Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors are most grateful to previous edition contributors to this chapter, including Paul S. Thornton, whose leadership and input on the chapter’s themes and data (particularly within the tables) were invaluable.
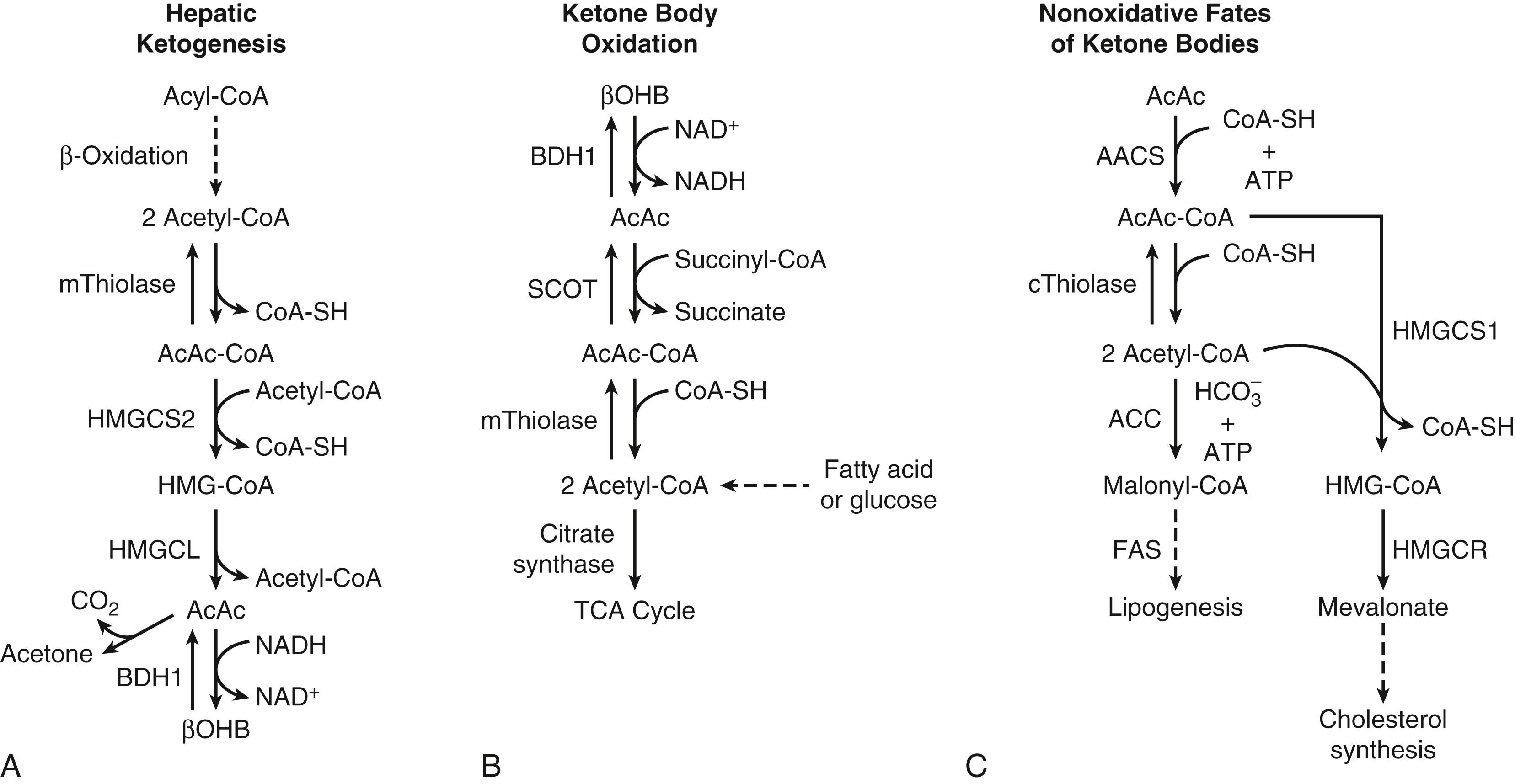
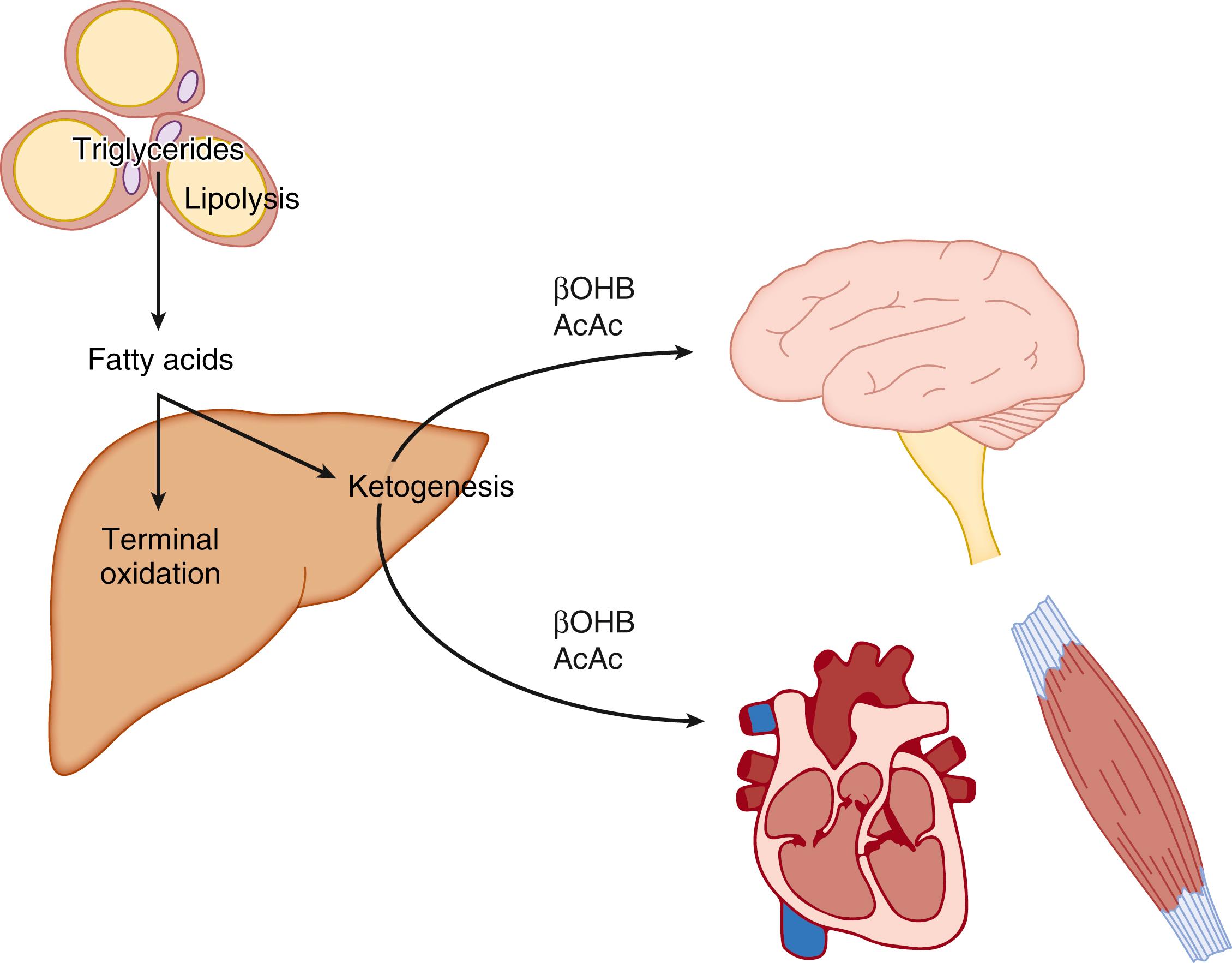
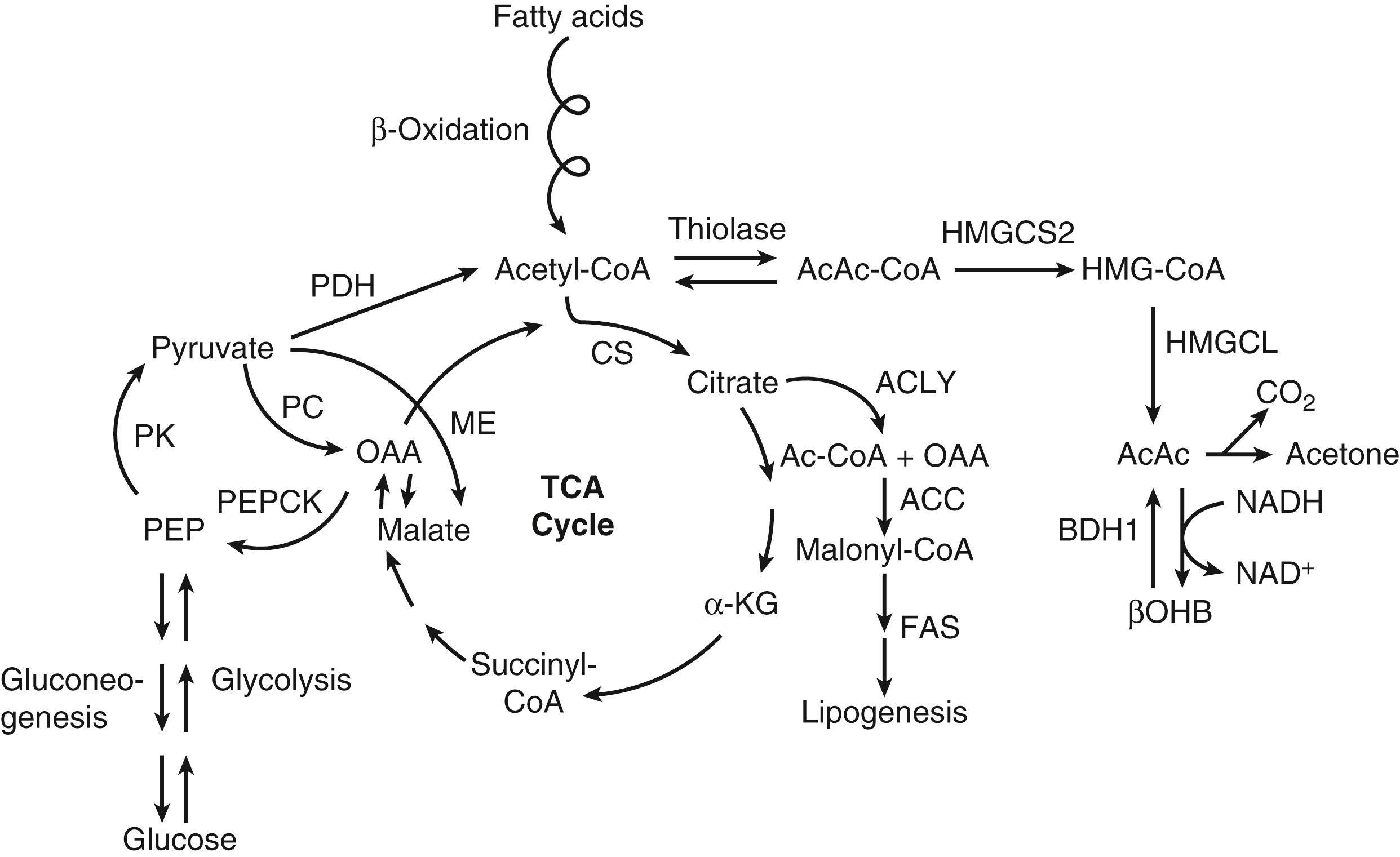
The metabolism of ketone bodies is evolutionarily conserved among all the domains of life on Earth. In mammals, ketone bodies are predominantly synthesized in the liver from acetyl coenzyme A (CoA) derived from fatty acid oxidation and are then transported to peripheral tissues for oxidation during physiologic states consisting of limited carbohydrate and surplus fatty acid availability (reviewed by Robinson and Williamson, McGarry and Foster, and Cotter and colleagues ; Fig. 34.1A and B). During the neonatal period, starvation, and adherence to low-carbohydrate diets, ketone body oxidation contributes significantly to energy metabolism within numerous extrahepatic tissues ( Fig. 34.2 ). In these carbohydrate-limiting states, circulating ketone body concentrations can increase from approximately 50 μmol/L in a normal fed mature human to up to 7 mmol/L. In neonatal humans, or after a prolonged fast in healthy adults, circulating ketone body concentrations can rise to approximately 1 mmol/L. In certain pathologic states such as diabetic ketoacidosis, ketone body concentrations can reach as high as 20 mmol/L if the state is left untreated. , , Although ketone bodies often serve energetic roles, ketone body metabolism also provides substrates for de novo lipogenesis and sterol biosynthesis in many tissues, including the developing brain, lactating mammary gland, and liver (see Fig. 34.1C ). Within the mitochondria, hepatic ketogenesis converges with the fundamental metabolic pathways of fatty acid β-oxidation, the tricarboxylic acid (TCA) cycle, and gluconeogenesis ( Fig. 34.3 ). In various disease states, including infantile ketoacidosis and type 1 diabetes, dysfunctional ketone body metabolism is observed and may even play a role in pathogenesis. Ketone body metabolism also shifts over the course of normal development and aging. The dynamic role of ketone body metabolism in the neonatal period will be the primary focus of this chapter.
Under physiologic conditions, the rate of utilization of ketone bodies is directly proportional to their concentration in the circulation, which in turn represents a balance between production (ketogenesis) by the liver and disposal by peripheral tissues (ketolysis). Ketone bodies are excreted in the urine (ketonuria) when the renal reabsorption threshold is exceeded. The relationship between production and disposal can be disturbed if the utilization of ketone bodies is inhibited by drugs, , with congenital absence of key enzymes required for ketone body utilization, or in insulin-deficient states secondary to a metabolic defect in utilization.
The concentration of ketone bodies in the blood is extremely sensitive to alterations in the physiologic state. The balance of ketone body production and disposal determines the steady-state circulating concentration of ketone bodies, and although it is inappropriate to apply the definitions universally among all individuals, in general terms, in humans normoketonemia is characterized by a serum total ketone body concentration of less than 0.2 mmol/L, ketonemia is characterized by a serum total ketone body concentration of greater than 0.2 mmol/L, and ketoacidosis is characterized by a serum total ketone body concentration of greater than 7 mmol/L. In adult humans, small but characteristic diurnal changes in blood ketone body concentrations have been observed. Larger increases in concentration occur with fasting (in both humans and rats), with consumption of a high-fat diet, after exercise, in late pregnancy, and during suckling ( Table 34.1 ). In pregnancy, blood ketone bodies are available to the fetus in prevailing concentrations because the placenta appears to be freely permeable to the ketone bodies acetoacetate (AcAc) and β-hydroxybutyrate (βOHB). ,
| Clinical/Experimental Condition | Ketone Body Concentration (mmol/L) a | |
|---|---|---|
| Human | Rat | |
| Fed normal diet | ∼0.1 | ≤0.3 |
| Fed high-fat diet | ≤3 | 4–5 |
| Fasted: 12–24 h | ≤0.3 | 1–2 |
| Fasted: 48–72 h | 2–3 | 2–3 |
| After exercise | ≤2 | ≤2 |
| Late pregnancy | ≤1 | ≤0.3 |
| Late pregnancy after 48-h fast | 4–6 | 6–15 |
| Neonate: 0–1 day | 0.2–0.5 | 0.2 |
| Neonate: 5–10 days | 0.7–1.0 | 0.5–1.1 |
| Hypoglycemia | 1.5–5 | — |
| Untreated diabetes mellitus | ≤25 | ≤10 |
a Concentrations of total ketone bodies (acetoacetate plus 3-hydroxybutyrate measured by enzyme assay) in whole blood.
The major substrate of the mammalian fetus is glucose supplied by the mother. However, the concentration of ketone bodies in maternal blood is increased in the last trimester of pregnancy, , and high concentrations are attained during delivery, particularly if it is prolonged (ketosis of labor). , This increase in the concentration of blood ketone bodies may be related in part to the decrease in food intake around parturition because hyperketonemia develops more rapidly with fasting in women in the second trimester of pregnancy than in nonpregnant control subjects. , Poorly controlled diabetes during pregnancy (including gestational diabetes) leads to wide fluctuations in the concentrations of blood ketone bodies.
During the suckling period the neonate is presented with a relatively high-fat and low-carbohydrate diet. Both humans and rats have marked hyperketonemia in the early suckling period, compared with the respective adult fed values (see Table 34.1 ). By contrast, the concentrations of ketone bodies are not increased to the same extent in the blood of puppies, piglets, and lambs. Fatty acids (both long-chain fatty acids [LCFAs] and medium-chain fatty acids [MCFAs]) are the major precursors of ketone bodies, and one contributing reason for the species differences in neonatal ketonemia may be the fat content of the maternal milk (rat milk contains 14.8 g fat/100 g; sheep milk contains 5.3 g fat/100 g). Some mammalian species, including pigs, do not leverage ketogenesis as a high-capacity conduit for disposal of excess acetyl-CoA, instead using acetate to export acetyl-CoA carbons. ,
Neonatal hyperketonemia in humans is a physiologic event; thus any marked deviation in blood ketone body concentration likely indicates underlying disease. Increased concentrations of ketone bodies may accompany the hypoglycemia associated with inborn errors of metabolism, and a decrease may occur in hyperinsulinism. It is therefore advisable to measure blood ketone body concentrations by a specific enzymatic method in neonates presenting with hypoglycemia or any other abnormality in the concentration of circulating substrates. For example, infants born small for their gestational age have increased blood concentrations of gluconeogenic substrates, in association with decreased blood ketone body concentrations. , The latter is due to defective development of the ketogenic capacity of the liver and decreased availability of peripheral adipose tissue to supply the key ketogenic substrate nonesterified fatty acids (see later).
Insight into the regulation of ketogenesis has increased dramatically with the use of novel genomic, proteomic, and metabolomic analyses. Earlier investigations were based mainly on studies in rats—in particular, during the fed-to-starved transition, as well as from work on perfused livers or isolated hepatocytes from adult rats. Consequently, in the review of regulation of ketogenesis in the adult presented in this section, comparison is made with the neonate or fetus whenever information is available. Further details are available in more detailed reviews. , ,
Regulation of ketogenesis is both extrahepatic and intrahepatic (see Fig. 34.2 ). The major precursors of ketone bodies in the postabsorptive state are LCFAs, and in all physiologic situations associated with hyperketonemia, including those of the neonatal period, the plasma concentrations of LCFAs are increased (see Table 34.1 ). Extraction of LCFAs by the liver is concentration dependent, and a direct relationship between their concentration and the rate of ketogenesis exists in both rats and humans.
In the adult, a key factor in determining the supply of LCFAs to the liver is their rate of release (lipolysis) from adipose tissue triacylglycerol stores. Lipolysis is initiated by activation of adipose tissue lipases, adipose triglyceride lipase, and hormone-sensitive lipase. Glucagon, epinephrine, norepinephrine, and thyroxine increase enzyme activity, whereas insulin (as well as some prostaglandins) has the opposite effect. Isolated human adipocytes from neonates are very sensitive to the lipolytic effects of thyrotropin, which increases in concentration immediately after birth and may therefore be involved in the regulation of lipolysis in the perinatal period. A decrease in blood glucose concentration (e.g., in starvation, fat feeding) along with a concomitant decrease in plasma insulin concentration leads to an increase in lipolysis and efflux of nonesterified fatty acids from adipose tissue. Carbohydrate provision increases insulin concentrations and thereby inhibits lipolysis. The rate of ketogenesis can therefore respond in a reciprocal way to the availability of glucose in the circulation, so an alternative fuel for the brain is provided when needed (see Fig. 34.2 ). During suckling in the rat, the plasma insulin-to-glucagon ratio is decreased, favoring lipolysis. ,
Ketone bodies regulate their own formation by feedback mechanisms on adipose tissue to decrease lipolysis. Current evidence suggests there are two mechanisms: (1) direct inhibition of lipolysis by ketone bodies via binding to the G protein–coupled niacin receptor GPR109A and (2) an indirect effect mediated by stimulation of insulin secretion. , , Work with the perfused rat pancreas suggests that ketone bodies increase insulin secretion only at concentrations greater than 1 mmol/L. These feedback mechanisms have not been investigated in neonates.
In the suckling neonate, the MCFAs (i.e., 12 or fewer carbons) of maternal milk are an important source of precursors for ketogenesis. These are in the form of triacylglycerols (one MCFA and two LCFAs per triacylglycerol) and are hydrolyzed by the action of lingual lipase. They are rapidly absorbed from the stomach into the portal venous system and are therefore directly available to the liver, unlike LCFAs derived from milk lipids, which are transported as triacylglycerols (within chylomicrons) by way of the lymphatic system and initially enter the peripheral circulation. In the neonatal period, chylomicrons may provide a direct source of LCFAs for the liver, as suggested by the presence of lipoprotein lipase (responsible for the hydrolysis of triacylglycerols contained in chylomicrons to LCFAs) in neonatal rat liver. However, the total lipoprotein lipase activity in liver constitutes only a small percentage (3%) of that in the whole body, the rest being contributed by muscle and adipose tissue.
The liver is the primary tissue capable of synthesis and release of ketone bodies into the circulation, primarily due to the relatively hepatocyte-specific expression of the fate-committing ketogenic enzyme mitochondrial 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase 2 (HMGCS2). Ketone bodies can also be formed by the intestinal mucosa of neonatal rats via enterocyte-derived HMGCS2. , Expression of HMGCS2 in intestinal mucosa is suppressed in weaned suckling rats but is regulated by the gut microbiome. The rate of intestinal ketogenesis is less than 10% of that in liver of suckling animals, but this mechanism does provide an additional strategy for supplying ketone bodies to developing tissues.
LCFAs hydrolyzed from triacylglycerols in adipose tissue are transported in the plasma bound to albumin, cross the cell membrane as free fatty acids, and then bind again to cytosolic binding proteins. Within the liver, the LCFAs either can be reesterified to form triacylglycerols (for subsequent secretion as very-low-density lipoprotein) and phospholipids or can enter the mitochondria by way of the carnitine palmitoyltransferase (CPT) system to undergo β-oxidation. The resulting yield of acetyl-CoA can (1) be converted to the ketone bodies AcAc and βOHB via a series of four mitochondrial enzymatic reactions referred to as the HMG-CoA pathway (see Fig. 34.1A ), (2) enter the TCA cycle, where it can undergo terminal oxidation, or (3) pass out of mitochondria as citrate to be used as a cytosolic substrate for fatty acid or sterol biosynthesis (see Figs. 34.2 and 34.3 ). By contrast, MCFAs are not converted to triacylglycerols in mammalian liver and directly traverse the inner mitochondrial membrane, bypassing the CPT system. Within the mitochondrial matrix, the MCFAs are converted to the corresponding acyl-CoA derivatives by acyl-CoA synthetases and undergo β-oxidation. Ketone bodies efflux from hepatocytes via SLC16 members of the monocarboxylate transporter (MCT) family, enter the circulation, are transported into cells of extrahepatic tissues through MCT-dependent mechanisms, and are ultimately oxidized. Mechanisms of ketone body transport into and out of mitochondria have not been definitively established.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here