Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Injuries to the anterior cruciate ligament (ACL) in skeletally immature athletes were traditionally thought to be rare. Historically, the incidence of ACL rupture in this population was reported as 1% to 3.4%. Avulsion of the tibial spine was reported to be more common than ACL rupture. However, as awareness of these injuries has increased, and with improvements in diagnostic modalities, especially MRI, ACL rupture is increasingly recognized in skeletally immature athletes who present with a knee injury. Injuries to the ACL have been reported in children as young as 4 years of age. In patients presenting with a hemarthrosis, the incidence of ACL rupture has been reported to be between 26% and 65%. It is now believed that the rate of ACL rupture in patients with open physes may exceed the rate of tibial spine fractures. Whether a young athlete sustains an ACL rupture or a tibial spine fracture may depend on the loading rate at the time of injury and the morphology of the intercondylar notch.
Skeletally immature athletes who have sustained an ACL rupture present very similarly to more mature athletes with similar injuries. As stated previously, the rate of ACL injury in a patient presenting with a hemarthrosis approaches 70%. Patients often state that during a pivoting or cutting activity they heard a “pop,” felt immediate pain and swelling in the knee, and were unable to return to full activity. As with other populations, the skeletally immature athlete may sustain an ACL rupture after a contact injury, but noncontact injuries are more common. After such an injury, athletes may describe continued instability with cutting or pivoting activities or can often have symptoms even with activities of daily living.
On examination, skeletally immature athletes should be assessed for the presence of an effusion, range of motion (ROM), a neurovascular examination, and standard knee ligament and hip and ankle assessments. Special attention should be directed to the Lachman, anterior drawer, and varus/valgus laxity tests both on the affected and contralateral knees because skeletally immature athletes can often have increased global laxity, and asymmetry in their examinations is an important diagnostic criterion. Patients with a suspected ACL rupture should also be closely examined for pathologic meniscal findings, with an assessment of joint line tenderness, flexion pinch, and McMurray tests. An assessment of patellar instability is also crucial in this population of young athletes who present with a painful effusion, because this diagnosis can often mimic an ACL injury.
Especially crucial to the examination of a skeletally immature patient with a potential ACL rupture is an assessment of physical maturity. Several methods may be used to assess physical maturity in adolescents. Tanner and Whitehouse correlated physiologic development signs with height, weight, height velocity, and weight velocity. Tanner staging is the most common means of assessing physical maturity on physical examination. Although many pediatricians are familiar and comfortable with this staging assessment, many sports medicine physicians may be unfamiliar or uncomfortable with this process. Self-Tanner staging, intraoperative Tanner staging, and growth relative to family members can be used to supplement the physical examination.
Standard sports-oriented radiographs of the knee should be obtained for skeletally immature athletes presenting with a suspected ACL injury. These radiographs include a standing anteroposterior (AP), standing notch or tunnel view, and lateral and sunrise views to assess for associated injuries such as osteochondritis dissecans (OCD; seen best on the notch view), patellar subluxation (seen best on the Merchant view), tibial spine fracture (seen best on the lateral view), and physeal injuries. In cases in which surgical intervention is likely, a mechanical axis radiograph for limb length and a standardized posteroanterior radiograph of the left hand is recommended to determine bone age using the Greulich and Pyle atlas. Further mention of bone age in this chapter refers to this technique. A simplified version of determining bone age has been recently developed. Often, contralateral knee views are necessary to better assess any asymmetry in the physes if a distal femoral or proximal tibial physeal injury is suspected. A Segond fracture or lateral capsular avulsion adjacent to the proximal tibia can be seen and is highly correlated with an ACL injury. This likely represents an avulsion of the anterolateral complex of the knee. The anterolateral complex/anterolateral ligament (ALL) is currently a hotly debated topic in ACL reconstruction at this time, and further study is needed to determine its importance, especially in the pediatric and adolescent patient with an ACL tear, because recent studies have shown variable anatomy in pediatric knees.
MRI has increasingly become the modality of choice for imaging the ACL. Direct evidence of ACL injury on MRI includes discontinuity of ACL fibers in any three imaging planes and the “empty notch sign,” in which fluid signal instead of ACL signal is seen at the proximal attachment site; this finding is best observed on T2-weighted images. In the acute setting, the injured or ruptured ACL can appear as an edematous mass with increased T2-weighted signal and abnormal morphologic features. In the subacute setting, it can appear discontinuous and less edematous with a more linear fragmented appearance, or it may even be completely absent in the chronic setting. In some cases a nearly normal ACL can be seen on the sagittal images when the ACL has scarred down to the posterior cruciate ligament (PCL). The axial and coronal images can be helpful, especially in evaluating the femoral footprint. The proximal ACL avulsion that scars to the PCL can be a point of confusion at the time of arthroscopy because the distal portion of the ACL can appear normal and can also create a pseudo–end point on examination.
Indirect evidence on MRI of an ACL injury can include a hemarthrosis and a pivot shift bone contusion, which is usually seen on the lateral femoral condyle and posterolateral tibial plateau. In addition, evidence of a deepened sulcus sign may be seen with an irregular-appearing lateral femoral condyle sulcus with a depth greater than 2 mm. Other indirect evidence of ACL injury on MRI includes a visible Segond fracture, the anterior drawer sign (i.e., increased anterior tibial translation), or buckling of the PCL, which may be nonspecific.
The decision to proceed with operative treatment of the ACL-deficient knee in a skeletally immature athlete is challenging and currently evolving for athletes, parents, and providers. The rationale for operative treatment of ACL injuries in skeletally immature patients is based on studies documenting the increased risk of meniscal damage, chondral damage, chronic instability, and inability to return to sports in these athletes. These risks must be balanced against the potential complications associated with ACL reconstruction in this population, which include growth arrest with resulting angular deformity or leg length discrepancy.
The risk of growth disturbance has been examined in numerous animals, with use of a transphyseal reconstruction technique across open physes. Guzzanti et al. first demonstrated this risk in a rabbit model. Houle et al. and Edwards and Grana also showed significant risk of deformity with soft tissue grafts placed under tension across both canine and rabbit physes. Stadelmaier et al. showed no evidence of physeal arrest when tension was not applied to soft tissue grafts.
Several clinical studies have reported growth disturbances associated with ACL reconstruction in the skeletally immature athlete. Risk factors associated with growth disturbances and angular deformities include transphyseal hardware fixation or bone plugs and lateral extra-articular tenodesis. A meta-analysis reported a 1.8% overall risk of growth disturbance in skeletally immature athletes undergoing ACL reconstruction.
ACL injuries in all athletes can have serious short- and long-term consequences, especially in the youngest populations. Strategies to prevent these injuries have been developed and are becoming increasingly refined and important both on an individual and public health level. These programs focus on education, stretching, proprioceptive training, plyometrics, sports-specific agility, jump and landing training, and progressive resistance weight training for the lower extremities. Sports medicine providers may want to consider implementing these preventive training programs, especially for high school athletes. A recent meta-analysis reporting on 24 studies and more than 1000 patients found that these programs are efficacious at preventing both knee injuries and specifically ACL tears. It is still unclear if pre–high school–aged patient populations are able to comply and take advantage of the benefits of these training programs.
Traditional recommendations for the treatment of the ACL-deficient knee in the skeletally immature athlete included delayed surgical treatment until skeletal maturity with interim functional bracing, physical therapy, and activity modification. Certain circumstances may dictate conservative treatment or a delay in surgical intervention, such as personal or medical reasons that would increase the risk of surgery or in the setting of an incomplete ACL tear without a secondary injury.
Treatment of partial ACL tears in the skeletally immature athlete entails decision-making principles similar to those used in athletes with complete ACL tears. If the clinical examination and the patient's symptoms are consistent with instability with activities and the inability to return to sport, or the patient has associated injuries such as meniscal tears or chondral damage indicative of an ACL-deficient knee or recurrent effusions are present, a partial ACL tear should be considered functionally incompetent. If the young athlete's symptoms and examination findings are not indicative of instability, then conservative treatment with functional bracing, physical therapy, and activity modification could be considered.
Milewski et al. have described an algorithm for deciding the operative surgical technique in skeletally immature athletes ( Figs. 137.1 and 137.2 ). After appropriate decision-making and conservative treatment has been considered, skeletal age is determined by bone age using radiographs of the hand.
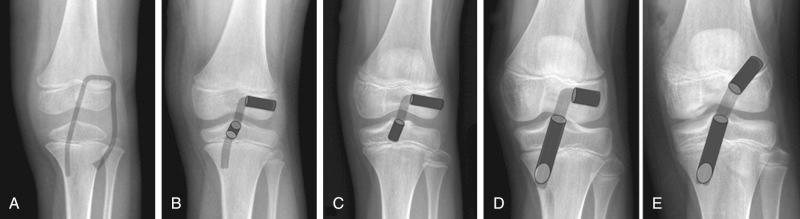
For prepubescent adolescents (i.e., those with a skeletal age <7 years), the physeal-sparing combined intra-articular and extra-articular reconstruction technique with an iliotibial band described by Kocher et al. is recommended. Older children with a small lateral femoral condyle may also be appropriate candidates for this technique.
For athletes with a skeletal age of 7 to 12 years, an all-epiphyseal reconstruction as described by Lawrence et al. is advocated. Ganley has recommended a modification to this technique, and the technique is described by Anderson for use in adolescents who have less than 20 mm of epiphyseal length. In these patients, cortical button fixation is used on both the femur and tibia for an all-epiphyseal tunnel procedure with a hamstring autograft.
For 13-year-old female athletes and 13- to 14-year-old male athletes, transphyseal reconstruction with a quadrupled hamstring autograft is recommended. This procedure can be modified using the hybrid anatomic technique with an all-epiphyseal femoral tunnel and a transphyseal tibial tunnel if a significant amount of growth is believed to remain and if the surgeon prefers to use the newer anatomic ACL techniques of centering the tunnels within the anatomic footprint.
After operative treatment of an ACL-deficient knee is elected, it must be decided whether to use a physeal-sparing or physeal-respecting technique or a traditional technique for ACL reconstruction. Physeal-sparing techniques for ACL reconstruction were initially described with use of patellar tendon grafts without drill holes by DeLee and Curtis and using hamstring tendon grafts by Brief and Parker et al.
Kocher et al. have described and advocated use of a physeal-sparing combined intra-articular and extra-articular reconstruction with an autogenous iliotibial band in prepubescent children who are at Tanner stage 1 or 2. This reconstruction technique has no bone tunnels and incorporates a lateral extra-articular iliotibial band reconstruction to help control rotation as described by Losee et al. ( Fig. 137.3 ).
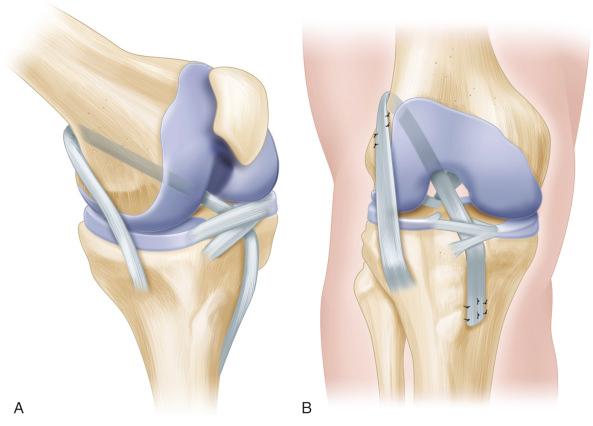
Anderson, as well as Guzzanti et al., have described physeal-sparing hamstring graft reconstruction techniques. Both techniques require extraosseous tensioning of the graft across the tibial physis. Anderson's reconstruction technique uses a hamstring graft tensioned across epiphyseal tunnels in the femur and tibia with the graft extending out of the tibial tunnel and across the tibial physis and attached with a screw and post construct in the tibial metaphysis.
Lawrence and colleagues at Children's Hospital of Philadelphia have recently described an all-epiphyseal ACL technique using femoral and tibial epiphyseal tunnels confirmed with intraoperative computed tomography (CT). Use of an intraoperative CT scan or fluoroscopy is necessary to avoid the physes (see Fig. 137.1C ).
More traditional transphyseal reconstruction techniques have also been used in skeletally immature athletes. These techniques become more appropriate as the patient approaches skeletal maturity. Aronowitz et al. and Kocher et al. have shown that Tanner stage 3 athletes have little risk of angular deformity or leg length discrepancy. Shea et al. used MRI of children's knees to show that drill holes could ideally affect less than 5% of the total volume of both the tibial and femoral physes and was central in the tibia but more peripheral in the femur, which was hypothesized to increase the risk of growth arrest.
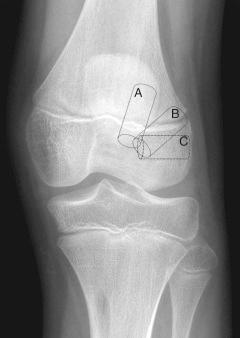
Investigators in many older series relied on traditional transtibial tunnel drilling for transphyseal reconstructions. The more vertical tunnels used in these techniques may minimize physeal damage by producing a hole of a smaller aperture in the physis. However, the vertical graft position may not fully restore the normal kinematics of the knee and may be less “anatomic” by centering less graft in the anatomic ACL footprint. Independent femoral drilling techniques such as those using an accessory medial portal, a two-incision technique, or a retrograde drilling outside-in technique have been advocated to place the femoral tunnel within the anatomic footprint. In skeletally immature athletes with open physes, Nelson and Miller have shown that the femoral tunnel produced by these drilling techniques crosses the lateral femoral physis obliquely and eccentrically and would potentially damage a much larger area of physis and perichondral ring than a more vertical, less anatomic tunnel. Given the increased risk of growth disturbance with these newer anatomic reconstruction techniques, an all-epiphyseal femoral tunnel combined with a traditional transphyseal tibial tunnel hybrid technique may be used ( Fig. 137.4 ). Recent anatomic studies have described anatomic and radiograph techniques for safer all epiphyseal femoral tunnel drilling. Specifically, radiographic landmarks have been obtained to guide avoidance of the lateral collateral ligament complex on the lateral aspect of the femur using a lateral radiograph of the knee ( Fig. 137.5 ).
Given the increased incidence of secondary injuries and less optimal outcomes with conservative or delayed reconstructive procedures in skeletally immature athletes, we attempt to schedule ACL reconstruction within the first 3–5 weeks after the injury.
Elimination of effusion and regaining full ROM are preferred.
Inability to gain full, painless ROM often denotes displaced meniscal fragments. In this case, earlier intervention is appropriate.
Patients should be encouraged to use crutches until ambulation without a limp is achieved.
Crutch use after ambulation without a limp is preferred to prevent meniscal injury.
Our graft choice is uniformly hamstring autografts in these primary ACL reconstructions.
Grafts smaller than 8 mm have been shown to have an increased rate of failure and should be measured after pretensioning ; it is still controversial as to the minimum graft diameter in a skeletally immature population. Augmentation with allograft was popular but may have an increased failure rate. Consideration for contralateral harvest or alternative graft sources (quadriceps vs. contralateral hamstring augmentation) can be considered.
The determination of which physeal-respecting approach we use depends on the skeletal age of the patient and relative remaining growth as determined by a posteroanterior radiograph of the left hand.
Our approach for prepubescent athletes is to perform an all-epiphyseal reconstruction with fixation performed with cortical button fixation. Combined intra-articular and extra-articular reconstruction that spares the physes can also be used in this population, especially if the patient's skeleton is very immature or the epiphysis is insufficient for adequate tunnel lengths.
Use of fluoroscopy or an intraoperative CT scan is essential to ensure that femoral and tibial tunnels appropriately respect the femoral and tibial physes.
Setting up the C-arm or intraoperative CT scanner before beginning the operation is helpful so that “real time” drilling with guidance can be performed.
The proximal tibial epiphyseal tunnel should generally be at least 20 mm to attempt an all-epiphyseal ACL reconstruction to ensure an adequate length of graft in the tunnel for fixation and incorporation.
Tibial and femoral drilling can be performed with a retrograde drill such as the FlipCutter, (Arthrex, Naples, FL) using fluoroscopic guidance.
Placing a small-diameter Kirschner wire prior to drilling with the retrograde drill is useful to ensure that tunnel placement successfully avoids the physes. These drill systems are not cannulated, and thus the guidewire is removed prior to drilling.
Fixation can be performed with interference screws as initially described or with loop fixation for both the femoral and tibial portions.
In pubescent patients, we perform a hybrid fixation with epiphyseal fixation on the femur and transphyseal drilling on the tibia and fixation distal to the tibial physis and apophysis.
Fluoroscopy is again key to successfully avoiding the femoral physis. It can also be used to measure the distance from the tibial aperture to the tibial physis for appropriate tibial screw length.
Tibial tunnel placement should be made as vertical as possible to minimize the aperture created in the physis.
In late pubescent patients, fixation is performed with loop fixation on the femur and expansion devices on the tibia.
Transphyseal femoral tunnels, when used, should attempt to cross the femoral physis as centrally as possible without jeopardizing the femoral tunnel footprint to lessen the possibility of differential physeal growth.
Postoperative rehabilitation after ACL reconstruction in the skeletally immature athlete is similar to the approaches used in more mature athletes. It is generally recommended that postoperative rehabilitation be divided into phases with specific criteria for progression. Emphasis on attainment of these goals rather than a precise postoperative time frame allows adaptations to be made as needed for the skeletally immature athlete.
The focus of the first phase of rehabilitation is to decrease postoperative impairments while protecting the patient and the surgical intervention. The value of postoperative bracing has been debated in the literature. Some benefits may include less pain, fewer complications due to postoperative effusion, and prevention of knee flexion contractions during the early phases of rehabilitation; however, no long-term benefits have been demonstrated with regard to ROM, joint laxity, patient-reported outcomes, or functional outcomes. During this phase, immediate ROM with an emphasis on knee extension is recommended to decrease pain, minimize patellofemoral complications, and reduce arthrofibrosis. A review by Beynnon et al. suggests that immediate weight bearing after ACL reconstruction is not detrimental to the graft and may decrease anterior knee pain. Goals for this phase are to decrease pain and joint effusion, obtain full knee extension and 90 degrees of knee flexion, and restore patella mobility and volitional quadriceps activation. Attainment of these goals indicates the athlete is ready to progress to the next phase of rehabilitation.
The goals of the second postoperative phase are to eliminate pain and effusion, progress toward full knee flexion, restore normal ambulation, improve proprioception, and progress strengthening of quadriceps and hamstrings. To obtain significant improvements in knee strength and overall function, strengthening exercises should be performed both in the open and closed kinetic chain position. Multiple studies have supported that open kinetic chain knee extension exercises initiated early on in the rehabilitation program increase strength and minimize patellofemoral joint stress while allowing for protection of the graft when performed isotonically between the range of 90 and 40 degrees of knee flexion and isometrically at 90, 60, or 0 degrees of knee flexion.
Closed kinetic chain strengthening exercises should be implemented as dictated by weight-bearing status, pain level, and available ROM. Closed kinetic chain exercises not only assist in strengthening but are functional and emphasize cocontraction and neuromuscular control. Early on in the rehabilitation program, knee flexion ROM should be limited to between 0 and 60 degrees during closed chain strengthening, to decrease stress placed on the ACL graft and patellofemoral joint. After postoperative week 9 the knee flexion ROM for both open and closed chain strengthening can progress due to increased tensile strength of the graft.
Postoperative rehabilitation should incorporate strengthening and neuromuscular control to allow the patient to initiate running and plyometric training during the third phase. Emphasis should be placed on strengthening and neuromuscular training of the hip and core of athletes after ACL reconstruction. Impairments in transverse plane hip motions, frontal plane knee motions, and trunk displacement have been attributed to increased risk of ACL injury, especially in female athletes. Injury prevention training with a focus on strengthening and lower extremity alignment has demonstrated improvements in hip and knee kinematics in female athletes.
Return to play (RTP) is not based on one specific criterion but instead on a constellation of criteria including ROM, proprioception, functional strength, and knee/graft stability. In the adult population, it is recommended that athletes return to sport when hop tests and strength of hamstrings and quadriceps are 85% of the noninvolved limb and when the calculated hamstring to quadriceps ratio is less than 15% compared with the noninvolved limb. Passing specific functional return to sport criteria decreases the risk of graft rupture after return to sport. In general, most skeletally immature athletes are able to return to sports approximately 6 to 12 months after undergoing their reconstruction. Although there is no convincing evidence to support the use of functional bracing for return to sport following ACL reconstruction, some authors have advocated for the benefit of bracing after combined extra-articular and intra-articular iliotibial extraphyseal reconstruction has been performed.
The most serious complications of ACL injuries and reconstructions in skeletally immature patients are those associated with growth disturbances. Findings of animal studies and anecdotal reports of human growth disturbances demonstrate that these concerns are valid. The possibility of growth disturbance, including the possibility of angular deformity, leg length discrepancy, and overgrowth, needs to be fully disclosed and discussed with patients and their families. The appropriate approach then needs to chosen given the patient's skeletal age, and long-term follow-up is necessary to watch for the earliest signs of any growth disturbance, including use of long leg mechanical axis radiographs until skeletal maturity is achieved.
The increased incidence of ACL injuries in skeletally immature athletes has increased the need to look deeper into the issues surrounding this devastating injury. The most important questions that need to be studied and answered are the long-term outcome with regard to function and arthritic conditions of the different techniques.
The incidence of meniscal tears in skeletally immature athletes has been increasing, likely because of improved awareness by sports medicine providers, increased youth sports participation, and increased use of advanced imaging such as MRI. Identifying and treating these meniscal injuries in young athletes is crucial to maintaining normal joint mechanics and preserving and protecting articular cartilage. Preserving as much of the meniscus as possible is crucial, especially in the adolescent athlete, because of the long-term consequences of aggressive débridement.
The meniscus forms by 8 weeks of embryologic development ( Fig. 137.6 ). By week 14 it has a normal anatomic appearance. The entire meniscus is vascularized during the fetal period, but this vascularization gradually recedes by age 9 months, with the central third already avascular at this point in development. By 10 years of age, the meniscus has developed its adult structure, with only the peripheral 10% to 30% of the medial meniscus and only the peripheral 10% to 25% of the lateral meniscus receiving a direct vascular supply.
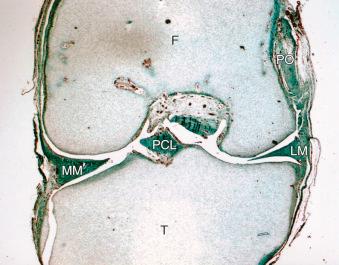
The meniscus consists of type I collagen fibers that are arranged in a circumferential pattern parallel to its long axis with radial, oblique, and vertical fibers that reduce hoop stresses. Inferior meniscal surfaces are flat, whereas the superior surfaces are concave. This shape allows for conformity of the femoral condyles with the tibial plateau surfaces. The meniscus is thus able to increase contact area and congruency, thereby reducing contact stresses and aiding in shock absorption. The medial meniscus is C -shaped; it covers approximately 50% of the medial tibial plateau and attaches to the medial joint capsule by the meniscotibial and coronary ligaments. It is able to translate approximately 2.5 mm posteriorly with femoral rollback during knee flexion. The lateral meniscus is circular, covering approximately 70% of the lateral tibial plateau. There are no attachments to the capsule at the popliteal hiatus, and the lateral meniscus translates 9 to 11 mm with knee flexion. The ligaments of Humphrey and Wrisberg are accessory meniscofemoral ligaments that are present in up to 84% of people.
In adolescents, nondiscoid meniscus injuries are usually a result of trauma during sports and typically involve a twisting injury or directional change. Pain is usually the chief complaint, but effusion, giving way, and mechanical symptoms such as snapping, catching, and locking also may be present. In one study, meniscal tears were found in 47% of preadolescents (aged 7 to 12 years) and 45% of adolescents (aged 13 to 18 years) with an acute traumatic knee hemathrosis. In both age groups the authors found that the medial meniscus was most commonly injured (70% in preadolescents and 88% in adolescents). A possible ACL tear should always be suspected in this population because 36% of the adolescents in this series with meniscal injuries also had an ACL tear.
On examination, effusion is usually indicative of intra-articular pathologic conditions in this age group. Skeletally immature athletes with meniscal injuries often have joint line tenderness on palpation. It is sometimes difficult to examine children with a painful knee, and they might not tolerate full ROM. One option is to use a modified McMurray maneuver, which involves pain with rotational varus or valgus stress at 30 to 40 degrees of knee flexion. It is always important to check ACL integrity with a Lachman test and compare it with the contralateral side because a high correlation exists with meniscus disease. The Lachman test is a reliable indicator of ACL injury. KT-1000 measurements were found to be increased in younger patients when measuring anterior tibial translation. When physical examination was performed by experienced examiners, Kocher et al. found that it was 62% sensitive and 81% specific for medial meniscus tears and 50% sensitive and 89% specific for lateral meniscus tears.
Initial workup should include standard AP, lateral, tunnel, and sunrise radiographic views. These views are helpful in detecting patellar dislocation, osteochondral fractures, physeal fractures, and loose bodies. The tunnel view is included to evaluate for OCD. The sunrise view may show patellar subluxation.
When the history and physical examination are suggestive of a meniscal tear and radiographs are normal, an MRI is usually ordered. Compared with adults, MRI in children has lower sensitivity and specificity in assessing meniscus tears because of the higher vascularity of a child's meniscus, which appears as intrameniscal enhancement, resembling meniscal tears. Children younger than 12 years were found to have 62% sensitivity and 78% specificity compared with 90% sensitivity and 96% specificity in children between the ages of 12 and 16 years. When evaluating an MRI for a meniscal tear, one should look for the enhancement to extend to the superior or inferior articular surfaces of the meniscus.
The basic principle in the treatment of meniscal injuries in children and adolescents is to preserve as much meniscal tissue as possible to minimize subsequent articular cartilage degeneration. The developing meniscus has an increased potential for healing because of its increased vascularity. When the history, physical examination, and imaging are consistent with meniscal injury and appropriate conservative options have been tried or considered, then surgery can be recommended in the skeletally immature patient.
After a meniscal injury is confirmed, initial management with conservative treatment including rest, activity modification, physical therapy for strengthening, and possible bracing can be considered. This approach is usually recommended for patients without mechanical symptoms or with imaging findings that are inconclusive or indeterminate. If the patient remains asymptomatic, then initial nonsurgical management can be continued.
Symptomatic meniscal tears in children and adolescents that have failed to respond to conservative treatment are usually treated surgically. Longitudinal tears in the red-red zone that measure 10 mm or less and are manually displaceable less than 3 mm may heal without repair. Arthroscopic partial meniscectomy is most commonly performed but does produce increased contact stresses. Cadaver studies showed a 65% increase in contact stress when removing small bucket-handle medial meniscus tears. Débridement of the posterior horn of the medial meniscus can increase contact stresses almost to the level of a total meniscectomy, which is 235% of normal. Authors of one study with a 5.5-year follow-up in 20 patients, with an average age of 15 years old, who underwent partial or total meniscectomy found that 75% were still symptomatic, 80% had radiographic evidence of osteoarthritis, and 60% were dissatisfied with their results. At 10- to 20-year follow-up, 50% of patients who underwent a total meniscectomy had evidence of osteoarthritis based on radiographic evidence, symptoms, and functional loss. Current indications for a partial meniscectomy in the skeletally immature athlete include irreparable meniscal tears such as radial tears, horizontal cleavage tears, and a complex degenerative tear in the white-white zone. It is preferred that as limited a partial meniscectomy as possible be performed for a damaged, unstable meniscus. A total meniscectomy is contraindicated.
Many meniscus tears in skeletally immature patients can be repaired. The types of tears most amenable to repair include longitudinal tears in the red-red zone or red-white zone, bucket-handle tears without significant injury to the bucket-handle fragment, and lateral meniscus posterior horn tears, which have a good vascular supply. All techniques first require débridement of loose degenerative edges and rasping of the perimeniscal synovial edges or trephination into the peripheral zone to promote vascular inflow to the repair site. The inside-out vertical mattress suture technique is still considered the “gold standard.” It uses absorbable or nonabsorbable suture with flexible needles that are placed through the meniscus in vertical or horizontal fashion and are tied down over the capsule through a separate incision. When repairing posterior horn tears, a posterolateral or posteromedial approach must be made to protect the posterior neurovascular structures with retractors. An outside-in technique is often helpful for isolated anterior horn tears or anterior portions of bucket-handle tears. If needed, open repairs can be performed through either posterolateral or posteromedial incisions for peripheral tears and are useful for posterior horn medial meniscal tears in patients with tight medial compartments. All-inside techniques have gained popularity again. Previously, these techniques included use of devices such as darts, arrows, and screws to anchor the meniscal tear to the capsule or peripheral meniscus. Unfortunately, they did not have the ability to compress across the tear site. A recent meta-analysis suggests a higher failure rate of all-inside devices compared with traditional inside-out repairs in the setting of ACL reconstruction. Newer repair systems are suture based and provide the ability to compress at the tear site. More contemporary data will be needed to compare these newer generation devices with inside out repairs at mid to long term.
Postoperative management after meniscal surgery in skeletally immature patients is similar to management in adults, with the understanding that both the young athlete and his or her family must be thoroughly informed of the expectations for activity restriction and rehabilitation after surgery. If bracing, weight-bearing restrictions, or specific rehabilitation protocols will need to be followed postoperatively, improved compliance will be achieved if both the patient and family are counseled appropriately before surgery.
Postoperative protocols are typically separated into meniscal repair and transplantation or meniscectomy. It is essential that the physical therapist be informed of the type of surgical intervention performed because variations in postoperative protocols are made according to the type, location, and size of the meniscal injury. Concomitant procedures such as ligamentous reconstructions or comorbidities such as articular cartilage damage also affect the potential for rehabilitation and the plan of care.
Following partial meniscectomies, most surgeons allow immediate weight bearing as tolerated with the use of axillary crutches, due to the postoperative sequelae of effusion and quadriceps inhibition. Rehabilitation after a meniscectomy can progress as tolerated with the initial goals of eliminating pain and effusion, attaining knee ROM of 0 to 90 degrees with good patellar mobility, ambulating without an antalgic gait, and regaining volitional neuromuscular control of the lower extremity. Full and painless knee ROM should be obtained approximately 6 weeks after the operation. Ongoing rehabilitation should emphasize strengthening of the lower extremities while progressing toward return to the previous level of function by the third postoperative month.
Depending upon the surgical technique and location of isolated meniscal repairs, many surgeons restrict weight bearing for 4 to 8 weeks and restrict ROM using a hinged knee brace for 4 to 6 weeks to minimize joint compressive and shear forces. The initial phases of rehabilitation emphasize decreasing pain and swelling, improving ROM, and regaining volitional quadriceps activation while protecting the surgical repair. Therapeutic exercises and neuromuscular training should progress in a manner consistent with postoperative ROM and weight-bearing guidelines. The range of knee excursion for open and closed kinetic chain exercises will be restricted to minimize stress to the meniscal repair and the patellofemoral joint.
Return to sport after isolated meniscal repair is generally allowed 4 to 6 months after the operation if the young athlete has regained full ROM and adequate strength and remains asymptomatic during functional activities. Regardless of the type of surgical intervention, patients should be monitored for pain in the tibiofemoral compartment, painful clicking at the knee, lack of progress with ROM, decreased patellar mobility, or persistent joint effusion.
The results of partial or total meniscectomies in children and adolescents are poor, with early onset of osteoarthritis. Although increased success rates are associated with meniscal repairs in younger patients, few long-term outcome studies have been performed regarding meniscal repair in this age group. In one series of 29 arthroscopic meniscal repairs in 26 patients with a mean age of 15.3 years, no meniscal symptoms were noted at an average of 5 years of follow-up, and 24 of 26 patients returned to their preinjury level of sports activity. Of note, 15 of 26 patients had a simultaneous ACL reconstruction, all the tears were in the posterior horn of either the medial or lateral meniscus, and 22 of the 29 tears were in the red-red zone. The mean time between injury and surgery was 6.7 months. Twenty-five repairs were performed with the inside-out technique, and four were performed with an all-inside technique. In another series of 71 meniscal repairs in adolescents with a mean age of 16 years, clinical healing was seen in 75% at a mean 51-month follow-up. All tears extended into the avascular white-white zone and were repaired with use of an inside-out technique. In patients undergoing a simultaneous ACL reconstruction, the success rate was even higher at 87%. Given the increased healing rate of meniscal tears in the setting of ACL reconstruction, many advocate for marrow stimulation techniques in the setting of an isolated meniscal repair to augment healing. Failure rates in patients with marrow stimulation to augment their isolated meniscal repairs are similar to those undergoing concomitant ACL reconstruction.
Complications from arthroscopic partial or total meniscectomy or meniscal repair are rare but may include painful neuroma, arthrofibrosis, complex regional pain syndrome, and iatrogenic chondral injury from the surgery or from a protruding implant.
Although short-term and midterm results are encouraging for meniscal repair, especially in the setting of simultaneous ACL reconstruction, long-term results are needed to determine if newer techniques for meniscal preservation are successful in decreasing the risk of osteoarthritis in the future for these skeletally immature athletes.
The discoid meniscus was first described by Young in 1887 after cadaveric dissection. It is almost exclusively found in the lateral meniscus and, in some studies, has been shown to have a higher prevalence of approximately 15% in Asian populations; however, it has a prevalence of only 3% to 5% in the US population. Approximately 20% of patients with a discoid meniscus have bilateral discoid menisci. The true prevalence of discoid menisci is unknown because many discoid menisci are asymptomatic.
Discoid menisci generally occupy greater than normal coverage of the lateral tibial plateau and are uniformly thickened. They can represent a spectrum of meniscal morphologic features and stability. Discoid menisci are thought to be a congenital anomaly because menisci are not discoid shaped during development. Increased meniscal thickness and width may also be due to compensatory changes for an unstable meniscus during development.
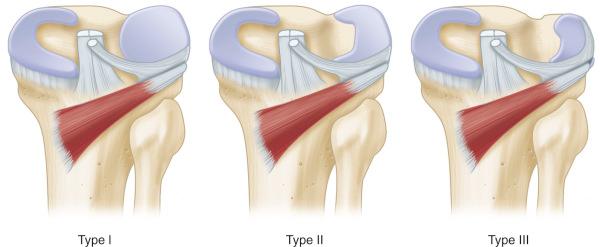
The Watanabe classification system is most commonly used ( Fig. 137.7 ). This classification system describes three types of discoid meniscus based on arthroscopic appearance and stability of the meniscus. Type I (stable, complete) menisci are stable to probing and complete; the meniscus is block shaped and covers the entire lateral tibial plateau. Type II (stable, incomplete) menisci cover 80% or less of the tibial plateau. Type III menisci, also known as the Wrisberg variant (unstable), have a thickened posterior horn but otherwise appear normal. They lack posterior meniscal attachments with the exception of the meniscofemoral ligament of Wrisberg, which causes the lateral meniscus to have posterior horn hypermobility. When the knee goes into extension, the posterior horn can be pulled into the intercondylar notch and can result in snapping knee syndrome. Klingele et al. examined peripheral rim instability patterns of 128 discoid menisci. They found that 62.1% were complete and 37.9% were incomplete. Peripheral rim instability was found in 28.1%, of which 47.2% occurred in the anterior horn, 38.9% in the posterior horn, and 11.1% in the middle third. Peripheral instability was more common in younger patients, with a mean age of 8.2 years, and in patients with complete discoid lateral menisci.
The clinical presentation of a discoid meniscus may vary. Stable discoid menisci usually are first noticed in older children who present with mechanical knee symptoms similar to those of a meniscal tear. These stable menisci are prone to tearing because of increased thickness and abnormal vascularity. Compared with normal menisci, discoid menisci have also been shown on transmission electron microscopy to have a decreased number of collagen fibers with a more disorganized pattern. Discoid menisci without an associated tear are often asymptomatic and are often found incidentally on MRI or during knee arthroscopy.
Children with unstable discoid menisci often present with intermittent snapping and popping within the knee. This phenomenon is called “snapping knee syndrome” and usually occurs when the knee is brought from flexion to extension; it may cause pain and apprehension. Unstable discoid menisci are usually found in younger patients with a Wrisberg type I discoid-appearing morphology, in which the meniscus is a solid block covering the entire tibial plateau.
Physical examination findings can include joint effusion, limited motion, terminal extension discomfort, a lateral joint line bulge, joint line tenderness to palpation, and pain or popping during the McMurray test. This popping during the McMurray test is a result of subluxation of the unstable lateral meniscus. Very often a lack of terminal extension mimicking a knee flexion contracture is present in children with discoid menisci. It is always important to examine and assess the symptom history of the contralateral knee as well because of the high rate of bilateral discoid menisci.
Standard knee radiographs are normal in most children with discoid menisci, but some radiographs occasionally indicate subtle findings, such as squaring of the lateral femoral condyle, a widened lateral joint line, cupping of the lateral tibial plateau, and mild hypoplasia of the tibial spine.
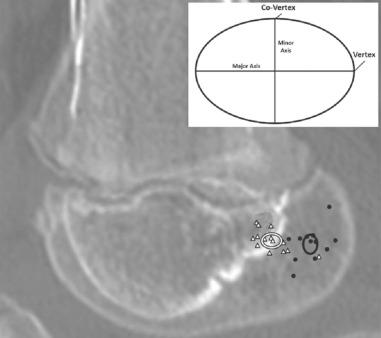
MRI is the radiographic study of choice for diagnosing a discoid meniscus. The diagnosis is made if the transverse meniscal diameter is greater than 15 mm or 20% of the tibial width on coronal views, or if continuity of the anterior and posterior horns of the lateral meniscus is seen on three or more consecutive sagittal images of 5-mm thickness. Types II (incomplete) and III (Wrisberg variant) discoid menisci may appear normal on MRI. However, type III discoid menisci may appear to have some minimal posterior horn anterior subluxation or have a high signal on T2-weighted images between the lateral meniscus and joint capsule, resembling a peripheral tear appearance. MRI has been shown to have low sensitivity (38.9%) in diagnosing a discoid lateral meniscus in children compared with an 88.9% sensitivity of physical examination.
If a discoid meniscus is asymptomatic and found incidentally, it should be treated with observation. The knee is functioning well and may have adapted to this abnormal anatomy. Surgery is indicated in patients with symptomatic discoid menisci for whom conservative management has failed, similar to the management of normal meniscal tears. This surgical indication includes discoid menisci with or without an associated tear as defined on MRI.
After it has been decided that a symptomatic discoid meniscus will be managed arthroscopically, the first issue to address is the size. This issue is addressed with a partial meniscectomy, also called saucerization . Saucerization is performed to create a stable and functional remaining meniscus that will provide adequate shock absorption. A minimum of 6 to 8 mm of the peripheral rim is left intact. Larger peripheral rims have been shown to have higher repeat tear rates. This remnant peripheral rim may be thicker than a normal meniscus and may require an undersurface débridement to recreate a more normal structure.
If peripheral instability occurs after saucerization, meniscal repair to the capsule is performed. Meniscal tears after saucerization are repaired in the same fashion as a nondiscoid meniscus tear. Remaining tears that cannot be repaired undergo débridement to a stable rim. If a horizontal cleavage tear remains, the unstable leaflet is usually débrided.
A total meniscectomy is rarely performed in children and adolescents; it is generally reserved for only the most difficult of unsalvageable cases.
Postoperative management is similar to that of other pathologic meniscal conditions. Following saucerization alone, immediate weight bearing and ROM are allowed as tolerated. It is crucial to work on extension exercises immediately because many of these patients have long-standing terminal extension limitations from their chronic discoid meniscus. Patients who undergo saucerization and meniscal repair are placed in a hinged knee brace with restricted ROM between 0 and 90 degrees and limited weight bearing for 6 weeks. Return to sports is generally allowed approximately 4 months after the surgical procedure is performed.
A total meniscectomy was traditionally used for the treatment of symptomatic discoid menisci, and although this approach is no longer recommended, the long-term results have been reported with mixed findings. Habata et al. and Okazaki et al. found excellent functional scores with minimal radiographic changes after a total meniscectomy at 14- to 16-year follow-up. It has been suggested that young patients adapt to increased articular cartilage stress after meniscectomy. Authors of another study examined 17 knees in children with a mean age of 9 years who were treated with a total meniscectomy. They found that 10 knees had clinical symptoms and radiographic changes of lateral compartment arthritis at 19.8-year follow-up. Two of the knees had lateral femoral condyle OCD lesions. Authors of another study compared long-term clinical and radiographic outcomes in a series of 125 complete and incomplete discoid menisci managed with partial or total meniscectomy. They found better radiographic results at 5-year follow-up with partial meniscectomy. The long-term prognosis was based on the volume of removed meniscus.
Saucerization has become more accepted as standard treatment for discoid menisci. Short-term and midterm results of this treatment have been reported. In a retrospective review, authors looked at 11 knees in children with a mean age of 11.5 years who were treated with arthroscopic saucerization for their discoid menisci. At 4.5-year follow-up, all patients had good to excellent clinical results with no radiographic degenerative changes. Authors of another study looked at 27 consecutive discoid menisci in children with a mean age of 10.1 years who were treated with saucerization and repair. Prior to repair, they found that 77% of the discoid menisci were unstable, with anterior horn instability being most common. At 3.1-year follow-up, 21 patients had excellent clinical results and 3 patients reported residual knee pain. In patients undergoing saucerization, smaller meniscal width (<5 mm) and the amount of meniscal extrusion postoperatively are correlated with degenerative change at a mean 39 months postoperatively. Although midterm results are encouraging, long-term results to demonstrate whether saucerization can prevent the development of clinical and radiographic osteoarthritis are still lacking.
Patients who have undergone a total meniscectomy may be candidates for meniscal transplantation. In a cadaveric study of nondiscoid knees, a total lateral meniscectomy was found to decrease the total contact area by 45% to 50% and increase peak local contact pressure by 235% to 335%. Even though the discoid knee has some anatomic and biomechanical differences compared with the nondiscoid knee, these changes are quite significant and should be considered when contemplating meniscal transplantation after a total meniscectomy. A meniscal transplantation may increase the total contact area and decrease the peak local contact pressure. In a study of 14 patients who underwent meniscal allograft transplantation after a previous total meniscectomy for a torn discoid lateral meniscus, Lysholm knee scores improved from 71.4 to 91.4 at a mean 4.8-year follow-up. Only one allograft tear was observed in six second-look arthroscopies. Similarly, in 36 patients aged 12 to 16, meniscal transplantation improved patients’ subjective knee symptoms and had only a 6% reoperation rate for meniscal intervention, with no transplant failures. These midterm results are encouraging.
Complications related to arthroscopic treatment of discoid menisci are similar to those of normal menisci. Perhaps most concerning are the risks of late arthritis, especially in patients with total or subtotal meniscectomies. A higher rate of retearing of the abnormal discoid meniscus tissue may be expected compared with normal menisci. In addition, an association of lateral femoral condyle OCD lesions has been reported with discoid menisci after meniscectomy.
Although it is not a complication, sports medicine providers should be aware of the high rate of bilateral discoid menisci. Patel et al. recently reported on children with symptomatic bilateral discoid menisci compared with children who had bilateral discoid menisci, only one of which was symptomatic. They found that patients younger than 12 years and those with complete or Wrisberg-type menisci were more likely to have symptoms and require surgical treatment for their contralateral discoid menisci.
The increasing understanding of the pathogenesis of the discoid meniscus has led to debate regarding the treatment of stable asymptomatic discoid menisci. Traditionally these menisci are managed with observation; however, prophylactic débridement may possibly prevent symptoms, instability, or future tears. In addition, long-term results are needed to address the risks of arthritis in the lateral compartment when counseling patients who have undergone meniscal saucerization with or without repair.
Patellar instability affects approximately 43 of 100,000 children. It has been generally accepted that it is easier to return to sport after a patellar instability episode compared with other knee ligamentous injuries or instability in other joints such as the glenohumeral joint. However, Atkin et al. demonstrated that only 66% of athletes were able to return to sport after patellar instability and 50% had some degree of limitation at 6-month follow-up. The rate of redislocation is high in pediatric and adolescent populations. Buchner et al. showed that patients younger than 15 years had a 52% redislocation rate versus a 25% rate for the entire cohort. Cash and Hughston found a 60% redislocation rate in younger adolescents aged 11 to 14 years versus a 33% rate for older adolescents aged 15 to 18 years. Authors of one study found a higher dislocation rate in women, but subsequent meta-analysis has shown no real sex predilection. A family history of patellar instability has been shown to be a major risk factor for repeat dislocation in patients with a previous dislocation. In a recent long-term follow-up study, patients with patellar instability had a 50% incidence of patella femoral arthritis at 25 years follow-up, which was worse in patients with recurrent dislocations and chondral injuries. This suggests that prevention of recurrent instability may be important for the long-term health of the joint.
The patella develops during the seventh week of gestation but does not begin to ossify until age 4 to 6 years. The patellar facets and trochlear sulcus are well formed in neonates but become more evident as the cartilage thins with age on both the patella and trochlea. The cartilaginous sulcus has been shown to measure between 134 and 155 degrees and remain consistent through development, but the osseous sulcus angle is inversely proportional to age ( Fig. 137.8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here