superior portions of the cut myotomy should move independently of each other ( Fig. 5-5 ).
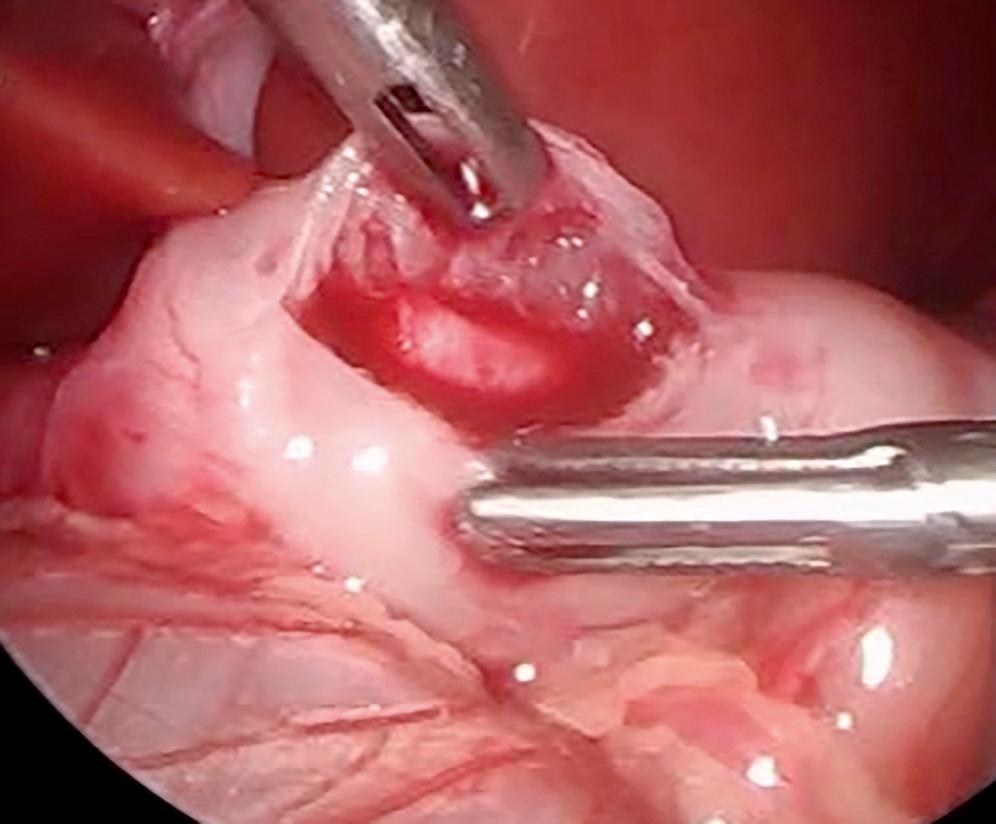
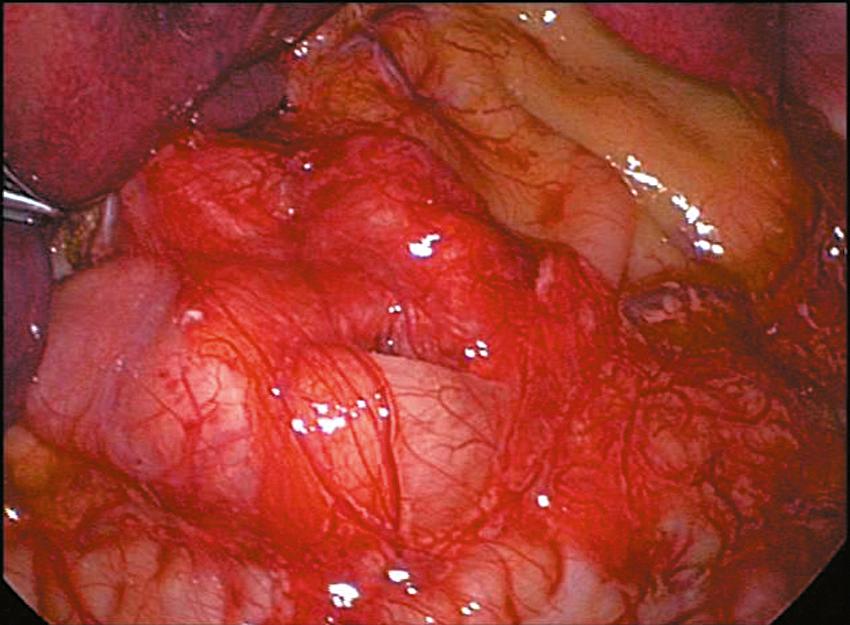
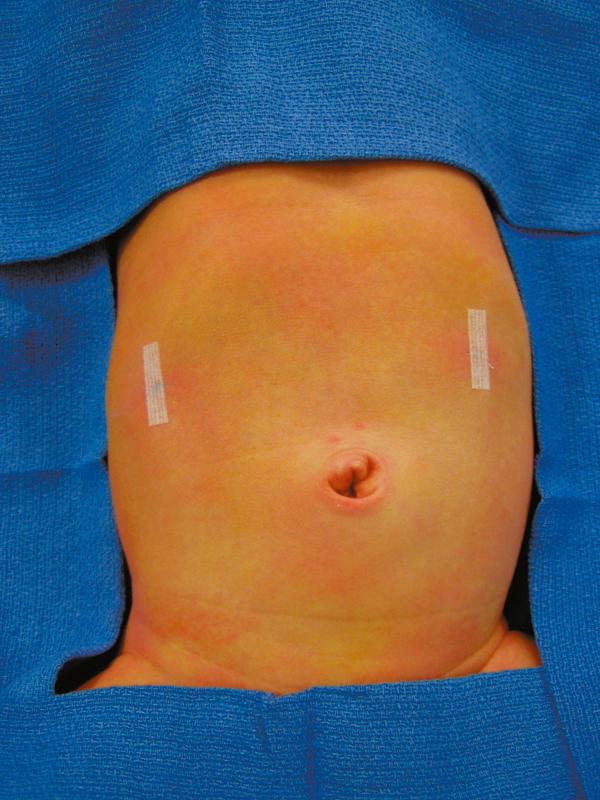
Once the myotomy is completed, the stomach is inflated with air ( Fig. 5-6 ). If not already placed, the anesthetist inserts a 14 Fr suction catheter into the stomach with a stopcock. A 60-mL syringe is attached to the stopcock. The stomach is inflated under visualization while the duodenum is obstructed with the instrument in the surgeon’s left hand. The myotomy site is then carefully inspected to make sure that there is no evidence of an occult perforation. The air is then evacuated from the stomach and the catheter is removed. Omentum can be placed over the myotomy if desired ( Fig. 5-7 ). The instruments are then removed. The CO 2 is allowed to escape through the umbilical cannula, which is done to prevent “stove piping” of omentum out the abdominal stab wounds. The umbilical fascia is closed with 3-0 monofilament absorbable suture. The umbilical skin and epigastric skin incisions are closed with sutures, surgical glue, or Steri-Strips (3M Co., St. Paul, MN) ( Fig. 5-8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here