Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The major task in cardiac development is to form a four-chambered heart that functions in a coordinated fashion from a straight tube that functions merely by peristalsis. Cardiac development can be thought of as proceeding along various phases: fusion of myocardium and endocardium in the ventral midline to form a simple tube, onset of function, looping to the right side, specification and formation of chambers, development of specialized conduction tissue, formation of the coronary circulation, innervation of the heart, and formation of mature valves. The approximate times at which these various events occur in human development are shown in Table 62.1 .
| Event | Gestational Age (wk) |
|---|---|
| Formation of a heart tube | 3 |
| Aortic arches form | 3 |
| Onset of function | 4 |
| Looping | 4 |
| Onset of chamber septation | 5 |
| Development of specialized conduction tissue | 5 |
| Formation of the coronary circulation | 5–6 |
| Onset of innervation of the heart | 7–8 |
| Formation of mature valves | 10 |
| Definitive venous system established | 12 |
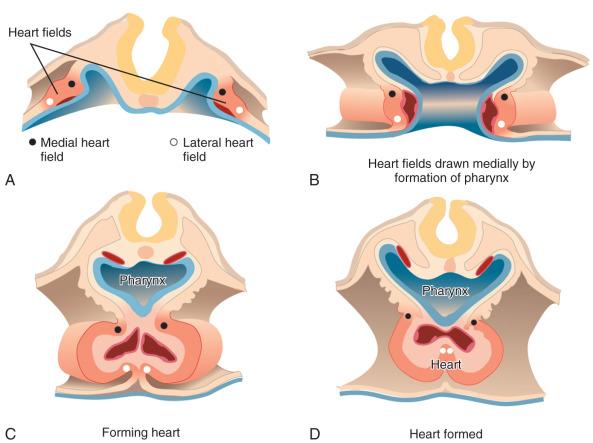
Three main groups of cells contribute to the morphogenesis of the heart. These main groups are the mesoderm of the primary heart fields (located in the splanchnic layer of lateral plate mesoderm bilaterally), the secondary heart fields (located in the pharyngeal mesenchyme), and the cardiac neural crest (a subdivision of the cranial portion of the neural crest). These tissues and their roles in the developing heart are illustrated in e-Fig. 62.1 , along with the two important extracardiac populations of cells described later in this chapter.
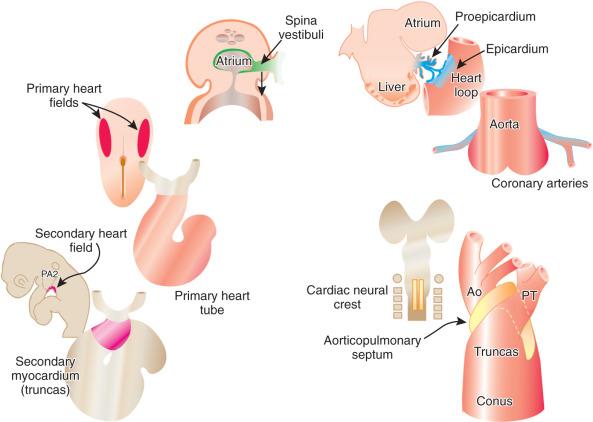
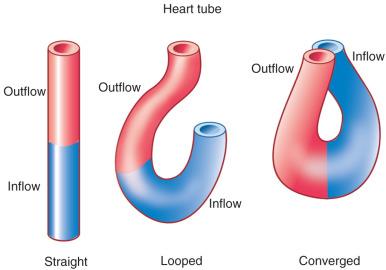
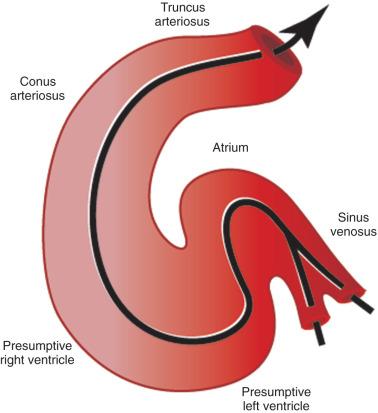
The first stage in the development of the heart is the formation of a single midline heart tube from the bilateral cardiogenic fields ( e-Fig. 62.2 ). The heart begins to beat even at this single tubular stage. Cells are then added to each end of the heart tube, and the tube begins to loop to the right. During looping, the tube is further lengthened by the addition of cells from the secondary heart field to the outflow pole. Looping and convergence bring the inflow and outflow poles of the heart into proximity ( Fig. 62.3 ), setting the stage for septation and definitive chamber formation. The traditional names of the various segments of the recently looped heart are illustrated in e-Fig. 62.4 . Note that the heart in this configuration is set up to become a double-inlet left ventricle and a double-outlet right ventricle; if septation proceeds properly, these forms of congenital heart disease are avoided. Formation of a four-chamber heart requires the right ventricle to obtain an inlet and the left ventricle to obtain an outlet.
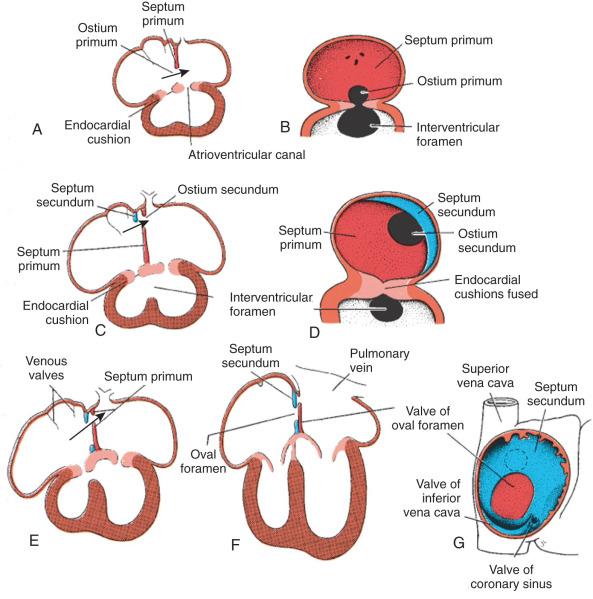
The atrioventricular canal of the heart is divided into right and left sides by endocardial cushions that develop at the atrioventricular junction and ultimately form the septum of the atrioventricular canal and the atrioventricular valves ( Fig. 62.5 ). The right atrioventricular canal and right ventricle expand to the right, and the atria are septated from one another. Septum primum, the primary atrial septum, is led by the spina vestibuli (vestibular spine) and ultimately fuses with the endocardial cushions to close the first interatrial communication or ostium primum. As the septum primum is growing, fenestrations begin to develop within it. These fenestrations coalesce to form the secondary interatrial communication, or ostium secundum (see Fig. 62.5 ). A second septum, or septum secundum, forms to the right of the septum primum much later, and postnatal fusion of the two septa obliterates any interatrial communication. Note that septum primum is ultimately a left atrial structure and that septum secundum is considered a right atrial structure, even though it expresses left-sided molecular markers. Note further that, in the common parlance of naming atrial septal defects, the defect is named for the embryonic ostium that persists, not for the embryonic tissue in which the defect exists. Thus, for example, a secundum atrial septal defect is persistence of the embryonic ostium secundum, even though the defect itself is most often in the septum primum. This naming practice is one of the more confusing points in the nomenclature of congenital heart disease.
Just as the atria and the atrioventricular canal portions of the heart are septated, so are the ventricles. The muscular ventricular septum arises from the deepest convexity of the cardiac loop and grows toward the atrioventricular septum. The membranous septum ultimately will bridge the gap between these two portions of the septum. The outlet, conal, or infundibular septum (i.e., the portion of the septum that ultimately will lie just below the semilunar valves and between the outflow tracts) forms from the conal cushions. Initially it is mesenchymal and only later muscularizes. Ventricular septal defects tend to occur at the locations where these various primordia of the ventricular septum fuse.
The aorticopulmonary septum is derived from the cardiac neural crest and functions to separate the aortic sac into the aorta and pulmonary artery and to separate the truncus into the aortic and pulmonic valve orifices. This septum is contiguous proximally with the conal septum. The aorticopulmonary septation complex segregates the ventricular outflows from one another just as the septation of the atrioventricular canal segregates the ventricular inflows.
The cardiac electrical system, which is composed of pacemaking cells and a specialized conduction system, develops from regions of specialized myocardial cells that are set aside from working myocardium for this purpose. One of the most important processes involved in the electrical coordination of cardiac function is the electrical isolation of the atria from the ventricles by the fibrous skeleton of the heart. Only the penetrating bundle of His normally electrically connects (via muscle to muscle) the atria and the ventricles. This atrioventricular discontinuity is established by the incorporation of nonmyocardial tissue into the atrioventricular junction.
The epicardium of the heart is derived from an extracardiac population of cells known as the proepicardium. These cells come from the mesenchyme of the septum transversum or liver and literally jump across the coelomic cavity to reach the heart (see e-Fig. 62.1 ). The proepicardium not only forms the definitive epicardium of the heart but also the endothelium and smooth muscles of the coronary arteries, as well as the connective tissue of the heart. The formation of the coronary arteries and the connective tissue of the heart is made possible by a process known as epithelial to mesenchymal transformation. The epicardium of the outflow tract is distinct from that of the remainder of the heart and is derived from the splanchnic mesoderm of the ventral pharynx. Because of the diversity of cells that the proepicardium provides, many investigators consider the proepicardium to be an important potential source of cardiac stem cells.
Before the development of the coronary arteries, the loosely packed myocardium of the embryonic heart is nourished from the cavities by sinusoids. As the myocardium becomes more compact, arteries and veins develop within the epicardial layer, with the intramyocardial circulation developing within the myocardium itself. The arterial channels then grow into the developing aortic valvar sinuses to form true coronary arteries arising from the aorta.
Innervation of the heart is a late development and is not complete until well after birth. Parasympathetic ganglia are intrinsic to the heart and develop from the cardiac neural crest. Sympathetic ganglia are paravertebral and develop from the truncal neural crest. All postganglionic neurons develop from the cardiac neural crest.
The final step in cardiac development is sculpting of the endocardial cushions at the ventricular inflow and outflow to form functional valves. This process occurs at the atrioventricular junction by undermining of the cell layers just underneath the luminal cells to free the leaflets from the myocardium and to create the support apparatus (papillary muscles and chordae tendineae) of the valves. At the ventriculoarterial junction, the sculpting occurs distal to the cushions themselves, and no such support apparatus is formed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here