Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intraoperative neurophysiological monitoring is a valuable tool for improved patient outcomes because it permits the surgeon to evaluate functional changes in structures at risk. Facial nerve monitoring has reached a level of consistency that makes it a state-of-the-art adjunct to lateral and posterior skull base approaches. The techniques for monitoring auditory function continue to evolve. Lower cranial nerve monitoring may be included for other complex neurotologic procedures.
Krause first described facial nerve monitoring in 1912, using a faradic stimulation during cochlear nerve section for tinnitus. The twitching of the ipsilateral facial muscles during stimulation helped him preserve the facial nerve, and the patient had transient facial weakness postoperatively. In the 1960s, dedicated facial nerve monitoring systems were developed. The Hilger stimulator was used principally in the assessment of facial paralysis, but was also used during surgery. Further developments in facial nerve monitoring occurred in the 1970s and 1980s. Delgado et al. described the use of electromyography (EMG) monitoring in cerebellopontine angle (CPA) surgery. Møller and Jannetta combined the specificity of EMG recording with the advantage of acoustic feedback to the surgeon.
The underlying principle for intraoperative monitoring is that some types of injury can be reversed. Although the introduction of facial nerve monitoring has improved the functional outcomes further, especially in medium and large CPA tumors, facial nerve monitoring should never replace anatomical knowledge, technical skill, and clinical judgment. Facial nerve monitoring can be useful to identify the facial nerve when it is not clearly visible in the surgical field. The other benefits include localizing distal nerve fragments in trauma cases and identifying sites of nerve compression, as long as Wallerian nerve degeneration has not completely occurred. The otologic procedures in which the nerve is at risk include cochlear implantation, revision tympanomastoidectomy, and repair of external auditory canal bony stenosis. Monitoring may augment safety of patients in whom the anatomy is altered by infection, trauma, or congenital malformation. It may also be beneficial in training centers where some portions of operations are performed by less experienced surgeons.
Stimulation of a motor nerve by electrical, mechanical, or thermal means results in depolarization of the nerve. A compound muscle action potential is the composite electric activity within the target muscle resulting from synchronous activation of a group of motor neurons within a nerve bundle. EMG monitoring essentially measures this electric activity in the portion of the muscle near the recording electrodes and converts the electric activity to sound via a loudspeaker with or without visual oscilloscope display.
Synchronous activity initiated by electric stimulation produces a biphasic and well-defined waveform. Asynchronous activity, as is generally produced by mechanical stimulation, produces a polyphasic pattern. When these response patterns are converted to sound, electric stimulation results in well-defined pulsed sounds, whereas mechanical stimulation yields a rough, almost scratchy, burst of acoustic energy.
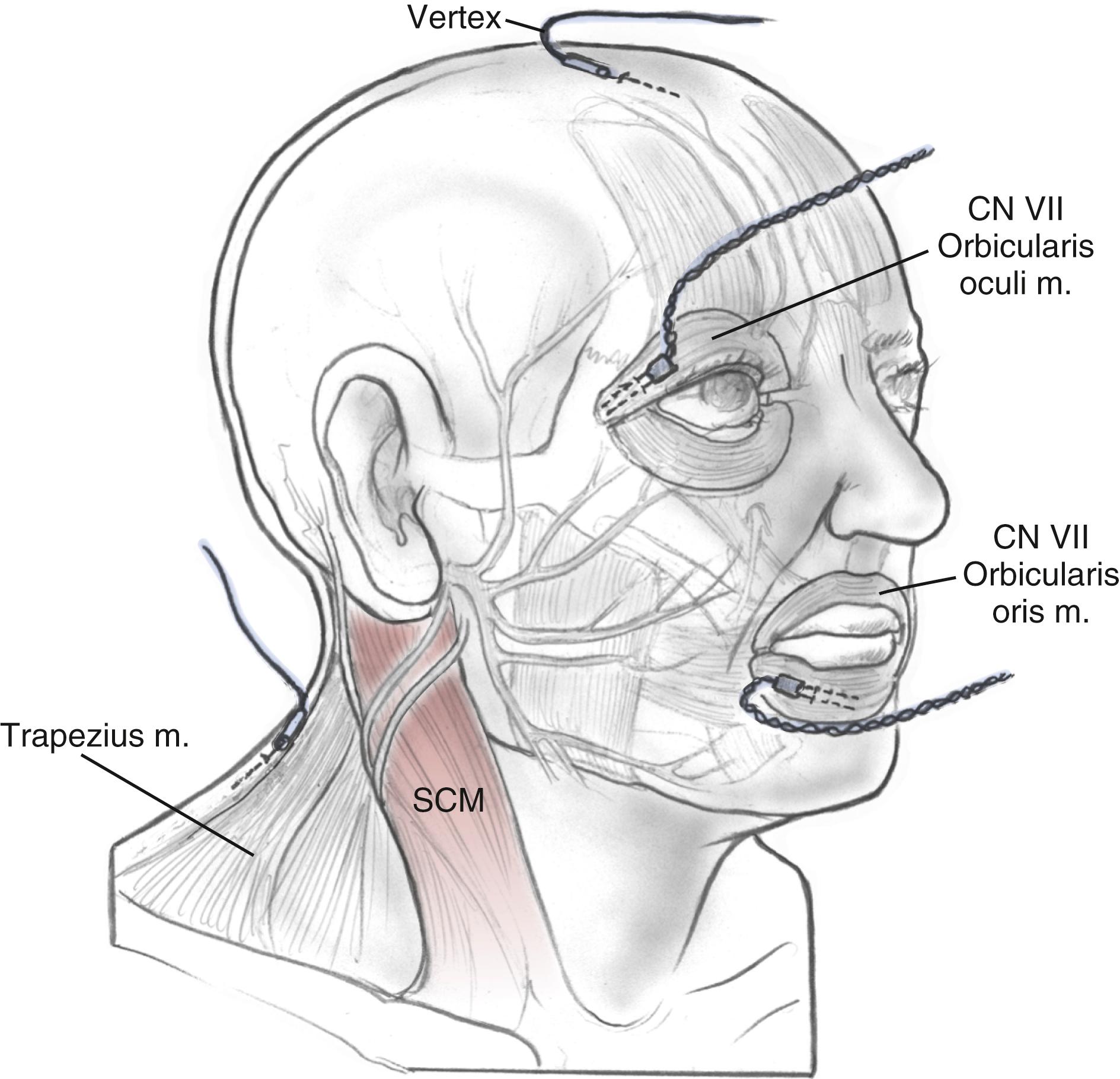
Subdermal needle electrodes are most commonly used for facial nerve monitoring. They have the advantages of ease of use, low impedance, and stability (less likely to be displaced than surface disks or cups). Monitoring more than one muscle provides additional sensitivity and redundancy. For two-channel bipolar recording, a typical sensing montage includes a pair of electrodes in the orbicularis oculi approximately 1 cm apart and another pair in the orbicularis oris ( Fig. 58.1 ). The ground electrode is placed in the forehead, and the anode for the monopolar nerve stimulator is inserted at the ipsilateral shoulder. The operating room is a noisy place with abundant electric interference. The connections are checked by tapping the electrodes and observing an audible and oscilloscopic response. This mechanical compound muscle action potential results in a characteristic sound from the monitor. A second check, when available, involves testing the impedance of the inserted electrodes.
Individual impedance values should be less than 5 kΩ to avoid electromagnetic interference from other devices in the operating room. Current instrumentation uses differential amplification techniques that improve signal/noise ratio optimally when impedance imbalance is less than 1 kΩ between the electrode pairs. Ideally, the imbalance/impedance ratio should be less than 10%. Personnel who perform intraoperative monitoring must be present in the operating room to ensure all equipment is functioning properly.
Many facial nerve monitoring systems are available commercially, including the Nerve Integrity Monitor (NIM; Xomed, Inc.; Fig. 58.2 ) and the Neurosign 100 (Magstim Company). All of these devices use EMG. The Silverstein S8 Facial Nerve Monitor (WR Medical Electronics) is an example of a motion detector device. Some systems, such as the Silverstein Monitor, have the ability to electrify instruments to aid with monitoring.
![Fig. 58.2, Facial nerve monitor screen (Nerve Integrity Monitor [NIM] Response 2.0; Medtronic ENT). Two-channel modes are typical for mastoid surgery. (A) Burst activity, occurring with mechanical stimulation or brief electric stimulation. (B) Pulsatile stimulus. (C) Train activity. Fig. 58.2, Facial nerve monitor screen (Nerve Integrity Monitor [NIM] Response 2.0; Medtronic ENT). Two-channel modes are typical for mastoid surgery. (A) Burst activity, occurring with mechanical stimulation or brief electric stimulation. (B) Pulsatile stimulus. (C) Train activity.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/IntraoperativeNeurophysiologicalMonitoring/1_3s20B978032369427800058X.jpg)
Although stimulation can be applied as either a pulsed or direct current, pulsed stimulation may be safer and more efficacious. The parameters of safe nerve stimulation are 100 to 250 μsec pulses with a range of 0.05 to 0.5 mA. The upper limit of safe nerve stimulation varies along the course of the nerve, but animal studies have shown myelin and axonal injury using 2 mA stimulus for 3 seconds. A 0.5-mA stimulus applied briefly 50 times was found to manifest no functional or histological evidence of injury in a mouse model. Most normal facial nerves should be stimulated with direct contact of the probe using a 100-μsec pulse of 0.05 mA. Settings of 0.05 to 0.1 mA are recommended when working close to the nerve. Farther from the nerve, currents of 0.2 to 0.3 mA may be used. Higher settings may be required when the nerve is covered by bone, connective tissue, or granulation tissue. Cerebrospinal fluid or blood may shunt current from a stimulator probe. In these cases, stimulation with a constant voltage may be used.
In an attempt to determine the threshold necessary to detect a surgical dehiscence of the facial nerve electrically, Choung et al. estimated the minimal threshold of electric current needed to change the EMG of facial muscles using the NIM-2 in 100 patients. Surgical dehiscence of the facial nerves was noted in 43 of 100 cases (43%), and all surgically dehiscent facial nerves responded to 0.7 mA or less of electrical stimulation. The mean threshold of minimal electric stimulation was 0.29 mA for tympanic segments and 0.41 mA for mastoid segments that were dehiscent.
It is crucial that patients be grounded to the monopolar electrocautery unit adequately to permit safe current return. Otherwise, the electrocautery current may find a route through the nerve monitoring electrodes, causing severe burns. All patient connections must be optically and/or electrically isolated to prevent patient injury. This isolation separates the patient from power line voltages and currents. Some monitoring devices achieve this using battery power.
During the course of surgery, many burst potentials may be observed and are usually not associated with significant trauma to the nerve. Bursts are brief discharges in which the stimulus and response are simultaneous ( Fig. 58.2 ). These single, polyphasic responses occur from simultaneous activation of multiple motor units. Burst activity occurring with gentle manipulation suggests a healthy nerve. Very similar responses may be obtained, however, when drilling close to or after complete transection of the nerve. A lack of burst activity during dissection may be associated with minor manipulation of a healthy nerve, significant manipulation of an already injured nerve, or a problem with the monitoring connections and instruments. Electrically stimulating the nerve at this point verifies the integrity of the nerve. Stimulation is ideally performed at a location proximal to the site of manipulation.
Episodes of repetitive EMG activity may occur several seconds to minutes after the stimulus, making it difficult to identify the initiating factor or to modify dissection technique. As seen in Fig. 58.2 , trains are caused by prolonged depolarization of the nerve beyond its threshold for developing an action potential. Subsequent repetitive firing continues until the nerve repolarizes or can no longer sustain the repetitive activation. The most common initiating factor is traction on the nerve. Trains may indicate significant trauma has occurred, although this is not always the case. Changes in temperature around the nerve may also precipitate train activity. When caused by cool irrigating fluid, the spontaneous activity usually subsides with warming. When a train pattern develops after laser application or cautery, however, thermal damage should be suspected. Elevated stimulation thresholds after repetitive nerve activity suggest significant injury. Higher-frequency train activity (>50 Hz) with an amplitude greater than 250 μV is associated with a more ominous outcome; in a series of 51 patients, Nakao et al. found that 86% of patients with train activity having an amplitude greater than 250 μV had severe facial nerve dysfunction. More than 10 seconds of cumulative train time is associated with postoperative facial paresis.
One of these train patterns, particularly the A-train, is associated with postoperative facial paresis following vestibular schwannoma surgery. An A-train is composed of sequential triphasic elements that have a high frequency and homogeneous appearance. The summation of the duration of all A-trains is termed the traintime. Prell et al. demonstrated a high correlation between traintime and postoperative facial nerve function in the short and long term in 30 patients who received vestibular schwannoma surgery. Traintime can easily be monitored in real time using special software, but more importantly, it can be used intraoperatively to estimate the postoperative facial outcomes.
It is common practice that when facial nerve monitoring is employed, neuromuscular blocking agents are avoided after the induction of anesthesia. In 2003, Kizilay et al. examined the effects of different levels of neuromuscular blockade (NMB) on electric stimulation thresholds of the facial nerve during otologic surgery. Minimal facial nerve stimulation causing EMG responses in the facial musculature was measured during recovery from the effects of muscular relaxants and with 25%, 50%, 75%, and 100% levels of NMB. All of the patients had detectable EMG responses of the facial musculature at the 50% and 75% levels of NMB in response to the electric stimulation (mean, 0.1 mA) of the facial nerve. No responses were measured in 31% of the patients when the level of peripheral NMB was 100%. The investigators concluded that a regulated 50% level of peripheral NMB provides reliable intraoperative EMG monitoring of the facial musculature in response to electric stimulation and adequate anesthesia, with full immobilization of the patient. However, chronic injury resulting from compression from a tumor may make the facial nerve more sensitive to the effects of NMB.
Spontaneous repetitive responses may be observed when the level of anesthesia becomes inadequate and the facial muscles contract. This activity is commonly the first indication that the patient is awakening and the prelude to larger movements such as coughing or bucking. The migration of inadequately secured electrodes may create auditory artifacts that mimic the spontaneous activity of light anesthesia.
Monopolar stimulation is especially useful for mapping the nerve throughout its course. Monitoring becomes more important in cases of large tumors. A stronger stimulus may need to be used to confirm that the nerve is not in close proximity to an area of tumor to be removed. False-positive responses may be obtained from stimulation of the superior vestibular and cochlear nerves owing to electric current dispersion, especially within the internal auditory canal where the nerves lie in close proximity to one another. Stimulation of the trigeminal nerve may elicit motor activity that can be interpreted as facial nerve stimulation. False-negative responses are usually related to technical errors such as failure to connect the stimulus probe, anesthetic-induced muscle paralysis, or impedance imbalances.
The electric current dispersion is most effectively overcome by using bipolar stimulation or a flush-tip monopolar stimulator on the lowest possible setting. A useful method to check the monitoring system is to stimulate the nerve where it is known to be available and intact. If no response can be obtained at that location, the entire setup should be inspected from the electrode placements in the facial muscles to the recording instrument. When a reliable response is elicited from the nerve, stimulation may be resumed with confidence.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here