Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Iron is an essential trace element required for energy production, oxygen transport and utilization, and cellular proliferation. Iron is a catalyst, acting as an electron donor and an electron acceptor by readily interconverting between ferric (Fe 3+ ) and ferrous (Fe 2+ ) forms. Iron functions mainly in association with proteins, either within prosthetic groups (heme, iron-sulfur clusters) or bound directly by amino acid side chains. Important heme-containing proteins include the oxygen carrier hemoglobin, oxygen storage protein myoglobin, and redox enzymes called cytochromes. Iron-sulfur cluster proteins are prominently involved in mitochondrial energy metabolism. Proteins that directly coordinate iron include a family of prolyl hydroxylases that stabilize collagen and mediate oxygen sensing. The ease with which iron can gain and lose electrons also allows the pathological formation of highly reactive oxygen species that can damage lipids, proteins, and DNA and injure subcellular organelles, resulting in cellular dysfunction, apoptosis, and necrosis. Animals have evolved mechanisms that tightly control total body iron and the amount of iron within each cell to ensure adequate iron availability and avoid its toxicity.
Humans have no regulated means for iron excretion, and obligatory losses are normally minuscule, 1 to 2 mg/day or less than 0.05% of the total body iron each day. The amount of body iron is therefore determined by controlling iron absorption. Human iron homeostasis is dependent on the highly efficient recycling of iron ( Fig. 36.1 ).
Although all cells require iron, most of the iron in the body is found in the hemoglobin of erythroid cells, and most of the daily movement of iron (approximately 80%) cycles through the erythroid compartment. Iron contained in nonerythroid cells is also recycled. The normal losses of iron of about 1 to 2 mg/day are attributable to iron contained within desquamated epithelial cells from the skin and the intestinal tract. These losses are physiologically compensated by the absorption of similar amounts of dietary iron in the duodenum. In women who menstruate, the losses of iron can be higher, and compensation for them requires more iron absorption.
Iron bound to the plasma iron carrier protein transferrin is carried into the erythroid marrow and incorporated into hemoglobin, thereby entering the circulation within red blood cells (RBCs). At the end of their lifespan, normally about 120 days, RBCs are phagocytized by a select population of macrophages in the spleen, liver, and marrow. Macrophages degrade the RBCs, releasing their iron to plasma transferrin. Hepatocytes and some macrophages in the liver, spleen, and marrow function as a dynamic storage compartment for iron. After examining the intricate interrelationship between intracellular and systemic iron homeostasis, this chapter considers in turn each portion of the pathway of iron transport, use, storage, and absorption (see Fig. 36.1 ). Altogether, iron homeostasis is maintained by effective use of iron for erythropoiesis, efficient recycling of iron from senescent erythrocytes, hepcidin-controlled storage and release of iron by macrophages and hepatocytes, and fine regulation of intestinal iron absorption.
Each cell in the body regulates its iron uptake, content, and utilization. The transport protein transferrin functions as the physiologic carrier of iron in the plasma and extracellular fluid. Each cell captures its share of circulating transferrin-bound iron using transferrin receptor 1 , a glycoprotein on cell membranes that binds the transferrin-iron complex. The transferrin receptor with its cargo is internalized in an endocytic vesicle, where iron is released, and then the complex of the receptor and iron-free (apo)transferrin returns to the cell membrane, liberating apotransferrin into the plasma. Within the cell, the iron released from the endosome is either used for the synthesis of iron-containing biomolecules or stored in cytosolic ferritin , a protein that holds iron in a nontoxic form ready for mobilization when needed. The main determinant of iron uptake by each cell is the number of transferrin receptors on the cell surface. Within each cell, the intracellular availability of iron homeostatically regulates its cellular uptake and storage through the iron-regulatory proteins 1 and 2 (IRP1 and IRP2) whose levels are negatively regulated by available intracellular iron ( Fig. 36.2 ). When iron is available in the cytoplasm, IRP1 acquires an iron-sulfur cluster to become a cytoplasmic enzyme, aconitase, and loses its iron-regulatory function, while IRP2 is degraded by an iron-dependent process. When available iron is low, the IRP concentrations rise and IRPs bind to RNA stem−loop structures called iron-responsive elements (IREs). IREs within the 3′-untranslated region of a messenger (m) RNA (e.g., transferrin receptor 1 mRNA) bind IRPs that protect the bound mRNA from degradation, making more mRNA available for translation, thus increasing protein production. Under the same conditions, IRP binding to IREs located in the 5′-untranslated region of an mRNA (e.g., cytosolic ferritin mRNA) impedes protein translation. Accordingly, a decrease in intracellular iron availability enhances transferrin receptor 1 protein synthesis, increasing iron import, and reduces cytosolic ferritin and iron storage. Conversely, an increase in intracellular iron availability reduces transferrin receptor 1 protein synthesis, inhibiting iron import, and augments cytosolic ferritin protein production and iron storage. In iron-replete cells with sufficient oxygen, F box and leucine-rich repeat protein 5 (FBXL5), a subunit of a ubiquitin ligase complex, monitors cytosolic iron and initiates iron-dependent degradation of iron-regulatory protein 2. Another system that contributes to cellular iron homeostasis is the regulated release of ferritin iron stores via a process called ferritinophagy. Here, a cargo carrier protein NCOA4 (nuclear receptor coactivator 4) delivers ferritin to the lysosomal compartment for the breakdown and release of its iron content. When cellular iron levels are high, NCOA4 itself is targeted for iron-dependent degradation via the ubiquitin proteasome system, so that NCOA4 concentrations in the cytoplasm are low, decreasing ferritinophagy and increasing ferritin iron storage. Conversely, under low cellular iron conditions, NCOA4 is increased, enhancing ferritinophagy and releasing more iron from ferritin to restore cellular iron levels.
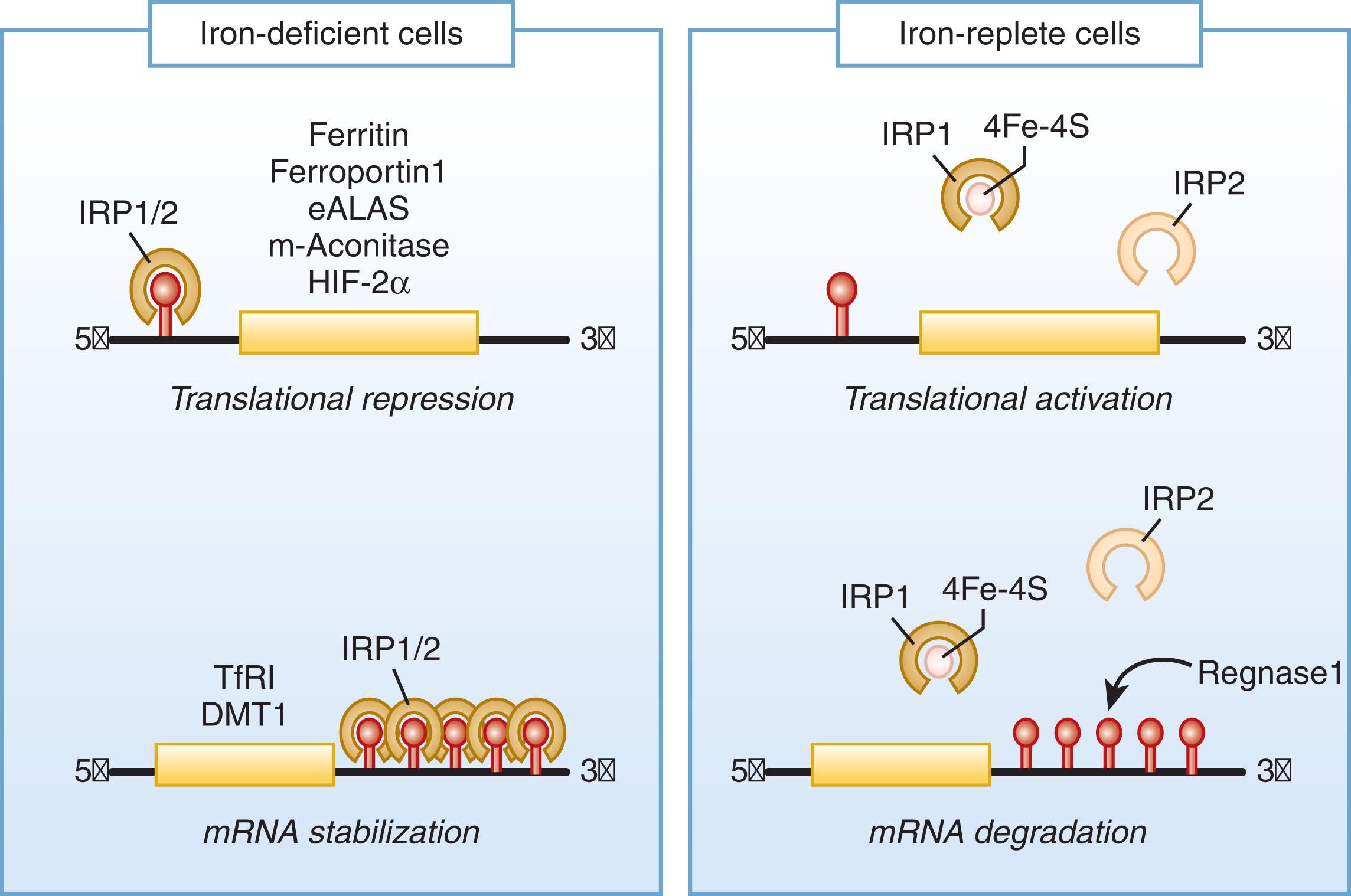
Altogether, regulation of intracellular iron homeostasis is mediated principally through iron-regulatory proteins 1 and 2 by their reciprocal control of the synthesis of transferrin receptor and ferritin and, in specialized cells, by controlling the synthesis of other essential proteins involved in iron homeostasis, including erythroid δ-aminolevulinic acid synthase 2 (eALAS), mitochondrial aconitase, hypoxia-inducible factor 2α (HIF-2α), intestinal divalent metal transporter 1 (DMT1) isoform I, and ferroportin. The NCOA4 system and other iron-dependent regulatory processes also contribute to cellular iron homeostasis.
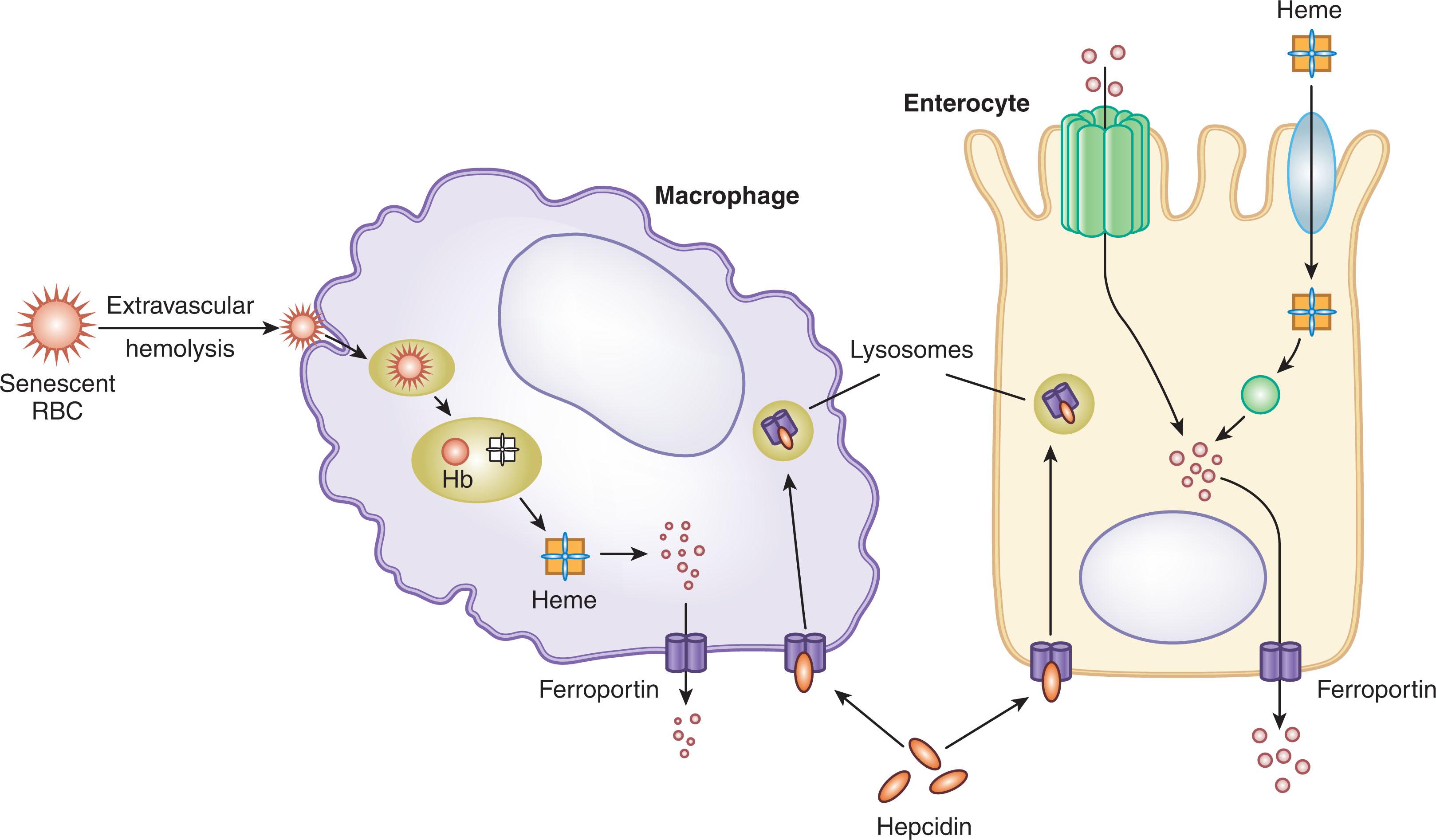
Regulation of organismal iron content and tissue distribution is accomplished by the control of the entry of iron into plasma for transport by transferrin. Circulating iron is delivered to transferrin by specialized cells that can export iron, primarily splenic and hepatic macrophages that recycle iron from senescent RBCs (“iron-recycling macrophages”), hepatocytes that can mobilize iron from stores, and duodenal enterocytes that provide iron absorbed from the diet. To enter plasma, iron in these cells must pass through ferroportin (SLC40A1, a member of the solute carrier family of transporters), a 12-transmembrane-segment protein that is the sole known cellular iron exporter. Hepcidin , a small 25-amino acid peptide hormone secreted principally by hepatocytes, posttranslationally controls ferroportin iron transport and ferroportin membrane concentration. Hepcidin acts by binding to and occluding ferroportin and inducing its internalization, ubiquitination, and degradation, thereby inhibiting iron export from duodenal enterocytes, iron-recycling macrophages, and iron-storing hepatocytes to plasma ( Fig. 36.3 ). Hepatic hepcidin synthesis is stimulated by increases in body iron stores, plasma iron concentration, infection, and inflammation and is inhibited by hypoxemia and increased erythropoietic demand. The amount of iron in plasma in adults at any given time is only about 2 to 4 mg, while the daily amount of iron required for erythropoiesis is about 20 to 25 mg, so the iron in plasma turns over approximately every 2 to 4 hours. Increments in plasma hepcidin reduce the export capacity of ferroportin and the amount of ferroportin in cell membranes, thereby decreasing iron delivery to plasma. Continuing iron consumption for erythropoiesis and other processes then causes a prompt fall in plasma iron concentration as the small amounts of iron in plasma are rapidly depleted. Conversely, decrements in plasma hepcidin concentration increase the amount of ferroportin, producing a rise in plasma iron concentration.
Less well understood is the role of microRNAs, short (approximately 22 nucleotides), noncoding RNAs that act as antisense regulators of target RNAs, providing a further degree of control of both cellular and systemic iron homeostasis.
The major traffic of iron in the body is to the erythroid marrow ( Fig. 36.4 ). Each day, almost 200 billion RBCs are produced in a normal adult to replace a similar number reaching the end of their lifespan, amounting to about 24 mL of packed RBC/day containing about 24 mg of iron. Erythroid precursors and most other iron-utilizing cells require iron complexed by transferrin. Apotransferrin, a form of transferrin not carrying iron, is a single-chain glycoprotein with two structurally similar lobes. The binding of a ferric (Fe 3+ ) ion to one of these lobes yields monoferric transferrin; binding of ions to both yields diferric transferrin. The transferrin saturation is the proportion of the available iron-binding sites on transferrin that are occupied by iron atoms, expressed as a percentage. In humans, almost all circulating plasma apotransferrin is synthesized by hepatocytes. Apotransferrin is not lost in delivering iron but is reused repeatedly as the half-life of the protein is about 8 days. After delivering iron to cells, apotransferrin is promptly returned to the plasma to function again as an iron transporter, completing 100 to 200 cycles of iron delivery during its lifetime in the circulation.
Transferrin receptors on the cell surface bind monoferric or diferric transferrin. Two different forms of the transferrin receptor exist, encoded by two separate genes. Transferrin receptor 1 is ubiquitously expressed and functions as the physiologic transferrin iron importer on all iron-requiring cells. Transferrin receptor 2 is expressed in hepatocytes, functioning in the control of iron supply by regulating hepcidin expression (see later), and in erythroid precursors, coordinating erythropoiesis with iron availability (see later). Transferrin receptor 1 is a transmembrane glycoprotein dimer composed of two identical subunits linked by a disulfide bond. Each transferrin receptor 1 can bind two molecules of transferrin; if each transferrin is diferric, the dimeric receptor can carry a total of four atoms of transferrin-bound iron. The affinity of transferrin receptor 1 for transferrin depends both on the iron content of transferrin and on the pH. Transferrin receptor 1 has a low affinity for apotransferrin; an intermediate affinity for monoferric transferrin; and the highest affinity for diferric transferrin, estimated at 2 × 10 −9 to 7 × 10 −9 M. Under physiologic conditions, the affinity of transferrin receptor 1 for diferric transferrin is more than fourfold greater than that for monoferric transferrin. At a pH of about 5 in the endosome, the affinity of transferrin receptor 1 for apotransferrin increases to that of diferric transferrin.
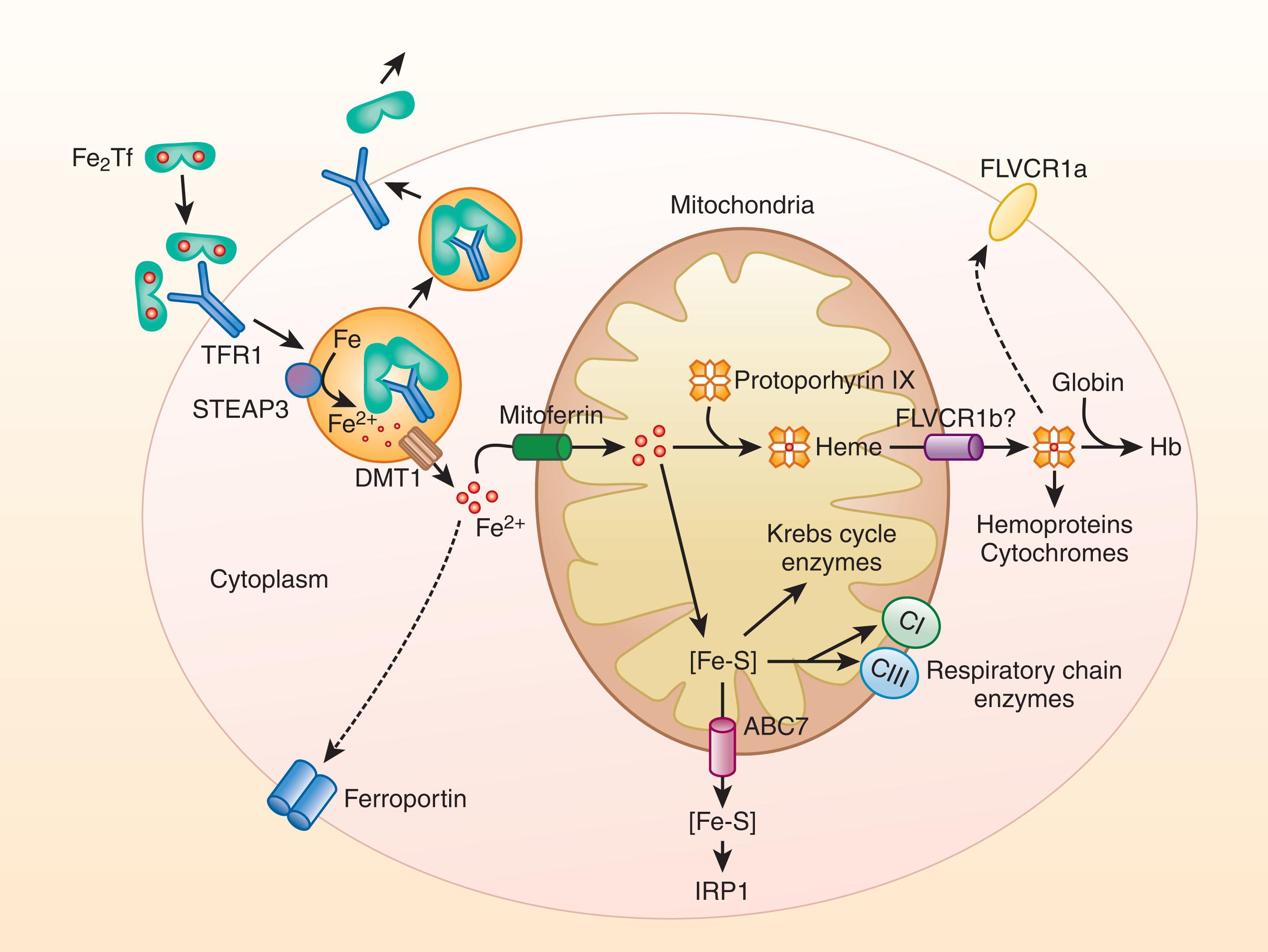
Iron delivery to an erythroid cell (see Fig. 36.4 ) begins with the binding of one or two molecules of monoferric or diferric transferrin to transferrin receptor 1. The efficiency of iron delivery to the cell depends on the amounts of monoferric and diferric plasma transferrin available, with the iron distributed between the two forms according to the principles of chemical equilibrium, which also govern the interaction of the two forms with transferrin receptor 1. During normal erythropoiesis and a normal transferrin saturation of about 33%, most of the iron supply to cells is derived from diferric transferrin, providing four atoms of iron with each cycle. At a transferrin saturation of about 19%, equal amounts of iron are provided by monoferric and diferric transferrin; at lower saturations, most of the iron is derived from the monoferric form. Whether monoferric or diferric, the fate of transferrin bound to the transferrin receptor is the same. When bound, the iron-bearing transferrin−receptor complex rapidly clusters with other transferrin−receptor complexes in a clathrin-coated pit that is promptly internalized. Within the cytoplasm, the coated vesicle is rapidly stripped of clathrin, and the uncoated vesicles fuse to become multivesicular endosomes. A proton pump then acidifies the endosome internal pH to less than 6. In the acidic environment of the endosome, both transferrin and transferrin receptor 1 undergo conformational changes that cause iron release. The released ferric iron is reduced by the ferrireductase six-transmembrane epithelial antigen of the prostate 3 (STEAP3) to the ferrous form and then transported across the endosomal membrane through DMT1 (SLC11A2). Low pH in the endosome increases the affinity of the now iron-free apotransferrin for the transferrin receptor so that the complex remains intact as it is transported back to the cell surface within the endosome. On exposure to the neutral pH of the plasma, the apotransferrin loses its affinity for the transferrin receptor and is released from the membrane, making both the apotransferrin and the transferrin receptor 1 available for reuse (see Fig. 36.4 ).
Most of the iron transported across the endosomal membrane through DMT1 is then directed to the mitochondria for use in the synthesis of heme and iron-sulfur clusters (see Fig. 36.4 ) with the rest destined for cytoplasmic proteins that coordinate iron through their amino acid side chains and for cytoplasmic ferritin that stores an inorganic iron complex in the cavity of a shell-like structure.
Iron can be imported from the cytosol across the mitochondrial membrane by the transmembrane protein mitoferrin 1 (MFRN1; SLC25A37). Transport of iron from endosomes into mitochondria for heme synthesis by direct contact between the organelles (“kiss and run”), avoiding the cytosol, also has been proposed. Heme (ferrous protoporphyrin IX), a planar molecule consisting of an atom of ferrous iron in the center of a tetrapyrrole ring, is then synthesized in eight biochemical reactions, with the first and final three reactions catalyzed by mitochondrial enzymes and the four intermediate reactions taking place in the cytoplasm (see Chapter 39 ). Most heme is then bound to α- or β-globin subunits that combine to form α–β dimers that in turn join to form the functional α 2 -β 2 -tetramer of hemoglobin (see Fig. 36.4 ). Small amounts of heme are incorporated into heme enzymes and cytochromes. Iron is also used for another class of prosthetic groups, iron-sulfur clusters, assembled within mitochondria and in the cytosol (see Fig. 36.4 ). The cytosolic iron chaperones poly(rC)-binding proteins 1 and 2 (PCBP1, PCBP2) may ferry surplus iron to multiple intracellular destinations: cytosolic ferritin for storage, to some cytosolic nonheme enzymes, and in specialized cell types for export into plasma to meet the iron needs of the organism. Transferrin receptor 2 (TfR2), which binds iron-loaded transferrin with an affinity some 25-fold less than that of transferrin receptor 1, functions as a sensor of iron bound to transferrin and is not involved in cellular iron uptake. In erythroid precursors, transferrin receptor 2 coordinates erythropoiesis with iron availability, a vital mechanism for adaptation to iron deficiency. Transferrin receptor 2, a component of the erythropoietin receptor complex, stabilizes the receptor on the cell surface and modulates the sensitivity of the developing erythroid cells to erythropoietin. By simultaneously sensing the concentration of iron-loaded transferrin in developing erythroid cells and in hepatocytes (see later), transferrin receptor 2 permits erythropoiesis to adapt to the level of the iron supply while modulating iron absorption and the release of iron from stores to meet the erythropoietic (and organismal) requirement for iron. Erythroid precursors have multiple other mechanisms to coordinate their iron usage with systemic iron availability, to conserve iron, and to share excess iron with the rest of the organism. These mechanisms likely evolved to avoid the adverse consequences of the erythropoietic system using more than its share of iron at the expense of the rest of the organism.
Reduction of responsiveness to erythropoietin: Iron regulation of erythroid differentiation helps match the rate of erythropoiesis to iron supply. With iron deficiency, an TfR2-dependent pathway also reduces the responsiveness of erythroid progenitors to erythropoietin. During iron deficiency, decreased erythroid use for RBC production helps preserve the supply of iron for vital functions in other tissues.
Inhibition of erythroid heme synthesis: Heme synthesis is coordinated with iron availability through an iron-regulatory element in the 5′-untranslated region of the mRNA for eALAS, the erythroid-specific initial enzyme in the heme synthetic pathway. If intracellular iron availability is low, binding of an iron-regulatory protein will inhibit heme synthesis by preventing translation of the mRNA.
Inhibition of globin and other protein translation: If the lack of iron leads to heme deficiency, the heme-regulated translational inhibitor (HRI) is activated and, acting through the α-subunit of eukaryotic initiation factor 2, slows most protein synthesis to coordinate the translation of globin mRNAs with the intracellular heme concentration. This action of the HRI is responsible for the physiologic adaptation that produces hypochromic, microcytic erythrocytes in iron deficiency. More recent data indicate that mTorc1 (mechanistic target of rapamycin complex 1), the master regulator of cell growth in proportion to available nutrients, coordinates with HRI to regulate the synthesis of cellular macromolecules and thereby determines the size of erythrocytes.
Export of iron through ferroportin: Developing erythroblasts synthesize ferroportin to export iron. Their expression of ferroportin is regulated principally by hepcidin, providing another means to coordinate erythroid iron use with systemic iron availability. In erythroid precursors (and in duodenal enterocytes; see later), two ferroportin transcripts are present: the ubiquitously expressed ferroportin (FPN1A), with an iron-responsive element in its 5′ untranslated region, and FPN1B, which lacks the iron-responsive element. During erythroid cell differentiation, FPN1B is therefore not subject to translational repression by the iron-regulatory protein system, thereby permitting the export of iron from erythroid precursor cells during the critical period when cells commit to proliferation and differentiation, express high levels of transferrin receptor 1, and rapidly accumulate iron. As a consequence, erythropoiesis may be partially suppressed when nonerythropoietic tissues are at risk for iron deficiency. Iron export from erythroblasts via FPN1B may account for the development of iron deficiency anemia as an initial, early manifestation of systemic iron deficiency. Nonetheless, when the cells begin to produce hemoglobin, FPN1B expression diminishes and FPN1A predominates, allowing erythroid cells to limit iron export through the iron-responsive element iron-regulatory protein system and to efficiently manufacture heme.
Export of excess heme: Erythroblasts have the capacity to export excess heme through the feline leukemia virus subgroup C cellular receptor and avoid heme toxicity.
Storage of excess iron: Cytosolic ferritin can be synthesized and utilized to deposit surplus iron in a safe form from which iron can be recovered when needed. Also, a mitochondrial ferritin, consisting of homopolymers of a nuclear gene-encoded H-type ferritin (see later), can be expressed to protect against mitochondrial iron accumulation in sideroblastic anemia and some other disorders.
Recovery of the nonfunctional iron content during terminal differentiation: Orthochromatic erythroblasts, with nuclei that are unable to synthesize DNA, gradually lose most mitochondria and halt RNA synthesis but continue to produce hemoglobin. The pyknotic nucleus is finally extruded through the erythroblast membrane with the loss of about 5% to 10% of the hemoglobin that had been synthesized previously, with the extruded material recycled by erythroblastic island macrophages. The resultant reticulocyte continues to synthesize hemoglobin for another 2 to 3 days until the cellular supply of mRNA is exhausted, producing as much as 30% of the total hemoglobin complement of the RBC. Eventually, the reticulocyte is released from the marrow, remodeled, and pitted of siderotic granules and debris within the spleen, and these excess materials are recycled by splenic macrophages.
The normal lifespan of erythrocytes is approximately 120 days. During their time in the bloodstream, RBCs undergo progressive modifications (oxidant damage, metabolic depletion, increasing intracellular calcium concentrations, dehydration, decrease in cell volume, phosphatidylserine exposure, formation of “senescent” antigens, and others) that lead to their selective removal by specialized recycling macrophages in the liver, and spleen ( Fig. 36.5 ). Macrophages in the bone marrow also cull defective immature erythroid cells to prevent their release into the circulation and remove some deposits of erythrocyte ferritin from developing RBCs.
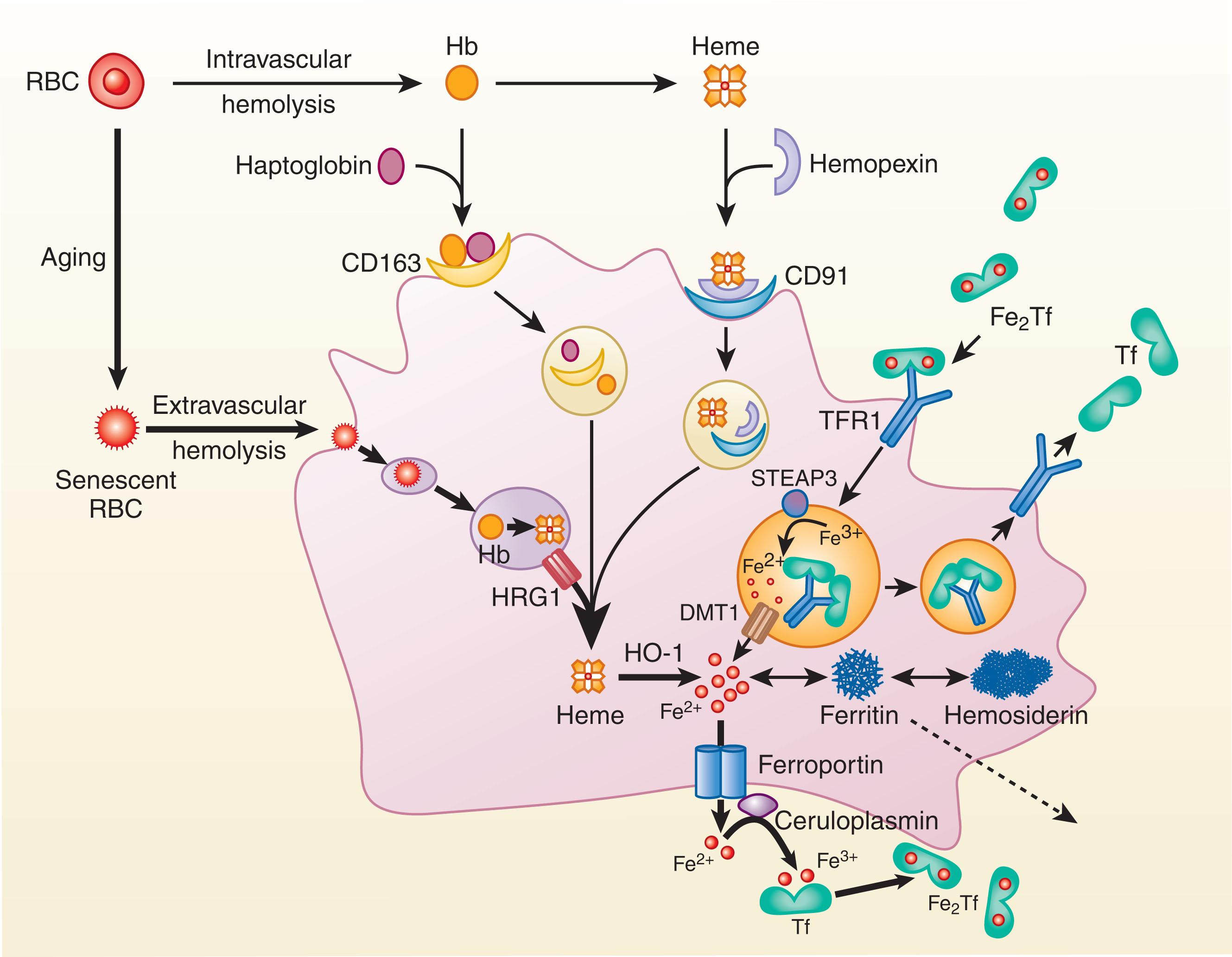
Quality control of RBCs is also performed by the spleen as evidenced after splenectomy by the appearance of abnormal erythrocytes in circulation. On average, each of these iron-recycling macrophages can phagocytize one erythrocyte per day. After ingesting the erythrocyte in a phagosomal vacuole known as an erythrophagolysosome , the erythrocyte membrane is lysed. The hemoglobin within then undergoes oxidative precipitation. proteolysis and liberation of heme (see Fig. 36.5 ). Heme is then transported from the erythrophagolysosome into the cytosol via the heme transporter HRG1 (SLC48A1), a heme-transporting permease.
A small proportion of aged or damaged erythrocytes undergo intravascular hemolysis. With normal erythropoiesis, this portion of the total iron flux is minor but can increase substantially in disorders with increased ineffective erythropoiesis or intravascular hemolysis. The hemoglobin released into plasma is rapidly bound by haptoglobin, a glycoprotein synthesized in the liver. The hemoglobin−haptoglobin complex (M r 150,000) is too large to be filtered by the kidneys, a feature that helps restrict the renal loss of iron with hemoglobinemia. Macrophages (and hepatocytes; see later) remove the haptoglobin−hemoglobin complex from plasma by binding through the cluster of differentiation 163 (CD163) receptor and after endocytosis digest the complex in lysosomes, liberating heme. In an analogous fashion, any heme released into plasma by intravascular hemolysis complexes with hemopexin and is removed by macrophages (and hepatocytes) expressing the low-density lipoprotein receptor−related protein 1 (LRP1).
In macrophages, heme from all these sources is degraded by an enzymatic complex containing nicotinamide adenine dinucleotide phosphate−cytochrome c reductase, the microsomal enzyme heme oxygenase 1, and biliverdin reductase, yielding carbon monoxide (this is its sole physiologic source in the body), bilirubin, and iron (see Fig. 36.5 ). Both DMT1 and natural resistance−associated macrophage protein 1 (NRAMP1; SLC11A1), a DMT expressed within the late endosomal and phagolysosomal membranes of iron-recycling macrophages, seem to be involved in the efficient recycling of this iron. The export of iron from the erythrophagolysosome may occur in parallel with that of heme, considering the evidence that heme export from the macrophage erythrophagolysosome is essential for iron recycling.
Ferroportin is the sole conduit for the export of elemental iron from macrophages in the bone marrow, liver, and spleen to plasma apotransferrin, normally the largest single flux of iron from cells in the body, greatly exceeding the flow of iron from dietary iron absorption in the duodenum. Ferroportin transcription increases in response to both iron and heme. FPN1A levels are also regulated posttranscriptionally through an iron-responsive element in the 5′-untranslated region, with increases in cytosolic iron resulting in increased ferroportin translation. Iron export through ferroportin is facilitated by ferroxidase activity, provided by the multicopper oxidase ceruloplasmin in macrophages and by hephaestin in duodenal enterocytes (see later). Ceruloplasmin oxidation may generate a concentration gradient that helps move the ferric iron out of the macrophage. The ferric iron can then be bound by transferrin and transported back to erythroid and other iron-requiring tissues.
Plasma hepcidin regulates iron efflux from macrophages by decreasing the number of functioning ferroportin molecules available for iron export. The multimeric composition of ferroportin has not been determined definitively, but there is immunohistochemical evidence for a dimeric structure, which is also the simplest explanation for the autosomal dominant inheritance of loss of function ferroportin mutations (see Chapter 37 ). For the most part, ferroportin mutations either interfere with iron export by decreasing the amount of functional ferroportin on the cell surface, resulting in retention and accumulation of macrophage iron, or produce ferroportin resistance to internalization and degradation by hepcidin, resulting in loss of control of macrophage iron export that leads to parenchymal iron loading. Following hepcidin binding, conformational change and ubiquitination, ferroportin is degraded after entering the multivesicular body that fuses with lysosomes. Hepcidin can also block the transport function of ferroportin directly by occluding the cavity involved in iron transport. This mechanism may be important in cell types that are not actively endocytic, such as mature erythrocytes.
Under normal circumstances, the macrophages in the liver, spleen, and bone marrow that are specialized in reprocessing hemoglobin iron from senescent erythrocytes partition the recovered iron between storage in ferritin and release through ferroportin. Synthesis of cytosolic ferritin is induced in response to erythrophagocytosis, and in the absence of iron deficiency, some of the iron derived from the ingested erythrocyte is retained within the macrophage inside cytosolic ferritin. With increasing amounts of storage iron within the macrophage, an increasing proportion of iron is stored within amorphous, insoluble masses as hemosiderin.
Iron preparations for intravenous use are carbohydrate-coated iron nanoparticles that are effectively targeted to macrophages. These preparations are relatively nontoxic because the release of iron from these nanoparticles to transferrin is subject to the same regulation as the release of iron from erythrophagocytosis. The use of intravenous iron at high doses would be expected to cause macrophage iron loading which accelerates the synthesis of ferroportin, thereby counteracting the depletion of ferroportin by hepcidin and promoting the export of iron.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here