Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intramedullary spinal cord tumors (IMSCTs) are challenging lesions that cause significant neurologic morbidity and mortality in patients of all ages. Gross total resection should be the mainstay of treatment, if possible, as survival is directly related to extent of resection in this subset of patients.
Careful evaluation of imaging to distinguish between the various IMSCTs is essential, and if hemangioblastoma is a possible diagnosis, preoperative vascular imaging is imperative to provide guidance for navigation around the tumor so as to minimize intraoperative blood loss.
Intraoperative monitoring is an important and necessary surgical aid to prevent postoperative neurologic deficits. However, if patients who harbor a holocord lesion with or without the presence of a syrinx lack transcranial motor evoked potentials and reliable D-waves, great caution must be taken, as these are the patients most prone to a poor neurologic outcome after surgical intervention.
Radiotherapy and chemotherapy have limited roles but are not without their own treatment-related morbidities.
Dr. Horsely's attempted surgical resection of an intradural extramedullary lesion that was producing progressive paraparesis in the late 19th century is one of the earliest reports of spinal tumor surgery. His successful resection of the fibromyxoma restored the patient's function and exemplified the potential promise of intradural spinal surgery. A few decades later, pioneers including Dr. Eiselsberg in Austria and Dr. Elsberg in New York began to describe techniques to remove intramedullary spinal lesions. By 1925 Elsberg was advocating a two-stage approach to spinal cord tumors involving laminectomy and myelotomy followed by reexploration and resection of tumor that had herniated out of the myelotomy. Despite these initial successes, lack of technology and high infection rates in the early 20th century hindered the rapid development of intradural spinal surgery. With the advent of microscopy, improved neuroimaging, and bipolar cautery, however, intradural spinal tumor surgery had a resurgence in the latter part of the 20th century, with results involving lower morbidity and improved outcomes.
Intradural tumors constitute a rare subset of tumors of the spine because most spinal tumors arise from extradural or osseous structures. Three main types of tumors exist in the spine: extradural extramedullary, intradural extramedullary, and intradural intramedullary tumors. The latter two make up a minority of spine tumors with an overall incidence of 1 and 2 per 100,000 persons. It is estimated that intradural tumors account for 5% to 10% of central nervous system (CNS) tumors. The most common intradural extramedullary tumors include meningioma and schwannoma; however, the differential diagnosis can be wide ( Table 31.1 ).
| Diagnosis | Magnetic Resonance Imaging Qualities | Signal Characteristics | Demographic |
|---|---|---|---|
| Nerve sheath tumors (schwannoma/neurofibroma/malignant peripheral nerve sheath tumor) | Enlargement of foramina Bony remodeling, with or without cystic features |
T1: Isointense T2: Hyperintense Enhancing |
Adults/pediatrics |
| Meningioma | Dural tail Isointense Circumscribed |
T1: Isointense T2: Isointense Homogeneous enhancement |
Mostly adults |
| Paraganglioma | Characteristic “salt and pepper” appearance Conus/caudal location With or without associated hemorrhage |
T1: Isointense T2: Hyperintense Avid enhancement |
Mostly adults |
| Solitary fibrous tumors | Well circumscribed | T1: Isointense T2: Hypointense Avid enhancement |
Adults |
| Myxopapillary ependymoma | Cauda equina Conus location Expansile |
T1: Iso/hyperintense T2: Hyperintense Enhancing |
Adults/pediatrics |
| Dermoid/epidermoid | Intradural well-defined mass Mimic cerebrospinal fluid intensities Restricting on diffusion (epidermoid) |
T1: Hypointense T2: Hyperintense Nonenhancing |
Pediatrics > adults |
| Metastasis | Variable Dural based Well circumscribed |
T1: Isointense T2: Hyperintense Enhancing |
Adults |
| Neuroblastoma | Heterogeneous lesion with necrotic or cystic areas Not well circumscribed |
T1: Heterogeneous T2: Hyperintense Variable enhancement |
Pediatrics |
Most patients with intradural extramedullary masses present with an insidious onset of symptoms that have gradually worsened over time. Most of these symptoms are due to spinal cord (meningioma) compression, nerve root (nerve sheath tumors) compression, or a combination of both. The constellation of symptoms may include sensory loss, nonspecific back pain, ataxia, motor weakness, and lack of proprioception. Radiculopathy followed by weakness may be more common in patients with nerve sheath tumors. Weakness in the setting of a nerve sheath tumor is a concerning sign for malignancy. Cervical lesions may also present with headaches and occipital pain due to involvement of the upper cervical roots. Clinicians can frequently miss thoracic tumors because they can present with chest wall pain that is often mistaken for referred visceral ailments. Lesions of the cauda equina may produce a local mass effect on lumbar roots that is alleviated when standing (less engorgement of the epidural venous plexus).
A thorough examination of patients with intradural extramedullary lesions is essential. The tumor's spinal level should correlate with the patient's signs and symptoms. Any incongruence in the spinal level should be investigated thoroughly with neuroimaging or nerve conduction studies to assess for additional lesions. A physical examination should also assess for signs of myelopathy including Hoffman signs, clonus, and hyperreflexia. For dorsal midline lesions, there may be an indolent progression of dysesthesia to frank myelopathy. When the lesion is associated with a syrinx, it is not uncommon to notice a suspended dissociated sensory level; however, this may be more conspicuous in patients with intramedullary lesions.
Previously, the diagnosis of intradural extramedullary tumors relied heavily on a detailed clinical examination and a computed tomography (CT) myelogram; however, with the advent of magnetic resonance imaging (MRI), earlier tumor discovery, localization, and tumor characterization have become feasible. CT myelography is now more commonly reserved for patients with contraindications to conventional MRI (eg, those who have pacemakers or foreign ferromagnetic objects).
In the assessment of spinal tumors, contrast MRI permits identification of the lesion and its proximity to adjacent vasculature. Additionally, a lack of contrast enhancement may portend a less ominous diagnosis such as spinal lipomas or arachnoid cysts. For patients with long-standing symptoms or patients with previous tumor resections, plain radiographs can be helpful for assessing sagittal and coronal imbalance or potential instability. Table 31.1 includes an overview of typical characteristic MRI imaging of intradural extramedullary tumors.
In cases with multiple intradural lesions or systemic disease, the use of positron emission tomography (PET/CT) may help to define the degree of metabolic activity within the tumor. Metastatic lesions to the dura such as lymphomas, renal cell carcinomas, or sarcomas may be hypermetabolic on PET/CT imaging; however, the resolution of PET/CT imaging is much poorer than that of conventional MRI.
The mainstay surgical approach for intradural extramedullary lesions is a posterior midline laminectomy that can be manipulated depending on the location of the lesion. Once fluoroscopic localization of the lesion is confirmed, laminectomies generally can be performed without disrupting the facet complex. Lateral extraspinal exposures (thoracotomy, costotransversectomy) are also helpful in some anterolateral-based thoracic lesions. In children, laminoplasties can be performed to maintain the integrity of the posterior columns. Once the bony exposure is complete, adjuvant imaging modalities such as ultrasound can help guide the dural opening. The dura can be opened widely and tacked up to the adjacent musculature. Depending on the adherence and tumor interface, an attempt at en bloc resection can be made. If the dissection planes are poor, piecemeal resection with bipolar cautery or ultrasonic cavitation can be performed. Maximal safe resection is always the goal of intradural tumor surgery; however, in some cases, sacrifice of a sensory nerve root is necessary for complete removal. Because of the risk of severe neurologic sequelae, intraoperative monitoring and appropriate preoperative counseling are crucial.
Meningiomas are the most common type of intradural extramedullary tumors, and 80% of all cases affect women. Patients classically present with myelopathy or sensory disturbances. CT imaging of meningiomas may demonstrate some degree of calcifications, and most meningiomas homogeneously enhance ( Fig. 31.1 ). Thoracic (70%–75%) and cervical (20%) meningiomas are the most common locations for intraspinal meningiomas. Although the pathophysiology is unclear, some authors have suggested that meningiomas arise from dural fibroblasts in the arachnoid layer. At times, meningiomas can be highly vascular; therefore careful evaluation of the preoperative imaging and adjacent vasculature is essential. In the spine, histology is typically benign, and gross total resection is the mainstay of treatment. With gross total resection, recurrence rates can remain quite low. Close follow-up is recommended with serial imaging if there is concern for a high Ki-67 mitotic index or atypical histology.
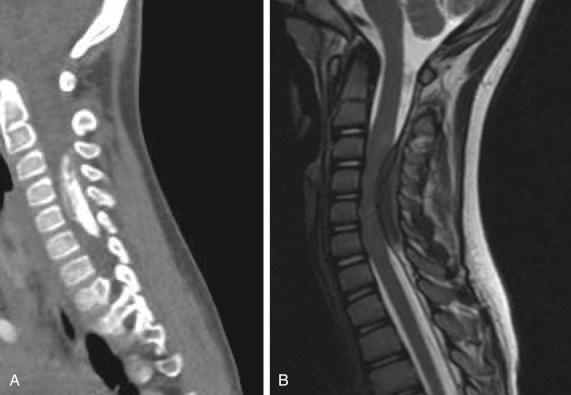
Given the histology, typically meningiomas can be removed by a combination of lesional cautery, debulking, and resection of the dural attachment. Meningiomas can frequently be calcified, which may complicate surgical excision; in these cases, ultrasonic cavitation may be helpful. If the tumor is densely adherent to adjacent neural elements, complete resection should be avoided. Evidence suggests that subtotal resection or coagulation of the dural attachment (Simpson grade II) should be considered to avoid neurologic injury in patients with difficult-to-treat spinal meningiomas. A complete tumor removal would necessitate excision of the dural attachment, which would demand a dural patch. Duraplasty with cadaveric dura and fibrin glue can be used to repair the dural defect in a watertight fashion in these cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here