Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurological and neuroanatomical injuries and disorders affect a large number of people worldwide, and often result in movement impairment and inability to perform everyday tasks, such as communicating, reaching, and grasping, independently. Persons who have experienced neurological injuries, such as spinal cord injury (SCI), amyotrophic lateral sclerosis, or stroke, can achieve partial restored function through cortical prosthetic systems. A cortical prosthesis is an end effector device that receives an action command to perform a desired function through a brain–computer interface (BCI) that records cortical activity and extracts (i.e., decodes) information related to that intended function. End effectors can range from virtual typing communication systems to robotic arms and hands or a person’s own limb reanimated by functional electrical stimulation (FES). BCI technology can range in levels of invasiveness, temporal and spatial recording resolution, and the types of recorded signals. Noninvasive brain imaging technologies, such as electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI), have been implemented in various simple BCI applications, such as low throughout communication spelling systems ( ). However, these noninvasive BCI options typically are too slow (e.g., fMRI), have low spatial resolution and low signal bandwidth (e.g., EEG), and/or are easily corrupted by external artifacts ( ). Thus they are not ideal for complex real-time applications, such as high-performance communication, control of multidimensional robotic limbs, and reanimation of paralyzed limbs for coordinated reaching and grasping. Invasive BCIs, because of the higher resolution and signal bandwidths available, have the potential to allow people with neurological injury to command more high-dimensional systems naturally and restore more complex functions.
In this chapter we review currently available invasive recording technologies used for BCI neuroprosthetic systems. We examine areas of cortex in which these recording technologies are typically implanted, the types of signals recorded from these areas, and the movement-related information that can be extracted. We review the most relevant algorithms for extracting (i.e., decoding) movement-related information from these cortical signals, as well as practical concerns when developing cortical decoders. Additionally, we discuss current applications of invasive BCIs in conjunction with technology for restoring communication and movement. Finally, we discuss hurdles to clinical translation and daily adoption, and current research aimed at overcoming these hurdles.
Invasive neural recording technologies are generally distinguished based upon the depth of cortical tissue penetration and the resolution of the recorded neural signals. Fig. 27.1 illustrates representative examples of the most common invasive neural recording technologies: stereoencephalography (sEEG) intracranial electrodes, electrocorticography (ECoG) sheet electrodes, and penetrating microelectrode arrays. It should be noted that there are different interpretations of the term “invasiveness.” One interpretation focuses on the depth of penetration into cortical tissue, in which case ECoG electrodes may be viewed as least invasive and sEEG may be viewed as most invasive. However, another interpretation focuses on the level of surgical intervention required, and more specifically the size of a craniotomy required for electrode implantation. In this interpretation, ECoG sheet electrodes may be viewed as the most invasive, due to the usually larger size of the recording sheets (and hence larger craniotomy), in comparison to penetrating microelectrodes and sEEG depth electrodes. Note that resection surgeries for persons with intractable epilepsy routinely require large craniotomies with relatively low rates of minor complication ( ). This section describes each recording technology, including neural features typically recorded, and current BCI implementations of each technology.
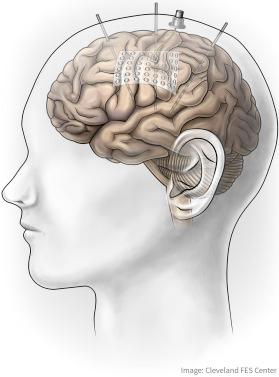
sEEG intracranial electrodes are long, thin, depth electrodes, similar to those used for deep brain stimulation, with large contacts spaced longitudinally along the shaft. Clinically, sEEG electrodes are routinely and predominantly used for presurgical seizure localization in persons with pharmacologically resistant epilepsy who are candidates for resection surgery. Surgical placement typically involves a small stab incision and burr hole for insertion of the electrode, rather than a larger craniotomy. Consequently, faster postoperative recovery, less tissue healing time, and smaller scars are observed in comparison to other invasive electrode technologies. Retrospective studies comparing complications of placement of sEEG intracranial versus ECoG subdural sheet electrode cases show that sEEG placement resulted in significantly fewer complication rates, and deem placement of sEEGs to be reliable and safe ( ). A major benefit of sEEGs is that they allow recording from potentially every cerebral structure, including difficult-to-access subsurface cortical areas and deep sulci walls where significant movement-related activity is routinely observed ( ). From these areas, sEEGs record field potential activity (0–200 Hz bandwidth), with observed modulation in specific spectral frequency bands (e.g., α: 2–12 Hz, β: 12–30 Hz, γ: 30–60 Hz) potentially conveying relevant movement information. Several recent studies have investigated the potential to use sEEG intracranial electrodes for BCI applications. reported sEEG electrodes recording α modulation from the insular cortex and broadband modulation from deep sulci walls of the motor cortex for prediction of hand grasp force. sEEG electrodes have also been used in standard two-dimensional BCI cursor control center-out tasks with moderate success ( ). Future research and electrode enhancements (such as high-resolution contacts for single neuron recordings) may enhance the performance of sEEG electrodes, potentially making them a very attractive option for invasive BCIs due to their already adopted clinical status.
ECoG subdural grid electrodes and strips consist of series of flat electrode contacts placed on a thin silicone sheet that either sits on the brain surface directly under the dura or is potentially placed within a sulcus. Like sEEG intracranial electrodes, ECoG grids and strips are used clinically for seizure localization, though in recent years with less frequency due to the required larger craniotomy and higher complication rates. However, ECoG electrodes offer broader coverage of the cortical surface and recording of neuron population-level field potentials, and have been demonstrated for closed-loop control of simple cursor movements ( ) and even control of a three-dimensional robotic arm ( ). Proponents of ECoG-based BCIs argue that the recorded field potential signals may have greater temporal stability, and hence greater clinical viability, than single neuron signals. However, this claim has not been rigorously validated.
Penetrating microelectrode arrays consist of a number of very small wires or shafts that penetrate into cortical tissue to allow recording of single neuron action potentials and local field potentials (LFPs). Currently the predominant penetrating microelectrode technology is the 96-channel microelectrode array (BlackRock Microsystems, Salt Lake City, UT) ( ). Ninety-six recording electrodes insulated with parylene-C are attached to a silicone platform substrate, which is then connected to a percutaneous pedestal through a wire bundle. The microelectrode shanks typically penetrate 1.0–1.5 mm into cortical tissue. Though only 4 × 4 mm in size, surgical implantation usually requires a larger craniotomy to ensure that the array shanks do not rupture cortical vasculature during placement. Implantation is usually performed using a pneumatic insertion technique ( ). These arrays have demonstrated efficacy in both nonhuman primate (NHP) and human invasive BCI applications, and are the focus of most of this chapter. Other penetrating electrode approaches have been widely used in preclinical studies; specifically, “microwires” and “Michigan probes” have been used successfully, each with unique advantages and disadvantages. Microwires are the lowest-profile penetrating electrodes (i.e., evoke the smallest immune response), and are promising for chronic multisite recordings ( ). Michigan probes have been developed in many formats, but typically include multiple electrode surfaces spaced longitudinally along the shaft and allow incorporation of active electronics ( ). However, these electrodes have some challenges in regards to mechanical stability ( ), and are not yet proven in chronic human applications.
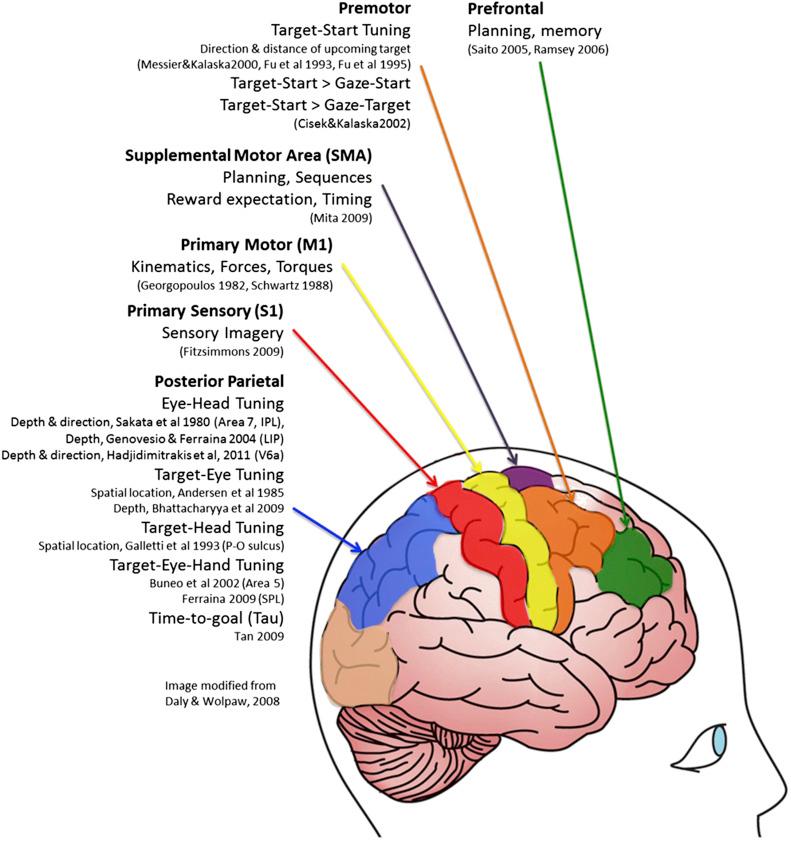
Invasive BCIs have the advantage over their noninvasive counterparts (EEG, MEG, fMRI) in that they allow high temporal and spatial resolution recordings of single neuron action potentials (“spikes”) and LFPs. The spikes and LFPs from recorded small cortical networks can often be directly related to specific aspects of movement. Several motor cortical areas have been investigated as possible recording sites for providing information relevant to decoding natural arm and hand movements, or BCI-commanded target-directed movements. This section presents a brief synopsis of the different types of movement information encoded in various cortical areas of interest ( Fig. 27.2 ), and the various cortical signal features recorded and used in invasive BCIs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here