Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Electrolyte absorption and secretion mediated by ion channels of the epithelial cells play an important role in determining basal and active fluid movement (i.e., absorption or secretion) in the GI tract. Ion channels that are involved in regulating fluid movement include Na + , K + , and anion (Cl − and HCO 3 − ) channels. Active Na + absorption and active Cl − secretion associate with fluid absorption and secretion, respectively, while K + channels maintain the negative membrane potential that is necessary for active anion secretion. Both cAMP- and Ca 2 + -activated K + channels are localized on both apical and basolateral membranes. The cAMP-activated K + channels named KCNQ1/KCNE2 complex that regulates H + (i.e., acid) secretion is localized on the apical membranes of the gastric parietal cells, while KCNQ1/KCNE1 complex is localized in the basolateral membranes of the entire GI tract. The Ca 2 + -activated K + channels named large conductance K + (BK) channels that regulate active K + secretion and intermediate conductance K + (IK) channels that maintain the negative membrane potential during active Cl − secretion are localized on the apical and basolateral membranes, respectively. Epithelial Na + channels (ENaC), which mediate active Na + absorption, are restrictively present in the apical membranes of colon. The cystic fibrosis transmembrane regulator (CFTR) Cl − channel is the only currently identified apical anion channel in all intestinal segments and mediates cAMP (i.e., cholera toxin)-, cGMP (i.e., heat-stable Escherichia coli enterotoxin, guanylin)- and Ca 2 + -activated Cl − /HCO 3 − secretion. Slc26a9 and CLIC6 are apical/apical recycling anion channels in the stomach, but their functional role for acid secretion is still under debate. The TMEM16 family members expressed in the GI tract are involved in the Ca 2 + signaling itself, and therefore, essential for stimulation of anion secretion by any agonist. The CLC family members regulate organellar function; a role for the membrane-localized CLC2 has been demonstrated in colonic Cl − absorption and it may also be involved in GI barrier function. The role and regulation of these epithelial K + , Na + , and anion channels during physiological and pathophysiological conditions are discussed in this chapter.
In the 1960s, several research groups exploited the new “Ussing chamber” technique, in which an isolated epithelium was placed between two lucite half chambers, and the potential difference (PD) between the serosal and mucosal side of the epithelium was recorded, which was generated by an active transport of ions through the mucosa. If continuously clamped to zero voltage by a current passed through the mucosa, this “short circuit current” (Isc) was recognized to represent net transport of charge across the epithelium in real time. A set of agonists, namely, vasopressin, theophylline, and cyclic-AMP analogues, increased the Isc in a variety of epithelia such as frog skin, toad urinary bladder, and the rabbit ileum. While the agonist-mediated Isc increase in skin and bladder was generated by the influx of Na + , Field et al. realized that in the ileum, the agonist-induced Isc was mediated by the secretion of Cl − . At the same time, investigators had found that crude supernatant of Vibrio cholera induced hypersecretion in isolated intestinal loops of rabbits and dogs and increased cAMP in enterocytes. The Ussing technique has been proved to be a powerful tool for bilateral isotopic flux studies. A number of investigators interested in the pathophysiology of diarrhea soon realized the importance of the stimulation of electrogenic Cl − secretion in the pathophysiology of enterotoxin-mediated diarrhea.
A period of very active research in a number of laboratories resulted in the establishment of a model for intestinal salt transport, based exclusively on electrophysiological, isotope flux, and in vivo fluid movement measurements, which already envisioned many of the key features that were later proved correct by immunohistochemical studies and knockout mouse models, although additional ions such as HCO 3 − and pathways such as the Na + -HCO 3 − cotransporters (NBCs) have more recently been recognized to also play a major role in intestinal electrolyte transport. This included, for example, the fact that the NaCl absorptive pathway resides in the villi and the anion efflux pathway in the crypts, that cAMP acts in two ways, by inhibiting the coupled NaCl influx pathway and by stimulating an electrogenic Cl − efflux pathway, and that stimulating Na + -solute cotransport by glucose or amino acids reduced enterotoxin-mediated intestinal fluid loss in vivo ( Fig. 58.1 ).
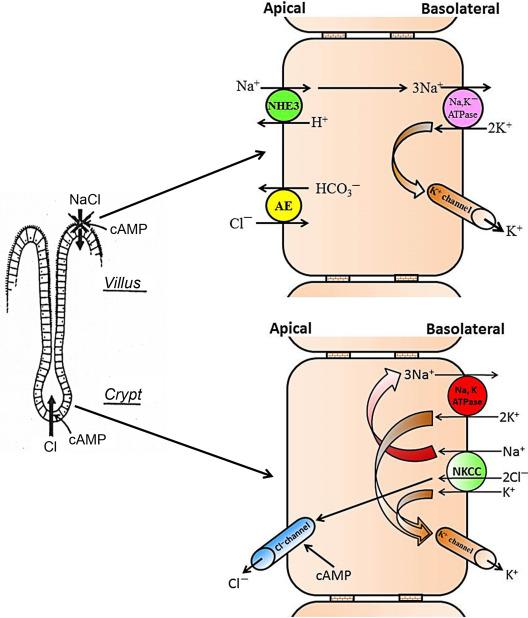
During these years, it also became clear that the intestine expresses yet another system for active Na + absorption in the distal part of the colon, which was electrogenic and sensitive to low concentrations of the diuretic amiloride. Edmonds and colleagues studied the amiloride-sensitive PD in humans and already realized that the transport process, which caused this PD was almost completely abolished in ulcerative colitis, and that the antiulcer drug carbenoxolone caused an increase in the PD, possibly a reason for the frequent edema formation observed with this drug.
A decade later, the energy requirements for active intestinal Cl − secretion had also been clarified. Suitable models such as the shark rectal gland or other amphibian intestinal epithelia, the advancement of microelectrode studies, as well as the development of brush border and basolateral membrane vesicle preparations resulted in the understanding that a Na + -K + -2Cl − cotransport (NKCC) system in the basolateral membrane of enterocytes allowed the uphill transport of Cl − and K + by utilizing the Na + gradient established by the basolaterally located Na + ,K + -ATPase, and that Cl − leaves the enterocyte via a cAMP-activated apical conductance along its electrochemical gradient ( Fig. 58.2 ).
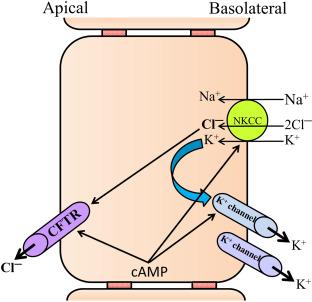
In the early 1980s, Dharmsathaphorn and colleagues described the use of a colon cancer cell line named T84 on permeable supports that could be placed in Ussing chamber systems, which appeared an ideally suited model for the cellular study of intestinal Cl − secretion. A large number of publications appeared in the following years that not only recapitulated the apical anion conductance and basolateral NKCC as important players in the secretory process, but also established the importance of activation of basolateral K + conductances by cAMP and Ca 2 + in maintaining the electrochemical driving force for electrogenic Cl − secretion. In addition, work in intestinal cell lines provided insight into the second messenger regulation of intestinal Cl − secretion, and it was recognized that cAMP- and Ca 2 + -elevating agonists had a synergistic action, suggested to occur via a cooperative action of Ca 2 + - and cAMP-activated basolateral K + conductance with an apical cAMP-activated Cl − conductance. Despite the fact that we now know that the intracellular regulation of secretion occurs through trafficking of transporters, cytoskeleton-anchored formation of multiprotein complexes, phosphorylation at multiple sites and by many kinases, and that the transmembrane transport of CO 2 and HCO 3 − , not represented in these models, also plays a major role in intestinal electrolyte transport regulation, the above model is what many of us still teach students today.
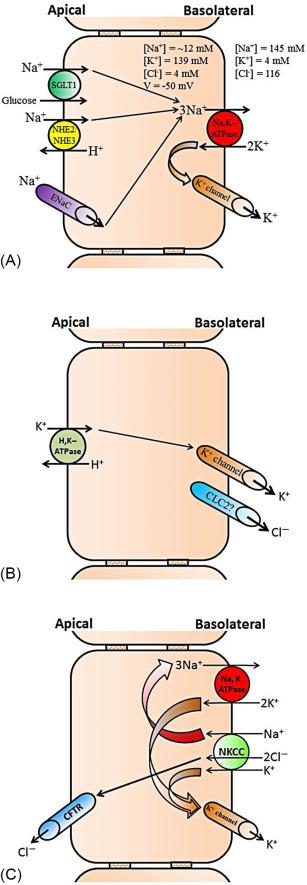
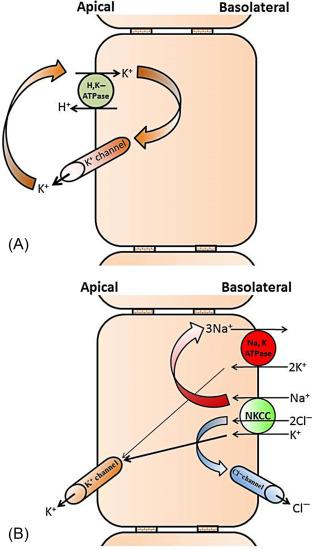
Epithelial K + channels that mediate cellular K + exit across plasma membranes help regulate several important physiological functions. The K + channels with distinct function are localized on the apical and basolateral membranes of the GI epithelial cells. Epithelial cells of the different part of the GI tract such as mouth (salivary secretion), stomach (acid secretion), intestine (nutrient absorption and electrolyte/water absorption/secretion), and colon (primarily electrolyte/water absorption/secretion) perform different functions. Thus, the K + channels present in different epithelial cells regulate different functions. Under basal condition , the basolateral K + channels regulate the Na + ,K + -ATPase (i.e., Na-pump) activity, which is the primary active transporter that catalyzes 3 Na + out of the cells in exchange for 2 K + into the cells. By regulating Na + ,K + -ATPase, the basolateral K + channels help maintaining intracellular low Na + and high K + concentration and a negative (~− 50 mV) membrane potential (i.e., electrochemical gradient). The electrochemical gradient provides the driving force for the secondary active transporters such as Na + -nutrient (e.g., Na + -glucose and Na + -amino acid) cotransporters, and electroneutral (e.g., Na-H exchange) and electrogenic (e.g., epithelial Na + channel, ENaC) Na + absorptive processes that are present in the apical membranes of intestinal and colonic epithelial cells ( Fig. 58.3 A ). Along with maintaining membrane potential, K + channels also regulate basolateral Na + -K + -2Cl − cotransporter (Cl − loader), which brings in Cl − during active Cl − secretion that is mediated through apical Cl − channels in salivary gland, intestine, and colon ( Fig. 58.3 B). In addition, the basolateral K + channels in coordination with apical H + ,K + -ATPase mediate active K + absorption in rat distal colon ( Fig. 58.3 C). In contrary to the basolateral K + channels, the apical K + channels regulate H + secretion (i.e., acid secretion), which is catalyzed by gastric H + ,K + -ATPase of the parietal cells in stomach ( Fig. 58.4 A ). Potassium secretion mediated by apical K + channels in part provides the driving force for secretion in salivary gland, while apical K + channels-mediated K + secretion regulates body K + homeostasis in colon ( Fig. 58.4 B).
Epithelial K + channels are classified into two major classes—Ca 2 + -activated (K Ca ) and cAMP-activated K + channels. There are five different K Ca channels in mammals: one large conductance K + (BK, also known as Slo, K Ca1.1 and KCNMA1), one intermediate conductance K + (IK; also known as SK4, K Ca3.1 and KCNN4), and three small conductance K + (SK1–3) channels. Only BK and IK, but not SK channels are expressed on the apical and/or basolateral membranes of the intestinal epithelial cells. Thus, this chapter will discuss the molecular identities and functional role of IK and BK channels. The BK channels exhibit K + conductance of ~ 150–230 pS, while IK channels exhibits K + conductance of ~ 12–39 pS. Furthermore, cAMP-activated K + channels with conductance of ~ 1–3 pS have also been characterized on both apical and basolateral membranes of intestinal epithelial cells.
Ca 2 + -activated K + channels are present in both apical and basolateral membranes of epithelial cells. Ion flux studies performed under voltage clamp condition have shown that K + channels mediate K + efflux across the apical membranes, while K + channels localized on the basolateral membranes provide the driving force for active Cl − secretion in trachea and colon. Subsequent patch clamp studies have characterized K + channels with conductance of approximately 39 and 200 pS that are designated intermediate K + (IK) and large conductance K + (BK) channels, respectively. BK channels are activated by Ca 2 + and cAMP and inhibited by Ba 2 + , tetraethyl ammonium (TEA), and quinidine. The IK channel is activated by both Ca 2 + and cAMP and inhibited by Ba 2 + , diphenylamine-2-carboxylic acid (DPC), and quinidine, but not by TEA. Patch clamp studies have also characterized BK and IK channels on the basolateral membranes of rat and guinea pig small intestine, and rat, guinea pig, rabbit, and turtle colon. Immunological studies have established the localization of both IK and BK channel-like proteins on both apical and basolateral membranes of epithelial cells of the intestinal tract.
Large conductance K + (BK) channels : The BK channels composed of pore forming α (BKα)- and Ca 2 + /voltage sensing β (BK β )-subunits. Two BKα splice variants (STREX and ZERO) that are transcribed from a single gene, and four BKβ-subunits (β 1–4 , known as KCNMB 1–4 ) that display tissue- specific distribution have been identified. Expression of BKα alone results in large-conductance (~ 150–200 pS) K + channel function, while coexpression with different β-subunits exhibit different sensitivity to Ca 2 + and voltage. The BKα splice variants STREX and ZERO have distinct sensitivities to cAMP (STREX being inhibited and ZERO being activated by cAMP). BK exon 18 is the splice target defining STREX (with exon 18) and ZERO (without exon 18). Only ZERO transcripts have been shown expressed in guinea pig and human colon. However, STREX and ZERO transcripts have been shown expressed in mouse colon. In addition, BKα (ZERO) has also been shown to exhibit three splice variants of COOH terminal in human brain and guinea pig colon. The BKα with exon-27 termination resulted in an amino acid sequence ending of QEERL, whereas the termination in exon-28 resulting in an amino acid sequence of EMVYR. The splicing of exon-28 has been shown to occur with two variants that differed by an omission of three base pair at the beginning of exon-28. The BKα variants that end with amino acid sequences QEERL and EMVYR are identified as BKα RL and BKα YR , respectively. Both BKα RL and BKα YR have been identified to express in guinea pig and rat colon (Rajendran, V. M., unpublished observation). The COOH-terminal variants of BKα RL and BKα YR contribute to the membrane (apical vs. basolateral membrane)-specific delivery of BK channels. BKα YR variant that has been localized on both apical and basolateral membranes of immature cells (i.e., bottom of the crypt) has been shown to be expressed only on the apical membranes of mature surface cells in guinea pig colon. Although the molecular identities of BKα splice variants of rat and human colon have not yet been established, the demonstration that cAMP-activated BK channel activity and forskolin (an adenylate cyclase activator that increases intracellular cAMP levels)-activated K + secretion suggest that the BK channel encoded by ZERO transcripts mediate the K + secretion in rat and human colonic epithelial cells. Aldosterone (dietary-Na + depletion) and high-K + diet enhance BKα-specific mRNA abundance and protein expression and stimulate iberiotoxin (BK channel specific blocker)-sensitive K + secretion in rat distal colon. It has been shown that aldosterone also stimulated K + secretion through ZERO isoform, as forskolin (cAMP) did not inhibit the aldosterone stimulated K + secretion in rat distal colon.
The BKα forms complexes with the auxiliary β-subunit, which has the ability to modify the activation kinetics (i.e., Ca 2 + and voltage sensitivity) and inhibitor sensitivity of BK channel function. Four isoforms of BKβ-subunits have been identified, each of which may associate with the BKα-subunit to modulate BK channel activities in a unique way. Earlier studies have identified the expression of all four β-subunits, while a recent study has identified only β2-subunit expression in mouse colon. Similarly, although different studies have shown all four β-subunits expressed, only β1- and β3-subunits have been identified as the predominant β-subunits expressed in the human colon. Only β1 and β4 are the only β-subunits detected in guinea pig colon. It is likely that BKα splice variants (BKα RL and BKα YR ) may coexpress with different β-subunits in mouse, guinea pig, rat, and human colon. Further studies are required to establish whether BKα RL and BKα YR isoforms coexpress with same or different β-subunits, and whether BKα RL and BKα YR express in same or in different cell types (villus vs. crypt cells) and different membrane (apical vs. basolateral membranes) in colonic epithelial cells.
Intermediate conductance K + (IK) channels : The IK channel (known as hSK4) that encodes 427 amino acids was originally cloned from human placenta. Human and rat colonic IK ortholog that encodes 424 amino acids has been cloned from T84 cells and rat colonic cDNA library, respectively. The rat colonic IK channel expressed in vitro exhibited Ca 2 + -activated K + current with a single channel conductance of 36 pS and was inhibited by clotrimazole (CLT, an antifungal inhibitor). Immunofluorescence studies have localized IK channel-like proteins on both apical and basolateral membranes of epithelial cells in esophagus, glandular stomach, duodenum, jejunum, ileum, and proximal and distal colon of rat, while immunogold labeling studies have localized IK-like proteins on both apical and basolateral membranes of rat and human colon. Further extensive cloning studies have isolated two additional IK splice variants that encode 425 and 395 amino acid proteins. The IK channel with 425 amino acid protein is identical to that of mouse IK channel (mIK1) ortholog that was cloned from smooth muscle. The IK channel isoform mRNAs that encode 425, 424, and 395 amino acid proteins were designated as KCNN4a, KCNN4b, and KCNN4c, respectively. It has been concluded that Kcnn4a encodes smooth muscle IK channels, while KCNN4b and KCNN4c encode basolateral (40 kD) and apical (37 kD) IK channels in intestinal epithelial cells, respectively.
cAMP-activated K + channels have been identified on the apical and basolateral membranes of gastric parietal cell and basolateral membranes of rat and human colon. This cAMP-activated K + channel is inhibited by chromanol-293B, a slow delayed rectifier K + current blocker. Molecular studies have established that this cAMP-activated K + channel is encoded by voltage-gated K v LQT1 channel, which is mutated in hereditary Long QT syndrome type-1. K v LQT1 composed of α-(KCNQ1) and β-(KCNE) subunits. Five different β-subunits (KCNE1–KCNE5) have been isolated. KCNQ1 expressed in different cells and membrane domain that exhibit various functions have been shown to coassemble with different KCNE isoforms. KCNQ1 coassembles with KCNE1 are localized on the basolateral membranes of trachea, pancreas, jejunum, and colon, while KCNQ1 coassembled with KCNE2 (i.e., KCNQ1/KCNE2 complex) forms apical membrane K + channels in gastric parietal cells. KCNQ1/KCNE3 complex have also localized on the basolateral membranes of mouse tracheal and intestinal epithelial cells. Thus, it is unequivocally established that KCNQ1/KCNE2 complex mediated K + exit is critical for apical H + ,K + -ATPase-regulated acid (i.e., H + ) secretion in gastric parietal cells. Although KCNQ1 is expressed in the basolateral membranes of colon, conflicting observations have been shown about its contribution to Cl − secretion. The chromanol 293 sensitive KCNQ1 K + channel has been shown to provide the cAMP active Cl − secretion in mouse colon, but it has been shown to be not essential for cAMP-dependent activated Cl − secretion in guinea pig colon. Since both cAMP and Ca 2 + have been shown to activate basolateral IK channels in human colon, it is likely that the basolateral IK, but not KCNQ1 channels play a central role in regulating infectious and other types of secretory diarrhea.
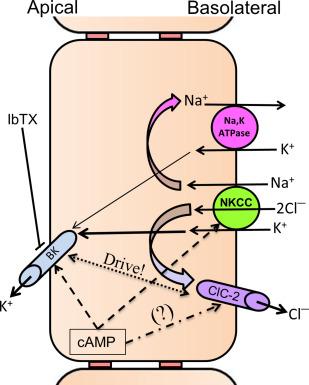
Slow marker perfusion studies have shown that the human colon, but not small intestine, secretes 4.7 mEq K + per day. Both passive K + permeation and active K + secretion contribute to luminal and stool K + concentration. Para cellular passive K + permeation occurs as a result of transepithelial lumen-negative (~ 20–30 mV) electrical PD, which may exist through the entire GI tract. In contrast, the transcellular active electrogenic K + secretion has only been localized in salivary gland, gastric mucosa, and large intestine of the digestive tract. Active K + secretion contributes to increased stool K + content during secretory diarrhea. Every day, normal humans excrete 10 mEq K + in stool, while patients with severe diarrhea such as cholera secrete 119 mEq/day. Active K + secretion requires K + uptake across basolateral membrane and K + exit across the apical membranes ( Fig. 58.5 ). In addition, electrogenic K + secretion also requires exit mechanisms for Na + and Cl − across basolateral membranes. Potassium uptake across the basolateral membrane is mediated by Na + ,K + -ATPase, and Na + -K + -2Cl − cotransport, while K + exit across the apical membrane is mediated by K + channels. The Na + brought in by Na + -K + -2Cl − cotransport is pumped out by Na + ,K + -ATPase, while Cl − brought in by Na + -K + -2Cl − cotransport exits via chloride channel-2 (CLC2) that has been shown to be localized on the basolateral membranes of mouse and guinea pig. Inhibition by serosal ouabain (Na + ,K + -ATPase inhibitor) and bumetanide (loop diuretic, Na + -K + -2Cl − inhibitor) has established that active K + secretion is regulated by basolateral Na + ,K + -ATPase and Na + -K + -2Cl − cotransport. Na + ,K + -ATPase regulates the active K + secretion under basal condition, while Na + -K + -2Cl − cotransport is the predominant regulator of the stimulated active K + secretion. In stoichiometric point of view K + uptake across the basolateral membrane far exceeds its transepithelial movement, thus suggesting the presence of K + recycling across the basolateral membrane. The observation of an increased K + secretory rate when Ba 2 + (nonspecific K + channel blocker) is added to the serosal bath suggested that K + recycling across the basolateral membranes of turtle and rabbit colon contributes to enterocyte K + homeostasis. Since Ba 2 + -sensitive K + channels have not been observed, it was suggested that K + -Cl − cotransport might be responsible for K + recycling across the basolateral membranes in rat distal colon. Since active K + secretion is enhanced in IK channel knockout mouse, and BK and IK channel-like proteins have been localized, it is likely that either BK and/or IK channel might regulate the K + recycling across basolateral membranes in guinea pig, mouse, and rat distal colon. The colonic K + secretion is activated by a cellular second messenger (e.g., cAMP and Ca 2 + ) and is stimulated by dietary-K + loading, dietary-Na + depletion (aldosterone) and dextran sulfate sodium (DSS)-induced colitis. Active colonic K + secretion is increased in patients with end-stage renal disease (ESRD), colonic pseudo-obstruction, and adenomas.
Ion flux studies performed under voltage clamp condition have shown that iberiotoxin or paxilline (BK channel-specific blockers) sensitive K + channels present on the apical membranes mediate both basal and stimulated K + secretion in guinea pig, mouse, and rat distal colon, while patch clamp studies have characterized K + channels with large conductance in rat and human colon. The absence of K + secretion in BKα knockout mouse has established that BK channel mediates both resting and activated K + secretion in mouse colon. It is current dogma that the absorptive (e.g., ENaC) and secretory (e.g., CFTR) transport processes are distributed differentially along the surface-crypt axis, as the absorptive process being localized on the surface cells, while the secretory processes are localized on the crypt cells. In contrast, conflicting observations exist regarding BKα protein localization in normal guinea pig, mouse, and human colon. In normal human colon, BKα-like proteins have been localized only on the apical membranes of surface cells in normal human colon, while in mouse it has been localized only to crypt cells. Absence of BKα proteins in BK channel knockout mouse justified the presence of BK channels in the crypt cell of mouse colon, while surface cell expression of BK channel has been established by extensive patch clamp characterization of large conductance K + channel in normal human colon. In contrary to both mouse and human, BKα proteins have been localized on both surface and crypt cells in guinea pig distal colon. BKα proteins have been localized on both surface and crypt (top 2/3rd of crypt) cells, while the bottom third of crypt cells are completely devoid of BKα protein, in human colonic biopsy specimens (Rottgen T, Nickerson A and Rajendran VM; unpublished observations). Despite the existing conflict on cell specific localization, it is well established that BK channels localized on the apical membrane mediate basal and activated K + secretion in mammals and human colon.
The K + concentration of protein-rich saliva is from 5 to 10-fold higher than that of plasma K + concentration. Micropuncture studies have shown that acinar cells are capable of K + secretion and that the salivary K + concentration is inwardly proportional to the perfusion rate in rat and mouse salivary glands. Although K + channels were originally described in the basolateral membranes, recent functional studies have shown TRAM-34 (IK channel blocker) and paxilline sensitive K + secretion, as evidence for the presence of IK and BK channels on the apical membranes of mouse parotid acinar cells. Molecular studies have established the expression of IK and BKα channels in mouse parotid acinar cells. BKα variant of salivary gland parSlo has been cloned and shown to express in both mouse and human parotid glands. As discussed, BKα coexpresses with different BK β subunit (BK β1–4 ) that expresses distinct Ca 2 + and voltage-sensitive K + current. Molecular studies have identified that both β1 and β4 expression in parotid glands. However, the biophysical characteristics of BK channels of parotid acinar cells have not been altered in β1/β4 double knockout mouse. As a result, further studies have identified leucine-rich repeat containing proteins 26 (LRRC26, known as BKγ1-subunit), which is highly expressed in salivary glands, as an accessory protein for parSlo. The parSlo coexpressed with LRRC26 has been shown to exhibit biophysical properties comparable to native BK channels in CHO cells. Thus, in contrast to other BKα, which coexpresses with β1–4, parSlo coexpresses with LRRC26 in salivary glands.
Although it has been hypothesized that K + channels are involved in the regulation of fluid secretion, the salivary gland fluid secretion was not affected either in IK or BKα channel gene knockout mice. However, the fluid secretion has been shown to be severely impaired in IK and BKα double gene knockout mice parotid gland. Based on these observations, it was suggested in the absence of one channel, the other channel compensates to regulate fluid secretion in salivary gland. However, although IK channels have been shown to provide the driving force for Cl − (i.e., fluid) secretion, it is not known whether BK channel can also provide similar driving force for Cl − secretion. Although fluid secretion was not affected, K + secretion was reduced by > 75% in submandibular salivary glands of BKα knockout mouse. Thus, the BK channel significantly contributes to the K + secretion in salivary glands.
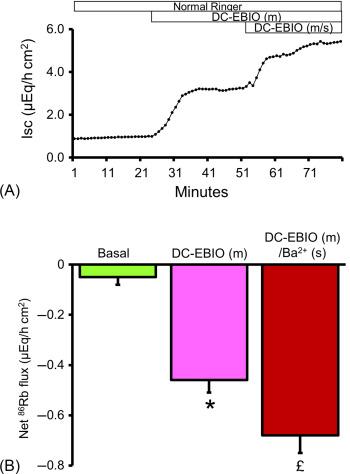
Patch clamp studies have characterized IK channels only in the basolateral membranes of crypts from rat and human colon. Immunofluorescence studies have localized IK channel proteins on the apical membranes of esophagus, glandular stomach, ileum (both villus and crypt cells), cecum, proximal colon and distal colon of rat, and human colon, while functional studies have identified IK channels in mouse parotid acinar cells. The apical IK channel has been shown to be encoded by KCNN4c transcripts (IK splice variant) in rat distal colon. Although the role of apical IK channels in small intestine is unknown, the role for apical IK channels has been shown in rat proximal and distal colon. Inhibition of carbachol stimulated K + secretion by clotrimazole (IK channel blocker) has been shown as evidence for the apical membrane IK channels in normal rat proximal colon. Mucosal DC-EBIO (IK channel opener) has been shown to stimulate TRAM-34 (IK channel specific blocker)-sensitive K + secretion, as evidence for apical membrane IK channels in normal rat distal colon ( Fig. 58.6 ). Further, the complete absence of mucosal DC-EBIO-induced K + secretion in dietary-K + depleted rat distal colon established the presence of IK channels in apical membranes. In addition to stimulating IK channel, mucosal DC-EBIO has also stimulated Inh- 172 (CFTR specific blocker)-sensitive Cl − secretion (measured as short circuit current). The TRAM-34, which blocked IK channel mediated K + secretion, also blocked the Cl − secretion. The inhibition of Cl − secretion by TRAM-34 indicates that apical membrane IK channel also provides the driving force for CFTR-mediated Cl − secretion. It is likely that in addition to basolateral membrane IK channels, the apical membrane IK channels also provide the driving force for active Cl − secretion in distal colon. The role of apical membrane IK channels need to be identified in the small intestine.
The human stomach secretes about 1–2 L of hydrochloric acid (~ 100 mmol/L) per day. This extraordinary function is catalyzed by an electroneutral apical H + ,K + -ATPase, which pumps out intracellular H + into the lumen in exchange for extracellular K + in gastric parietal cells, requires a continuous luminal K + supply. More than three decades ago, experiments with vesicles isolated from parietal cells have suggested that K + replenishment and accompanied Cl − secretion are accomplished through apical membrane localized K + and Cl − channels, respectively. Several other studies, however, have suggested that K + -Cl − cotransport, instead of K + and Cl − conductive pathways, as a possible mechanism for K + exit across apical membrane. Although K + conductance has not been observed in vesicles isolated from resting parietal cells, K + conductance that is augmented by phosphatidylinositol-4,5-bisphosphate (PIP2) has been shown in vesicles isolated from preactivated parietal cells. This apical K + channel has unexpectedly been identified as KCNQ1, although Kir Channels are also localized in the tubulovesicular membrane in resting state and secretory membrane in secreting. KCNQ1 knockout mouse exhibited a stomach phenotype (i.e., enlarged stomach as a result of hyperplasia of the antrum and fundus mucosa), decreased number of parietal cells, hypochlorhydria with increased stomach pH of 6–7 (wild-type mouse stomach pH is 1–2) and elevated plasma gastrin level. Immunofluorescence studies have localized KCNQ1 on gastric luminal compartment and have suggested a KCNQ1 recycling mechanism in mouse and human. Further a functional study has demonstrated that chromanol 293B (KCNQ1 inhibitor) almost completely inhibited the acid secretion in mouse, rat, and dog, but a later study revealed nonspecific effects of chromanol 293B and an incomplete inhibition of acid secretion by a more specific KCNQ1 inhibitor. In addition, Kir channels 4.1 and 5.1 × 100% cotrafficked with the H + /K + -ATPase, while KCNQ1 expression only partially overlapped with H + /K + -ATPase, raising the question of a complementary role for the two K + channels. However, the newborn KCNQ1-deficient but not Kir 4.1-deficient gastric mucosa is unable to secrete acid but its secretory rates are restored to normal levels when a high K + concentration was applied to the luminal bath, demonstrating an absolute dependence of acid secretion on the presence of KCNQ1. High K + rescue was not possible any more in adult KCNQ1-deficient stomach, which displays severely abnormal parietal cell ultrastructure and an enormous foveolar hyperplasia. Kir 4.1 channels, on the other hand, appear to play a role in apical membrane recycling. As discussed, KCNQ1 complexed with a different regulatory subunit (i.e., KCNE1–5) have been shown to exhibit various functions in different tissues. Of the five regulatory subunits, only KCNE2 and KCNE3 have been shown abundant in human stomach. This study has also shown that KCNE2 coexpressed with KCNQ1 exhibited voltage insensitive, luminal acid pH: 5.5 (compared to alkaline pH: 7.5) activated, chromanol 293B-sensitive whole cell current in COS cells. KCNE2 has been identified to colocalize with H + -K + -ATPase in stimulated, but not in nonstimulated rat gastric parietal cells. Studies with gene knockout animal have shown that similar to KCNQ1 knockout, KCNE2 knockout mice also exhibited abnormal parietal cell, hypergastrinemia, glandular hyperplasia and impaired gastric acid secretion, and have established that the regulatory subunit KCNE2 is critical for the proper function of KCNQ1 in gastric parietal cells. Thus, the KCNQ1/KCNE2 complex expressed on the apical membranes mediates the electrogenic K + secretion that is required for the continuous operation of H + ,K + -ATPase in gastric parietal cells. Intracellular second messenger (cAMP, Ca 2 + , and PIP 2 ) and extracellular acid pH that activated the luminal K + conductance of parietal cells have all been shown to activate the in vitro expressed KCNQ1/KCNE2 activity. The H + ,K + -ATPase location has been shown to have dramatic difference in resting and stimulated conditions in the parietal cells of the gastric mucosa. Most of the H + ,K + -ATPase has been shown localized in cytoplasmic tubulovesicles with no access to the canaliculi system in parietal cells. Upon stimulation, the H + ,K + -ATPase-containing tubulovesicles have been shown to exocytotically fuse into canaliculi that involve complex vesicular trafficking. Under a resting state (i.e., nonstimulated condition), H + ,K + -ATPase has been shown to most of the evenly distributed in cytoplasmic tubular vesicles, which is devoid of K + conductance, throughout the parietal cells. In contrast, in stimulated parietal cells, H + ,K + -ATPase has been shown localized in canalicular space, where KCNQ1 has also shown colocalized. Based on these observations, the cellular models have been proposed for both acid and K + secretion in parietal cells. In that the agonists (e.g., acetyl choline, gastrin and histamine) stimulate acid secretion by actively recruiting the H + ,K + -ATPase-containing tubulovesicles into canaliculi compartment. In contrast, KCNQ1/KCNE2 K + and CFTR Cl − channels, which are readily present on the canaliculi, are activated by second messenger (cAMP, Ca 2 + , and PIP 2 ) regulated phosphorylation. In addition to second messengers, luminal H + secreted through H + ,K + -ATPase also stimulates KCNQ1/KCNE2-mediated K + secretion. The K + secreted through KCNQ1/KCNE2 channels utilized by H + ,K + -ATPase for active H + secretion. Immunofluorescence studies have observed that H + ,K + -ATPase and KCNQ1 are unevenly expressed along the gastric gland, as H + ,K + -ATPase and KCNQ1 are predominantly localized on the parietal cells of the upper and lower part of the gastric gland, respectively. Thus, acid secretion (i.e., H + ,K + -ATPase) and K + and Cl − channels have been illustrated to localize in different cell types (i.e., top and lower cells, respectively) of gastric glands.
Potassium channels that exit K + across basolateral membranes into the systemic circulation regulate both the Na + ,K + -ATPase and the Na + -K + -2Cl − cotransporter. Under basal condition, constitutively open basolateral K + channels maintain a negative membrane potential, which is the driving force for Na + -dependent nutrient absorption and Na + /H + -exchanger mediated electroneutral Na + absorption in the small intestine. The constant K + loss is counterbalanced by the regulation of the Na + ,K + -ATPase, which maintains intracellular high K + levels. The electrochemical gradient also provides the driving force for electrogenic Na + absorption mediated through epithelial Na + channel (ENaC) in the distal colon. In contrast, under stimulated conditions, basolateral membrane Na + -K + -2Cl − cotransporter brings in Cl − , which exits through apical membrane Cl − channels and depolarizes the membrane potential. Potassium exits through agonist-activated basolateral membrane K + channels and maintains the negative membrane potential that provides the driving force for sustained electrogenic Cl − secretion. Patch clamp studies have identified characteristically different population of K + channels in the basolateral membranes of various epithelia of digestive tract (i.e., salivary acinar cells, gastric parietal cells, and intestinal and colonic crypt cells) of different species ( Table 58.1 ). Although it is difficult to generalize the properties and physiological roles of the basolateral K + channels between different species, and between different tissues, these K + channels can be divided into three categories: (1) small (< 6 pS), (2) intermediate (19–35 pS), and (3) large (67–240 pS) conductance K + channels. Molecular studies have identified that the small, intermediate, and large conductance K + channels are encoded by KCNQ1/KCNE3, IK (Kcnn4), and BK (KCNMA1) K + channels, respectively. In addition to these three categories, nonselective cation channels with 27–30 pS that conduct Na + and K + have also been identified in turtle and rat colon. However, the molecular identities of the nonselective cation channels are not known. Small conductance (KCNQ1/KCNE3) and BK K + channels are activated by cAMP and Ca 2 + , respectively, while IK channels are activated by both Ca 2 + and cAMP. Despite the electrophysiological and immunofluorescence localization, physiological functions of basolateral BK channels have not been identified. Since the contribution of KCNQ1/KCNE3-mediated K + conductance has been shown small in the basolateral of human colonic crypts, it is likely that the highly abundant IK channel that is activated by both Ca 2 + and cAMP plays critical role in activated Cl − secretion that drives fluid secretion.
| Tissue | Species | Agonist | Conductance (pS) | Inhibitor | Reference |
|---|---|---|---|---|---|
| Mouth | Sheep, rat and human | Ca 2 + | 30 | TEA, | |
| 165–240 | CTX | ||||
| Stomach | Necturus | Ca 2 + | < 6 | Not known | |
| cAMP | 21–33 | Not known | |||
| Ca 2 + | 67 | Not known | |||
| Duodenum | Rat | cAMP and Ca 2 + | 19–28 | Ba 2 + and TEA | |
| cAMP | 84–99 | TEA | |||
| Colon | Rat | cAMP | < 3 | Chromanol 293B | |
| Ca 2 + | 12 | Ba 2 + and TEA | |||
| Ca 2 + | 187 | Ba 2 + | |||
| Nonselective | 27 | Not known | |||
| Rabbit | Ca 2 + and cAMP | 90–220 | Ba 2 + and TEA | ||
| Turtle | Nonselective | 30 | Quinidine and DPC | ||
| Ca 2 + | 35 | Ba 2 + , quinidine and DPC | |||
| 188 | Ba 2 + and quinidine | ||||
| Human | cAMP | 6.8 | Not known | ||
| cAMP and Ca 2 + | 23 | Ba 2 + , quinidine and DPC | |||
| Ca 2 + | 138 | Ba 2 + , quinidine and TEA | |||
| Cell line | T84 | cAMP and Ca 2 + | 28 | CLT | |
| Ca 2 + | 161 | Ba 2 + |
Role of basolateral IK channels in active Cl − secretion : Electrogenic Cl − secretion, which is the critical event underlying in secretory diarrhea, occurs predominantly from crypts of small intestine and colon. Secretory diarrhea may be caused by a variety of infective and neurohumoral factors that stimulate intestinal Cl − secretion and water secretion by activating intracellular protein kinase A (PKA, Ca 2 + -dependent) and protein kinase C (PKC, cAMP-dependent) signaling pathways. The current model for electrogenic Cl − secretion requires activation of basolateral K + channels to promote hyperpolarization, and recycling of K + taken into the cell via basolateral Na + -K + -2Cl − cotransporter and Na + ,K + -ATPase. Ion flux studies have shown that basolateral K + conductance is important for cAMP-stimulated Cl − secretion in rat and human colon. Activated K + channels that exit K + across basolateral membranes generate the membrane potential gradient required to drive the sustained Cl − secretion via cAMP-stimulated CFTR Cl − channels localized on the apical membranes. Two pharmacologically distinct K + channels have been shown, one that is activated by Ca 2 + -dependent, while the other is activated by cAMP-dependent Cl − secretory agonists, in the basolateral membranes of T84 cells. Blockage of these basolateral K + channels has been shown to inhibit active Cl − secretion in T84 cells. Electrophysiological studies have characterized low-conductance basolateral K + channels that are activated during cAMP-stimulated Cl − secretion and inhibited by chromanol 293B in rat colonic crypts, while it has been identified as cAMP-stimulated K + channel encoded by KCNQ1/KCNE3 complex in T84 cells. In contrast, however, intermediate conductance K + (IK) channels have been shown abundant and activated by Cl − secretagogues carbachol (a Ca 2 + -mediated muscarinic agonist) and dibutyryl-cAMP (a membrane permeant cAMP analogue) in basolateral membranes of T84 cells rat and human colonic crypts. Activation of basolateral IK channels stimulates active Cl − secretion, while cAMP-stimulated Cl − secretion is completely inhibited by TRAM-34 (an IK channel inhibitor) in rat distal colon. Thus, the membrane potential maintained by basolateral IK channels provides the driving force for both Ca 2 + -stimulated and cAMP-stimulated Cl − secretion in human and mammalian colon.
Selective blockade of basolateral IK channels as a means of controlling epithelial Cl − secretion is being explored. Basolateral IK channels can be inhibited by Ba 2 + in human colonic crypts, and thus it has been shown to block both Ca 2 + and cAMP-stimulated Cl − secretion when added to the basolateral surface of T84 monolayer. The IK channel-specific inhibitors CLT and TRAM-34 have also been shown to inhibit both Ca 2 + and cAMP-stimulated Cl − secretion in mouse and rabbit intestine and rat colon. Somatostatin, a tetradecapeptide normally present in intestinal mucosa, has long been recognized as a potent antisecretory peptide that inhibits all forms of Ca 2 + and cAMP-stimulated Cl − secretion. Somatostatin has been shown to exert its effects via G-protein coupled receptors. Although somatostatin has been shown to reduce intracellular cAMP levels via a G-protein dependent inhibition of adenylate cyclase, it has also been shown to have additional antisecretory effects distal to the intracellular second messenger production cascades. Further studies have shown that somatostatin markedly inhibited basolateral IK, but not BK channels in human colonic crypts. Since multiple somatostatin receptors exist, identification of specific receptor subtypes linked to basolateral IK channels in intestinal and colonic epithelia may provide a basis for the development of new antidiarrheal drugs, which might act on a specific receptor.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here