Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A rapid and accurate diagnosis of thrombotic thrombocytopenic purpura (TTP) is critical, as initiation of therapeutic plasma exchange (TPE) can be lifesaving. To date, the diagnosis of TTP is dependent on the demonstration of an otherwise unexplained microangiopathic hemolytic anemia and thrombocytopenia, which may or may not have concurrent end organ damage, such as neurologic symptoms or renal dysfunction. Laboratory findings consistent with a hemolytic anemia include an elevated lactate dehydrogenase, increased reticulocyte count, and elevated indirect bilirubin. A microangiopathic cause of the hemolytic anemia is supported by the presence of schistocytes on peripheral blood smear (see Chapter 107 ). This chapter will review both the assays for ADAMTS13 activity and the assessment of inhibitory antibodies against the ADAMTS13 enzyme.
As early symptoms and laboratory findings can also be found in thrombotic microangiopathies, such as disseminated intravascular coagulopathy, a specific and sensitive test for the diagnosis of TTP is desirable. With the discovery of ADAMTS13 and its association with TTP, laboratory assessment of ADAMTS13 enzyme activity has been an attractive test to fill this role. Unfortunately, no assay is sensitive or specific enough to allow reliable diagnostic use; however, there is evidence that ADAMTS13 activity and inhibitor titer have prognostic value.
Different preanalytical variables are crucial for the quality of ADAMTS13 testing. First, as treatment alters ADAMTS13 levels, diagnostic samples should be collected before starting therapy. Second, EDTA plasma is discouraged as EDTA chelates the metal ions of the metalloproteinase that are necessary for ADAMTS13 function. Third, plasma samples are preferred because thrombin degrades ADAMTS13. Fourth, although there is some degree of plasma-free hemoglobin during the initial diagnosis, hemolyzed plasma induced in vitro (collection and preparation) with a hemoglobin concentration >2 g/L should be avoided because free hemoglobin inhibits ADAMTS13. Fifth, plasma should be frozen if it cannot be tested within 4 hours of collection and multiple freeze-thaw cycles should be avoided.
A variety of tests of ADAMTS13 activity have been developed using the natural substrate, VWF. Such assays require two steps. The first step incubates test plasma obtained from the patient with purified VWF that has been treated to cause partial unfolding. The second step determines the ADAMTS13 activity in the patient’s plasma by measuring proteolysis of VWF. This has been accomplished through direct measurement of the VWF protein itself (multimers or proteolytic fragments) or via indirect measurement of the VWF activity (glycoprotein Ib-binding, ristocetin cofactor activity, or collagen binding). Many of these assays are time-consuming and cumbersome to perform. However, a semiautomated method for indirect measurement has been developed and shows promise, with an interassay coefficient of variation of 14% and an intraassay coefficient of variation of 9%.
The cloning of ADAMTS13 and detailed mapping of its recognition sites on VWF has facilitated the development of modified ADAMTS13 substrates, leading to rapid and reproducible assays.
The most widely used assays are based on ADAMTS13 substrate, vWF73, which contains the 73 amino acid residues that are the minimum amino acid sequence required for ADAMTS13 cleavage of VWF between Y1605 and M1606 in the A2 domain ( Fig. 157.1 ). Using the vWF73 substrate eliminates the need to treat purified plasma or recombinant VWF to cause unfolding. To facilitate detection of ADAMTS13 cleavage of vWF73, fluorescence resonance energy transfer (FRET) is utilized. One side of the peptide has a fluorescent molecule, whereas the other has a quenching molecule; thus, cleavage of the peptide dequenches the fluorochrome, resulting in a positive signal. The fluorescence signal is detected every 5 minutes for 1 hour and, as more substrate is cleaved, the fluorescence increases. The change in fluorescence over time (reaction rate) is calculated by linear regression analysis. Pooled normal plasma is used as a reference. ADAMTS13 activity is expressed as a percentage of the reaction rate found in the test plasma compared with the reaction rate found in pooled normal plasma.
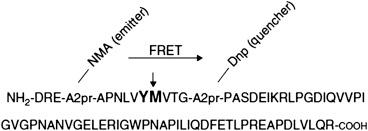
The assay reproducibility is excellent (coefficient of variation 6%). The FRET-vWF73 assay is now considered superior to other assays because it is a one-step assay that can be completed in 1 hour with excellent precision. When directly compared with other assays, there is good correlation with reported correlation coefficients ranging from 0.898 to 0.971.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here