Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
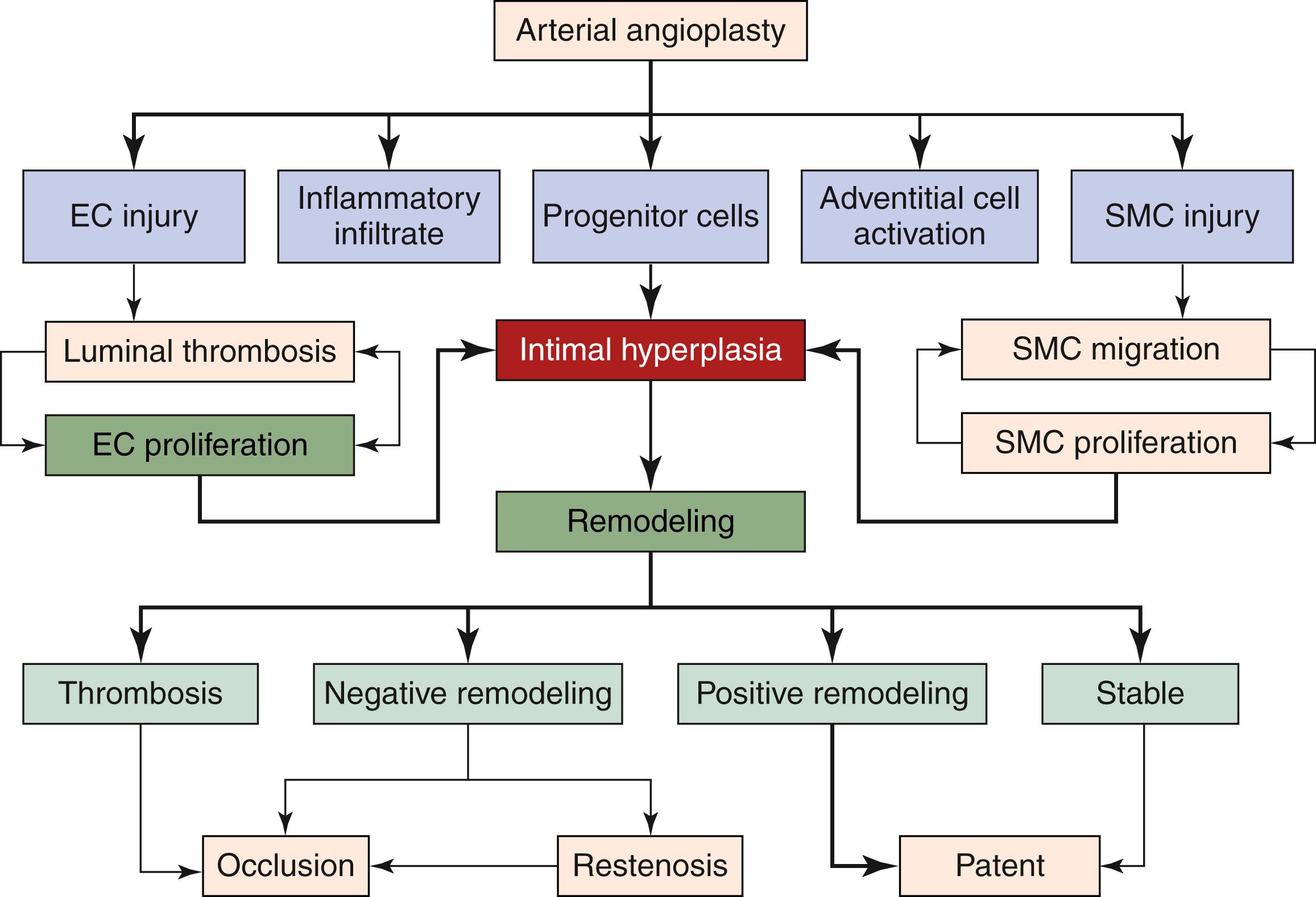
Intimal hyperplasia represents the healing response to vascular injury as well as the adaptive response to developmental or physiologic events. Injury induces a progressive structural change within the blood vessel lumen that begins with the disruption of endothelial barrier function and damage to the underlying medial smooth muscle cells, initiating platelet deposition and inflammatory changes. These changes begin a cascade of events that lead to smooth muscle cell migration and proliferation with associated extracellular matrix deposition that ultimately results in neointimal lesion formation that compromises blood vessel patency. Its development can be chronologically subdivided into hyperacute, acute, and chronic stages, reflecting the timelines of classical wound healing ( Table 5.1 ). Macroscopically, this lesion appears firm, pale, and homogeneous and is located in the subintimal location between the endothelium and the internal elastic lamina of an artery. Intimal hyperplasia may be focal at a site of injury or at an anastomosis or it can be diffuse in nature. While clearly adverse in the setting of vascular injury, intimal hyperplasia and vascular remodeling are necessary responses during development and growth. It plays an essential role in important developmental events including the closure of the ductus arteriosus and the ductus venosus after birth. It is also important in the normal growth of arteries. Similarly, vein bypass grafts undergo adaptive remodeling when taken out of the venous circulation and placed into the arterial system. The biology of intimal hyperplasia is complex and it still remains unclear how an adaptive response becomes pathologic. Adaptive and pathologic intimal hyperplasia, such as following therapeutic injury and bypass surgery, share many common mechanisms (coagulation, inflammation, cell proliferation, cell migration, proteases and extracellular matrix [ECM], and remodeling) but also have unique characteristics.
| Vessel Lumen | Vessel Wall | |
|---|---|---|
| Stage 1 | ||
| Hyperacute (minutes–hours) | Endothelial cell denudation Platelet aggregation Release of growth promoters |
SMC injury Activation of SMC Proto-oncogene expression Release of growth promoters |
| Stage 2 | ||
| Acute (hours–weeks) | Organization of thrombosis Endothelial cell ingrowth Release of growth inhibitors Progenitor cell deposition |
Medial SMC replication Medial SMC migration Infiltration of leukocytes Infiltration of adventitial cells Infiltration of progenitor cells Synthesis of growth promoters Synthesis of growth inhibitors |
| Stage 3 | ||
| Chronic (weeks–months) | Reendothelialization change of luminal dimensions | Intimal SMC replication Intimal SMC synthesis of ECM Remodeling of ECM Synthesis of growth inhibitors Vessel remodeling |
Vascular remodeling that occurs as normal adaptive responses is evident during the developmental process. It is essential to change fetal circulation to the post-fetal configuration. The functional change that directs blood through the pulmonary and systemic circulation is driven by changes in vascular resistance and hypoxia that leads to vasoconstriction of the ductus arteriosus and ductus venosus. These changes occur rapidly to eliminate blood flow through these vessels. The anatomic closure of these structures occurs over the subsequent few weeks. The hypoxia in these excluded vessels stimulates medial smooth muscle cell apoptosis followed by the release of growth factors that simulate intimal hyperplasia and fibrosis that result in the obliteration of these vessels.
During growth and aging, arteries increase in diameter and wall thickness. Between the ages of 20 and 90 years, the arterial intimal medial thickness increases nearly threefold even in the absence of atherosclerosis. , While medial thickness remains stable during the aging process, the intimal thickness gradually increases with age. Outward remodeling results in growth in lumen size despite the intimal hyperplasia, adapting to the changes associated with aging. It is thought that lower rates of intimal hyperplasia are consistent with less aging while higher rates suggest accelerated aging. In the presence of disease such as hypertension and diabetes, the intimal thickness and composition of the arterial wall change to include more extracellular matrix and result in increased stiffness.
Most forms of arterial injury arise from therapeutic interventions such as angioplasty or arterial bypass, which create local areas of vessel wall injury. Angioplasty creates a controlled focal injury to the vessel wall ( Figs. 5.1 and 5.2 ) and is the basis of a popular experimental model of intimal hyperplasia. The immediate response of the vessel to injury is to achieve hemostasis. Endothelial injury and denudation expose the subendothelial matrix which leads to platelet adherence and aggregation. Platelet accumulation occurs rapidly for approximately 8–10 hours after injury. The use of aspirin and other antiplatelet agents following therapeutic vascular interventions is aimed at reducing this early platelet response. Platelet levels on the vessel surface decrease from days 3 to 7. Platelets interact with subendothelial collagen through platelet membrane glycoprotein receptors (GPIb, GPIc/GPIIa, and GPIa/GPIIa), plasma von Willebrand factor, and fibronectin. Glycoprotein IIb/IIIa inhibitor drugs are used to reduce platelet aggregation following percutaneous coronary interventions. , The coagulation cascade (tissue factor, factor V, factor VIIa, factor Xa, or α-thrombin) is also activated and contributes to the initiation of intimal hyperplasia. Controlling the coagulation cascade early is key to preventing early stent thrombosis. Following injury, apoptosis (programmed cell death) can be identified in the cells in the arterial intima and media within 1 to 2 hours and abates after 4 hours. It then increases again by day 7 with 50% of medial cells undergoing apoptosis, potentially linked to increased proliferation at that time. By day 14, apoptosis is again markedly decreased.
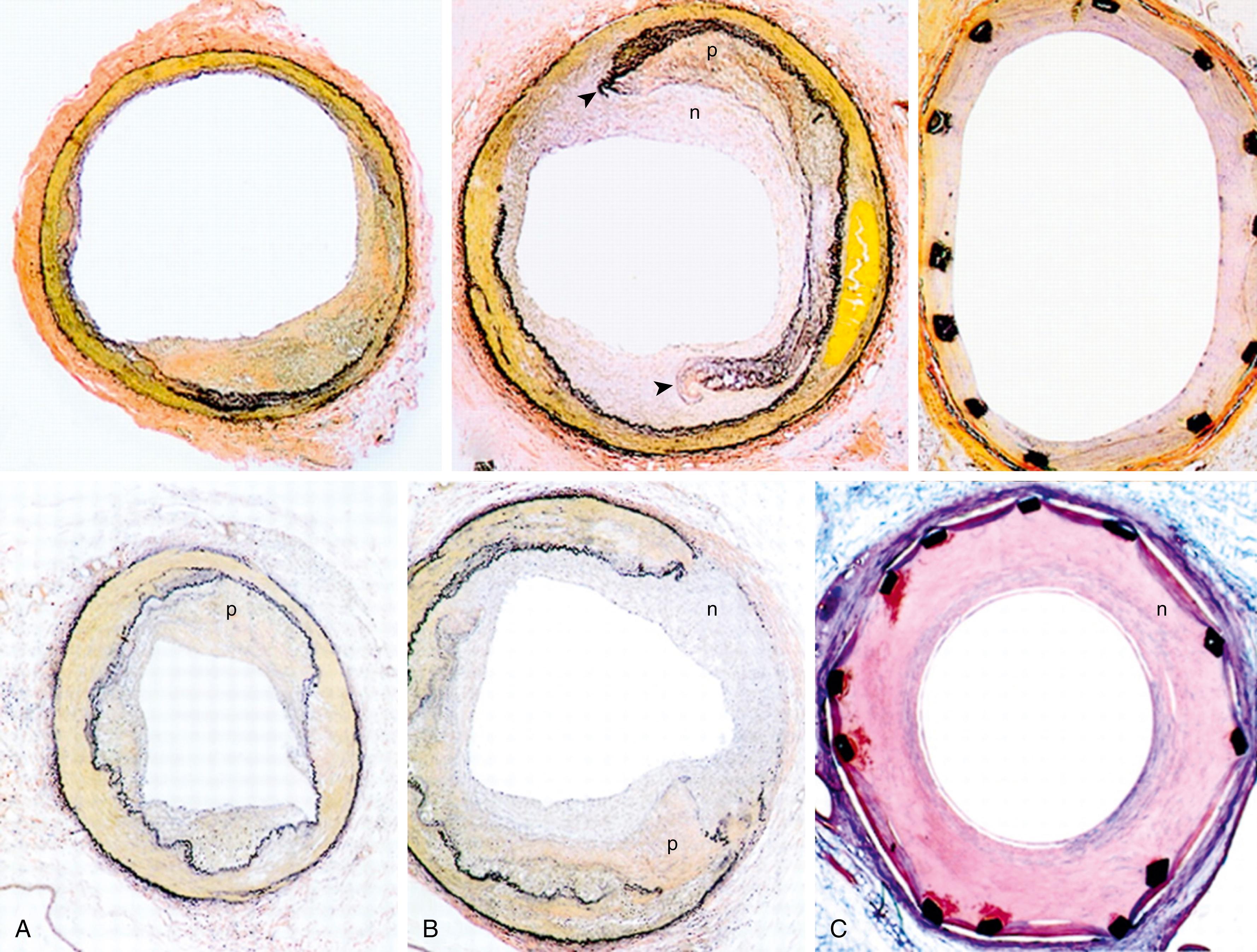
Following these early events, a robust inflammatory response ensues with the recruitment of polymorphonucleocytes (PMNs) and monocytes to the site of injury by the adherent platelets, activated endothelial cells, and the exposed matrix and smooth muscle cells. There is a sequential expression of inducible cell surface molecules in both endothelial cells and smooth muscle cells after experimental angioplasty. , Compared with the normal endothelium, injured and activated endothelial cells express high levels of vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM). Within 10 days, smooth muscle cells express both ICAM and MHC class II antigens. The upregulation of these adhesion molecules returns to baseline levels by 30 days post-injury. Chemokines and their receptors participate at every step of the vascular remodeling process. These chemokines signal monocytes to infiltrate into the injured vessel wall, further stimulating inflammation and smooth muscle cell proliferation. The monocyte chemotactic protein (MCP)-1/CC motif receptor 2 (CCR2) axis induces monocyte infiltration and smooth muscle cell proliferation. The RANTES (regulated upon activation, normally T-cell expressed, and presumably secreted) receptors CCR1 and CCR5 also regulate monocyte infiltration and neointimal growth. Reendothelialization and intimal growth is mediated by the chemokine CXCL1, which is augmented by stromal cell-derived factor-1 alpha (SDF-1α) and its receptor CXCR4 that recruit circulating progenitor cells as well as medial smooth muscle cell progenitors to the subintimal location.
Cytokines including tumor necrosis factors (TNF), interleukins (IL), lymphokines, monokines, interferons, colony-stimulating factors, and transforming growth factors (TGFs) are produced by both infiltrating inflammatory cells and injured cells of the vessel wall. These cytokines propagate the inflammatory response, cell adhesion, proliferation, migration, and apoptosis. Cytokines are also linked to increased mitochondrial reactive oxygen species production, activation of Ca 2+ , and multiple intracellular protein kinase pathways. Cytokines interact with integrins and matrix metalloproteinases (MMPs) to modify extracellular matrix composition, a key component of the developing neointima. The inflammatory response to arterial injury can be modulated by inducing changes in macrophage phenotype with a reduction in intimal hyperplasia. Administration of low-dose inhaled carbon monoxide (CO) for a brief exposure prior to angioplasty injury results in a significantly attenuated intimal hyperplastic response by modifying leukocyte function. In humans, targeted therapy against IL1α showed a trend toward reduced restenosis following SFA interventions. Exogenous granulocyte colony-stimulating factor (GF) increased circulating endothelial progenitor cells (EP) and increased reendothelialization, resulting in reduced vascular inflammation and neointima size.
Medial smooth muscle cells are normally quiescent with <1% in a proliferative state. This increases to >20% within 48 hours after injury. The exact mechanisms by which vascular injury induces and promotes smooth muscle cell proliferation remains an area of investigation. The first phase of smooth muscle cell proliferation appears to be driven by basic fibroblast growth factor (bFGF) released from dead and damaged cells in the injured vessel. Extracellular matrix degradation by matrix metalloproteinases (MMPs) allows the medial smooth muscle cells to migrate into the subintimal space. Smooth muscle cell migration is regulated by receptor tyrosine kinase-linked agonists (PDGF, bFGF, and hepatocyte growth factor) and G-protein coupled receptor agonists (vascular endothelial cell growth factor, chemokines, LPA, thrombin, and uPA). Inhibition of the PDGF receptor PDGFR-β/β with blocking antibodies inhibited intimal hyperplasia in animal models but was not effective in human trials. Circulating mesenchymal stem cells also contribute to the subintimal cells, differentiating into smooth muscle cells.
Once within the subintima, the smooth muscle cells begin to proliferate around day 7 and reach a peak at 14 days before returning to baseline by 28 days when vascular healing is complete. However, proliferation may continue for up to 12 weeks in areas where reendothelialization is delayed. While the total number of neointimal smooth muscle cells stabilizes after 12 weeks, the neointima continues to grow through the elaboration of extracellular matrix (collagen and proteoglycans) by the smooth muscle cells. In human intimal lesions, hyaluron content was inversely related to collagen I and III staining. Little matrix accumulation occurs over the first 1 to 2 months following balloon injury but dramatically increases from 3 to 6 months in restenotic human coronary arteries. As collagen increases in the ECM, the intimal and adventitial smooth muscle cells shift to a contractile phenotype, leading to arterial contraction and “negative” remodeling.
Myofibroblasts represent an important cell population in intimal hyperplasia. A marked infiltrate of myofibroblasts, some derived from circulating mesenchymal stem cells, is observed by day 2 and may represent up to 50% of cells within the intima by day 14. , The presence of myofibroblasts is common in wound healing and contributes to wound contraction. A similar phenomenon may occur in the healing vessel. Injured vessels may undergo chronic elastic recoil or negative remodeling, reducing lumen size without increasing neointimal area. Retrieved atherectomy specimens from restenotic lesions showed low proliferative activity but the smooth muscle cells from these lesions exhibited elevated migratory activity and collagen synthesis. These findings support the important role of intimal remodeling in the final determination of luminal diameter. ,
Traditionally, the regulation of vascular development and response to injury has been attributed to the endothelial cells and the medial smooth muscle cells in an “inside-out” fashion. The adventitia was believed to serve as structural support and transporting nutrients as well as maintaining sympathetic innervation to the vessel wall. It is now recognized as a source of cells that regulate all the layers of the vessel wall, supporting an “outside-in” hypothesis of vascular inflammation and healing. The cells in the adventitia include fibroblasts, smooth muscle cells, adipocytes, pericytes, and resident inflammatory cells. It is rich in stem and progenitor cells that can migrate and differentiate into a variety of cells in the media and neointima during vascular healing and disease. The adventitia is also home to the vasa vasorum, lymphatics and perivascular nerves. These cells and structures are embedded in an extensive network of extracellular matrix rich in collagen, elastic fiber nets, proteoglycans, fibronectins, and tenascin-c. The adventitial collagen fibers protect the vessel from over distention at high pressures while physiologic responses are mediated by the medial elastin layers. Evidence supporting outside-in healing is that early after injury, adventitial myofibroblasts express high levels of signals including monocyte chemoattractant protein-1 (MCP-1) that recruit and activate monocytes to the adventitia. Following arterial injury, adventitial fibroblasts become activated through sonic hedgehog signaling and increased reactive oxygen species production from upregulated NADPH oxidase and leads to increased neointima formation. The adventitial myofibroblasts, while contributing to the neointimal cells, are also important for negative remodeling through vascular contraction. Finally, aging results in increased resident inflammatory cells in the adventitia and may explain the greater susceptibility of aged arteries to atherosclerosis and intimal hyperplasia.
The degree of intimal hyperplasia that develops in a vessel is dependent on the degree of injury. Intimal proliferation is minimal when the media is uninjured but increases in proportion to the depth of the medial injury, indicating that the severity of smooth muscle cell injury regulates the magnitude of the proliferative response. , Further evidence suggests that smooth muscle cell distention without endothelial cell injury can also stimulate smooth muscle cell proliferation. The length of the injury correlates with the extent of endothelial injury. Reendothelialization occurs from the edge of the denuded area and possibly from the endothelial cells of the vasa vasorum. Until reendothelialization is complete, the underlying smooth muscle cells are without the modulating influence of homeostatic endothelium derived factors such as nitric oxide and prostacyclin. , After severe arterial wall injury, luminal compromise results from neointima formation as well as negative remodeling. Medial damage is accompanied by massive proliferation of adventitial myofibroblasts that mediate negative remodeling.
Changes in hemodynamic parameters affect both normal and diseased vessels. Clinical studies suggest that femoral angioplasty in patients with compromised outflow is associated with increased restenosis. Hehrlein et al. confirmed that reduced vascular runoff increased intimal hyperplasia following angioplasty. Blood flow and shear stress are best associated with the development of intimal hyperplasia, whereas circumferential deformation of the vessel wall correlates with medial thickening. Kohler , reported reduced intimal thickness with increased flow while increased intimal hyperplasia occurred with decreased flows, suggesting a direct impact of flow on smooth muscle cell function. However, they did not detect an ability of flow changes to alter established neointimal lesions.
The development of intimal hyperplasia in diseased blood vessels is exaggerated compared to normal arteries subjected to injury. In animal models of hyperlipidemia, balloon injury increased vascular inflammation and intimal hyperplasia. The resultant neointimal lesions had significant components of atheroma formation. Clinically, similar associations between hyperlipidemia with higher rates of restenosis have been reported. , Hyperlipidemia may result in the expansion of a subset of CD14/CD16 rich monocytes that may target sites of vascular injury more significantly. These cells have increased myeloperoxidase activity that contributes to increased oxidative stress and promotes further inflammation and the proliferation and migration of smooth muscle cells. Risk factors that contribute to atherogenesis also increase intimal hyperplasia. Cigarette smoke increases experimental intimal hyperplasia by twofold while cholesterol reduction with statins has been shown in some studies to reduce restenosis. , However, there is also evidence that statins improve outcomes through other mechanisms but do not impact rate of restenosis.
Diabetes is a predictor for augmented intimal hyperplasia and restenosis in response to vascular injury. In animal models, diabetes is associated with increased inflammation and intimal hyperplasia following injury. In the setting of metabolic syndrome or pre-diabetes, arterial injury upregulated adhesion molecules ICAM-1 and P-selectin with increased macrophage infiltration of the arterial wall and increased neointima formation. The expression of oxidized LDL receptor was also upregulated. Advanced glycosylation end products (AGEs) accumulate in blood vessels during aging and is further enhanced in diabetes. AGEs are particularly abundant at atherosclerotic lesions. AGEs interact with specific receptors (RAGEs) present on inflammatory cells and smooth muscle cells to stimulate inflammation, smooth muscle cell proliferation and migration, and ECM production. RAGE expression is upregulated in diabetes as well as following arterial injury. RAGE is also a receptor for other ligands released from injured cells including damaged associated proteins such as HMGB1 and S100. Inhibition of RAGE reduced neointima formation in nondiabetic and diabetic animals. , Higher rates of restenosis are observed in diabetic humans. Diabetic patients with restenosis have a higher rate of subsequent in-stent restenosis. Atherectomy specimens collected from restenosis lesions from diabetic patients had lower cellularity and increased collagen rich matrix than nondiabetic patients. These findings suggest that negative remodeling and recoil may contribute to restenosis in diabetes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here