Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Despite the first successful surgical treatment for correctable biliary atresia (BA) being reported in 1928, very few patients with correctable BA survived long term. In 1959, Professor Kasai described his hepatic portoenterostomy procedure for the first time and ended a long, hopeless era for patients with BA. Unfortunately, Kasai’s original portoenterostomy (KOPE) technique was published in Japanese and received little attention until it was published in English in 1968. However, the original KOPE was soon modified in an attempt achieve better rates of jaundice clearance (JC) and survival with the native liver (SNL), to the point that the current Kasai portoenterostomy (KP) involves an extended lateral dissection with a very wide anastomosis (extended portoenterostomy [EPE]) that hardly resembles Kasai’s original procedure. Nevertheless, this modified technique is still probably the most widely used open technique for treating BA.
The first laparoscopic KP procedure was described in 2002. Since then, few other studies have been reported, probably because of a combination of technical difficulty and negative research findings showing compromised liver perfusion during pneumoperitoneum causing liver cell damage, and elevated intra-abdominal pressure causing decreased proliferation and inducing apoptosis in hepatocytes in a rat model, as well as several reports of worse early clinical outcomes compared with the open KP.
The laparoscopic KP performed at Juntendo and described in this chapter is a modified version of KOPE, executed according to Kasai’s principles using minimally invasive surgery (MIS). In laparoscopic KOPE (LK), dissection of the porta hepatis is confined to the area around the base of the fibrous biliary remnant, which is transected shallowly to limit the extent of the transected surface and sutured shallowly around the outer edge of the biliary remnant to prevent injuring the microbile ducts at the 2- and 10- o’clock positions. These are KOPE principles. Interestingly, since embarking on our mission to incorporate the principles of KOPE rather than persevere with EPE, some centers have taken our lead and started applying our concepts when performing the open KP.
Signs suggestive of BA include jaundice, pale stools, dark urine, and hepatomegaly. Unfortunately, there is no single clinical feature with sufficient sensitivity and specificity to differentiate BA from other causes of neonatal jaundice. Thus neonatal jaundice that persists for more than 2 weeks should be investigated at a tertiary health care facility. Meconium staining may be normal, and feces may be yellow during the neonatal period in more than half of patients, but the urine gradually turns dark brown. Although the liver is diseased, the bilirubin is conjugated, and kernicterus is not usually a concern in BA. Early diagnosis is mandatory, and investigations should not be protracted because treatment will be delayed.
Many protocols have been suggested, but the diagnostic workup should always include ultrasonography, biochemical exclusion of α1-antitrypsin deficiency and cystic fibrosis, liver scintigraphy, and a percutaneous liver biopsy. The histological appearance of the liver is characterized by portal tract edema, bile duct plugging and proliferation, a small cell infiltrate, and variable amounts of giant cell formation. Bridging fibrosis is a late feature but in some cases may be present even at diagnosis. Using this protocol, about 80% of affected infants will have a positive diagnosis prior to operation. However, a liver biopsy can be traumatic and specimens require histopathologic assessment for accuracy, which can be time consuming.
While percutaneous liver biopsy is often performed, laparoscopic inspection provides the opportunity to diagnose liver cirrhosis and gallbladder atrophy. Diagnostic laparoscopy also allows cholangiography to be performed if the gallbladder is not atrophic to identify the intrahepatic bile ducts and common bile duct (CBD) to diagnose/exclude BA. Liver scintigraphy is typically abnormal in BA, with rapid uptake by hepatocytes and virtually no excretion into the bowel, even on delayed images. Laboratory investigations should include conventional liver function tests plus γ-glutamyl transpeptidase, TORCH (toxoplasmosis, other, rubella, cytomegalovirus, and herpes simplex) titers, and coagulation tests to exclude vitamin K–dependent coagulopathy.
All infant cases of BA should have parenteral vitamin K (phytomenadione, 1.0 mg/day) supplementation, usually given for several days prior to operation. The bowel should be prepared with oral kanamycin. Oral feeding is discontinued 24 to 48 hours before the operation and glycerin enemas are given. Broad-spectrum antibiotics are administered preoperatively. Our protocol is to administer antibiotics intravenously in the operating room and continue them postoperatively until the C-reactive protein (CRP) is less than 0.3 mg/dL or the leukocytosis has resolved.
The baby is positioned at the foot of the operating table, and the operating surgeon stands at the infant’s feet. An assistant/camera operator is situated slightly behind and to the left of the operating surgeon, and another assistant is located on the patient’s right to assist with exposing the porta hepatis and the anastomosis. The anesthetist is positioned near the baby’s head and the scrub nurse is situated to the left of the baby ( Fig. 22-1A ). The monitor is located above the infant’s head facing the operating surgeon. Vascular access, nasogastric tube insertion, and bladder catheterization are then performed.
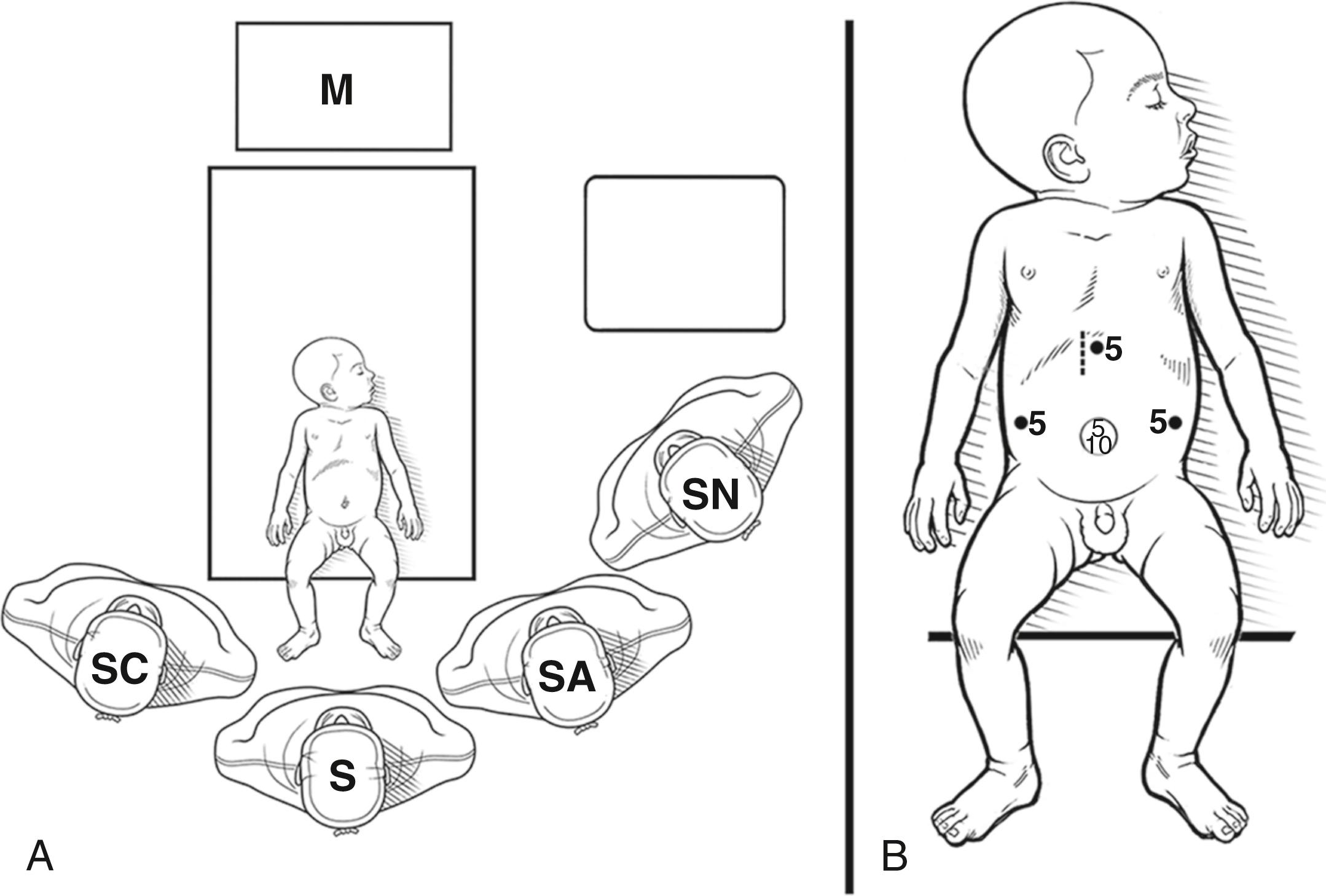
A flexible silicon wound retractor (LAP PROTECTOR) with a silicon cap (EZ Access; both Hakko Medical, Nagano, Japan) is inserted through a 20-mm umbilical incision ( Fig. 22-1B ). A 10-mm cannula, for a 30-degree 5- or 10-mm laparoscope, and a 5-mm cannula for retraction are placed via the EZ Access/LAP PROTECTOR. Three additional ports are then positioned under laparoscopic visualization. Two 5-mm cannulas are inserted on either side of the upper quadrant port for the surgeon’s right and left hands, slightly above the level of the umbilicus, and just lateral to the rectus abdominis. Another 5-mm port is introduced in the epigastrium for the Ligasure device (Valley Lab, Boulder, CO) or 3-mm Vessel Sealing System (Bolder Surgical Inc., Louisville, CO) (see Fig. 22-1B ). Cannulas are stabilized by fixing with sutures and rubber O-rings to prevent dislodgement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here