Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although many patients encountered in clinical practice complain about intestinal gas, systematic investigation of this subject did not begin until the 1960s, when studies by Michael Levitt, MD, and colleagues began to shed light on the pathophysiology of intestinal gas.
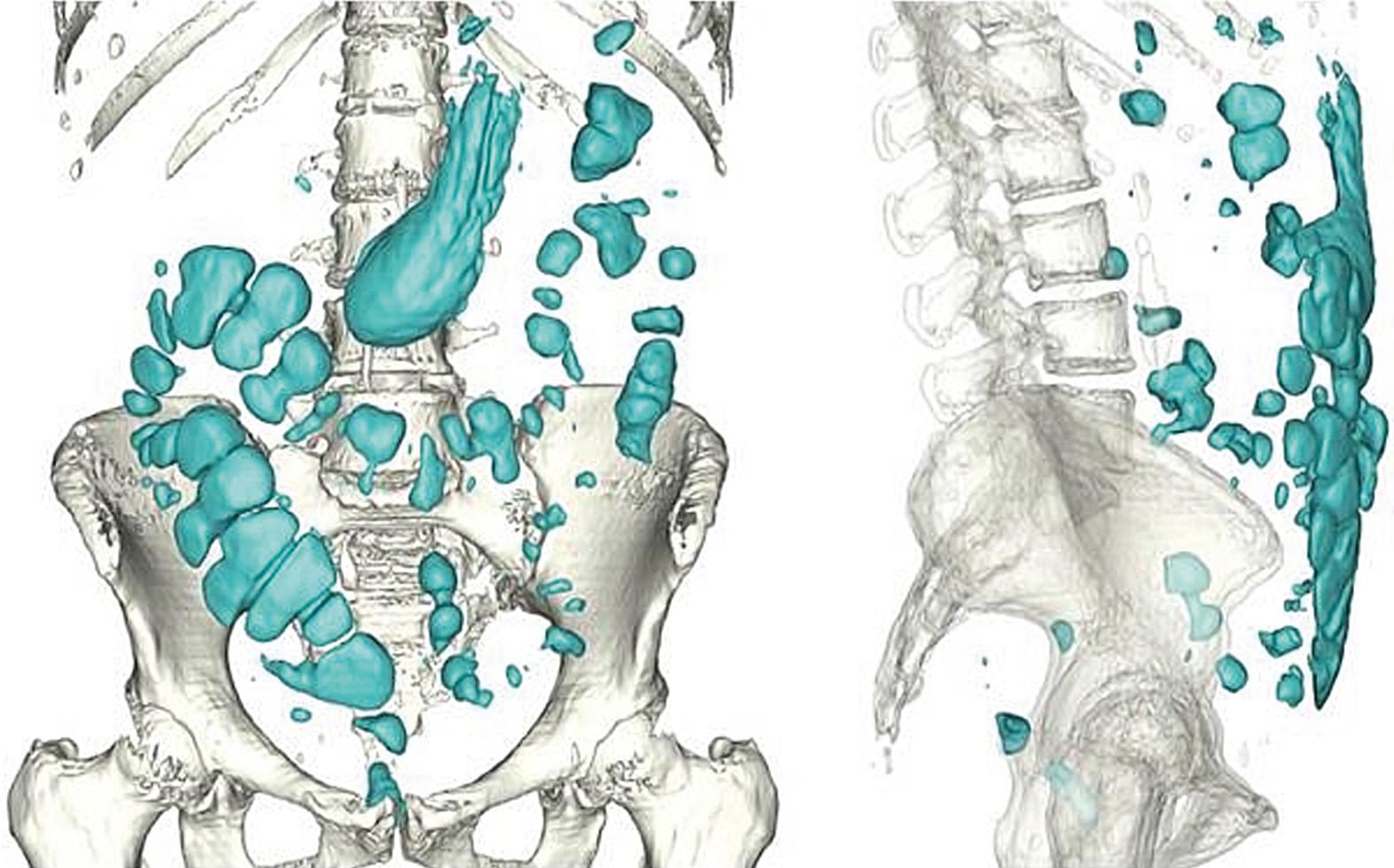
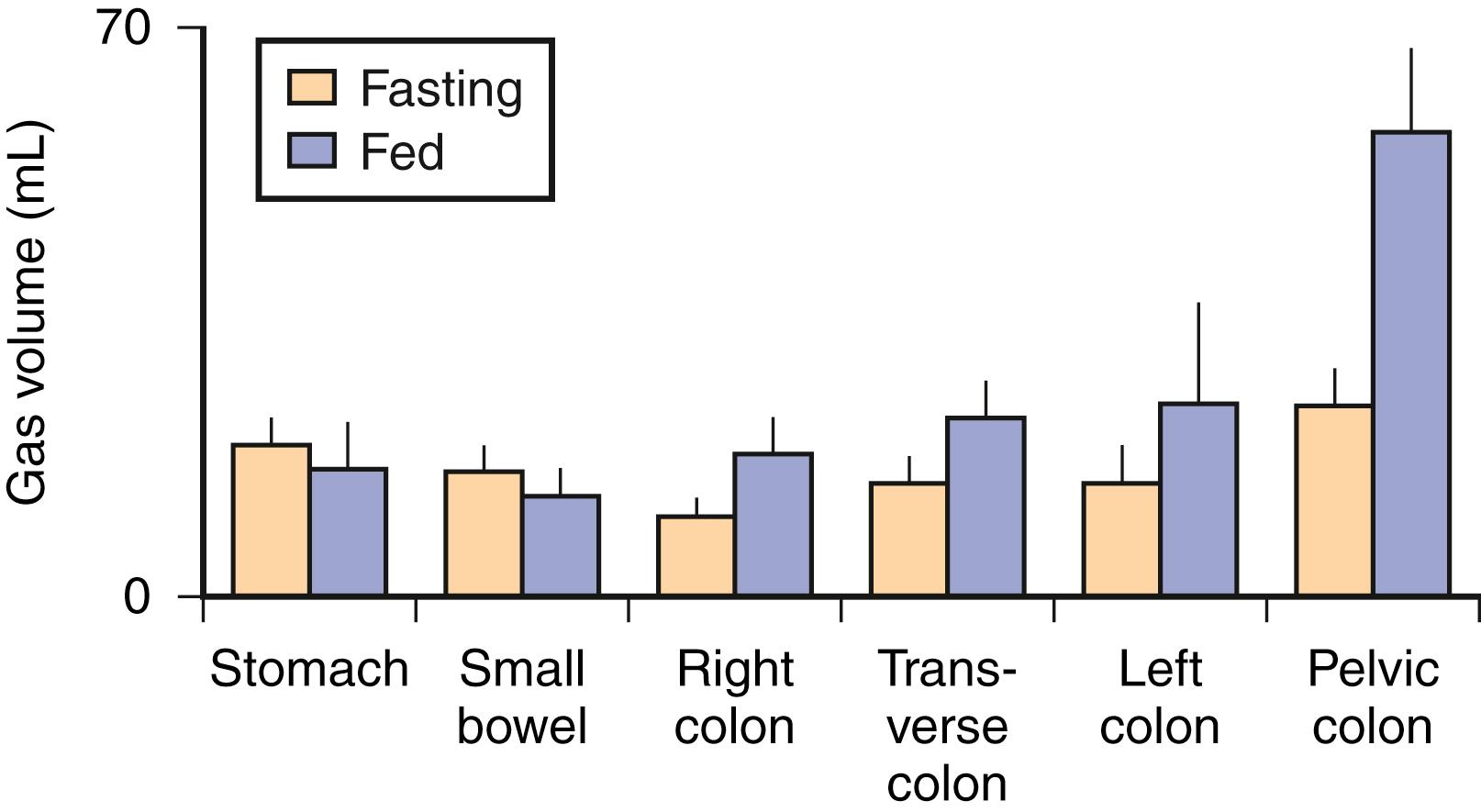
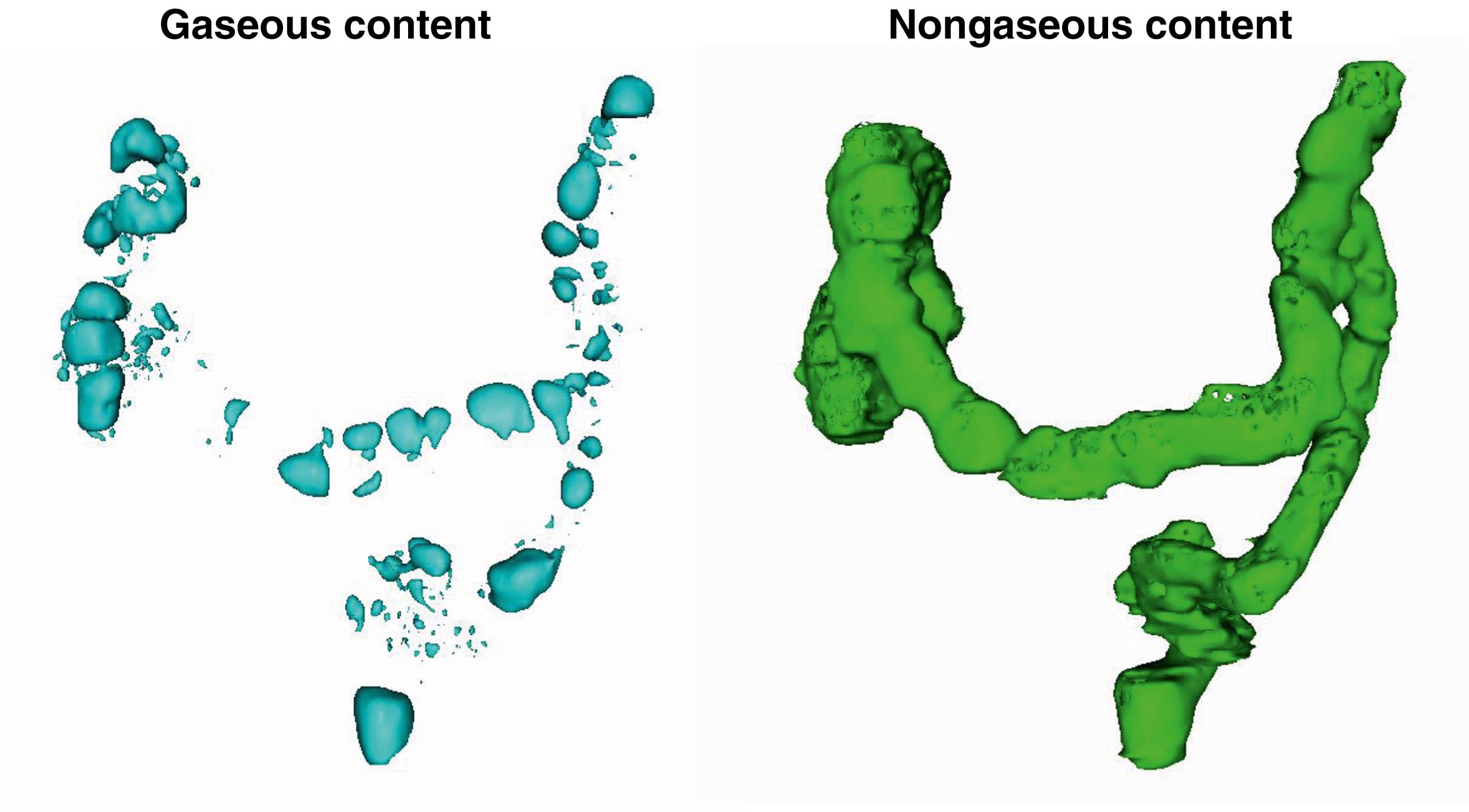
The GI tract normally contains a relatively small amount of gas. The volume of gas within the intestinal lumen is determined by the balance between gas input and output, a highly dynamic process. Gas input may result from swallowing, chemical reactions, bacterial fermentation, and diffusion from the blood, whereas output involves belching, bacterial consumption, absorption into the blood, and anal evacuation. Despite this variety of factors, the volume of gas within the GI tract is remarkably constant and similar among healthy persons, suggesting that it is under tight homeostatic control. Measurements of intestinal gas volume have been performed using a specifically designed CT technique. Data acquisition is relatively simple, but analysis is fairly elaborate and has been carefully validated. This technique has confirmed previous measurements by other techniques showing about 100 to 200 mL of gas in the fasting state and somewhat larger volumes (65% increase) after ingestion of a meal ( Figs. 17.1 and 17.2 ).
Analysis of gas composition is technically challenging, and still only few and relatively old data are available. In a study of 10 healthy fasting subjects, the overall composition of gas within the GI tract was assessed using a washout technique in which a rapid infusion of argon into the jejunum via an intraluminal tube was used to flush out the intestinal gases. Gas effluent was collected via a rectal tube and analyzed. Five gases—N 2 , O 2 , CO 2 , H 2 , and methane (CH 4 )—were found to account for more than 99% of intestinal gas; many additional gases were present in trace concentrations. During fasting, N 2 was usually predominant, O 2 was present in low concentrations, and the concentrations of CO 2 , H 2 , and CH 4 were highly variable. The latter 3 gases are related to fermentation of meal residues and may predominate in the postprandial period (see later).
GI gas is distributed in 3 compartments: stomach, small intestine, and colon. In each compartment the volume and composition of gas depend on gas metabolism and diffusion of gas between the lumen and blood. Part of the gas in one compartment is propelled to the next, and the end product is evacuated per anus. Fig. 17.3 schematically depicts gas homeostasis in various segments of the GI tract.
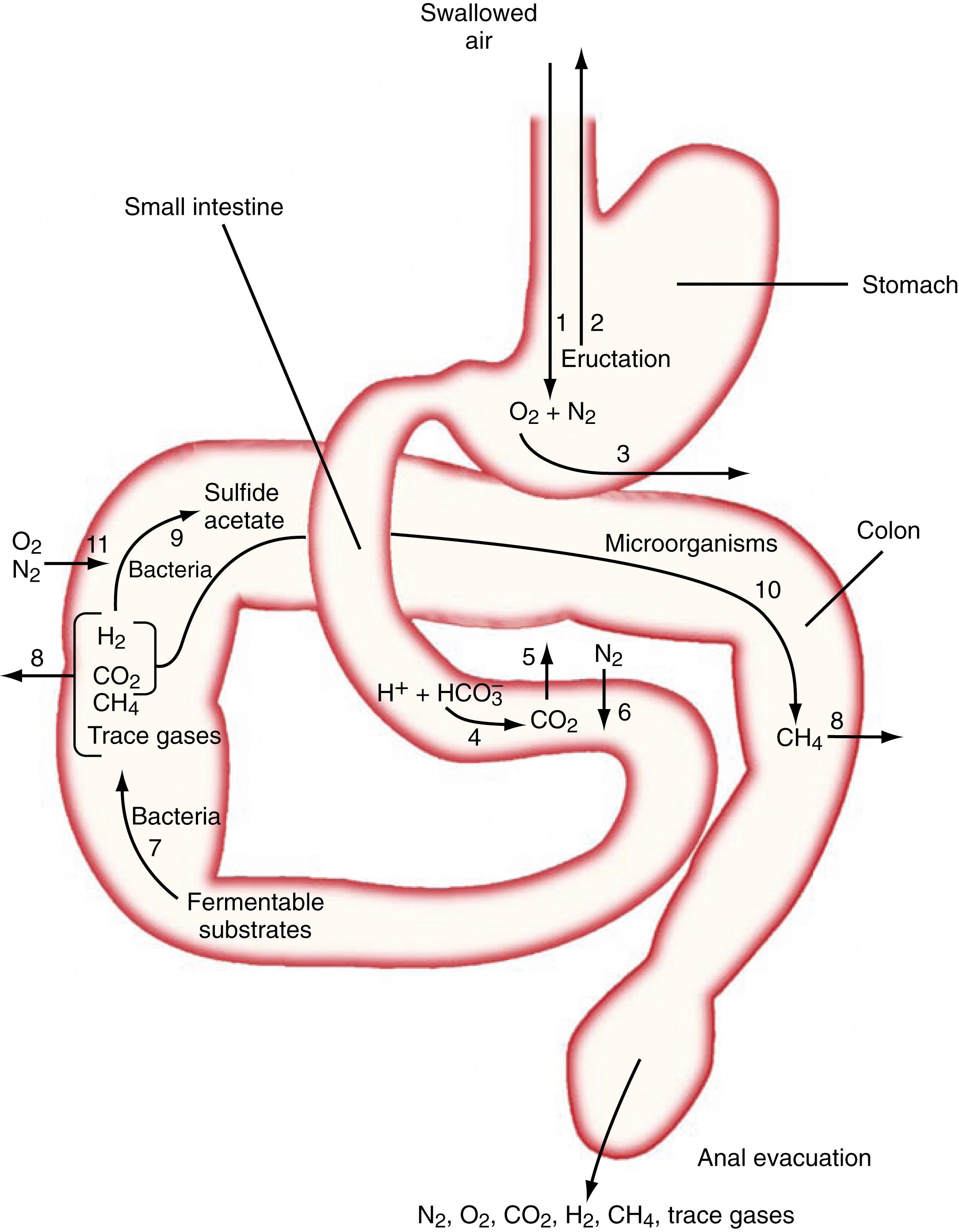
The rate and direction of diffusion of each gas is a function of the diffusivity, partial pressure difference between lumen and blood, and exposure of the gas to the mucosal surface. The diffusivity of a gas across the mucosa of the GI tract depends on its solubility in water. For a given partial pressure difference, CO 2 diffuses much more rapidly than H 2 , CH 4 , N 2 , and O 2 . Luminal gases with a partial pressure (concentration) higher than that in venous blood pass into the circulation and vice versa. Gas absorption also depends on the extent of the mucosal area and the time of exposure. H 2 and CH 4 absorbed from the bowel are not metabolized by the host and are excreted in expired air. Breath analysis provides a simple means of estimating the production of these gases in the GI tract. Breath excretion of these gases is the product of the alveolar ventilation rate and their alveolar concentrations. Because alveolar ventilation is relatively constant in sedentary conditions, the end-alveolar breath concentration of H 2 and CH 4 can be used as a simple indicator of their total breath excretion.
The stomach normally contains a relatively small amount of gas (≈10 to 20 mL). Its location within the stomach is determined by flotation and gravitational forces. In the upright position, gas forms a bubble in the fundus of the stomach. By contrast, in the supine position, gas forms a thin film lining the anterior wall of the gastric corpus and antrum close to the abdominal wall (see Fig. 17.1 ). Air swallowing (rather than intraluminal production) is believed to be the major source of stomach gas, as suggested by the absence of the gastric bubble in patients with advanced achalasia (see Chapter 44 ), but the normal amount of air swallowing is not clear. Gas leaves the stomach via belching, absorption, or emptying into the duodenum. The belching process has been well documented, but there is hardly any information related to the passage of gases across the gastric mucosa or the emptying of gas into the duodenum. Given the lower proportion of CO 2 and a higher proportion of O 2 in swallowed air compared with that in blood, in theory, CO 2 should diffuse from blood into the stomach bubble and O 2 from the lumen to blood. Because N 2 diffuses poorly across the mucosa, luminal N 2 throughout the GI tract presumably derives from swallowed air.
From 10 to 20 mL of gas is normally present in the small intestine, usually in the form of small bubbles scattered along the intestinal lumen. Radiologic images of intestinal air-fluid levels in the erect position indicate that liquid is present below the gas bubble and is seen only with abnormal fluid retention, as in an ileus or obstruction (see Chapters 123 and 124 ). Theoretically, in the upper small intestine, a large amount of CO 2 is liberated from the interaction of bicarbonate and acid; however, studies using CT-based volumetric analysis did not detect changes in gas volume within the small bowel in the postprandial period. The gas produced should diffuse into the blood (which is plausible for CO 2 ) or be transported to the colon. An increase in colonic gas, although relatively small, is observed after a meal (see later). Gas production by small bowel microbiota is considered negligible in normal conditions, but direct evidence of this conclusion is lacking. Patients with intestinal obstruction or pseudo-obstruction (impaired motility) develop large amounts of gas within the small bowel, but the origin and mechanisms of this accumulation remain unclear.
The colon normally contains around 50 to 100 mL of gas (see Fig. 17.2 ). A study comparing gas content based on CT images taken during fasting and 99 ± 22 minutes after a meal showed an increase in gas in the pelvic colon (see Fig. 17.2 ), earlier than would be expected for gas derived from colonic fermentation of food substrates. Therefore, gas of proximal GI tract origin is presumably propelled into the colon after ingestion of a meal, possibly by a gastroileal reflex. However, colonic gas originates primarily by the metabolic activity of the microbiota and is eliminated by mucosal absorption, microbiota gas consumption, and anal evacuation. With the growing interest in intestinal microbiota, the study of intestinal gas production and evacuation has become particularly important because it reflects the metabolic activity of intestinal microbiota.
The upper part of the digestive tract primarily has a nutritional function: useful components, such as nutrients, water, and minerals, are extracted from ingested material by a process of digestion and absorption. Nonabsorbed residues pass into the colon and serve as substrates for colonic microbiota, which carry out key functions related to host development and homeostasis (see Chapter 3 ). Therefore, the colon serves as a kind of “marsupial pouch” that provides the appropriate environment to host the largest proportion of the body’s microbiota and thereby serves as a complex metabolic organ.
The metabolism of fermentable meal residues by microbiota results in the release of a series of metabolites, including gases, that in turn serve as substrates for other subsets of microbiota. Therefore, the colon contains an active mass of living matter that consists of microbiota, meal residues, and secondary metabolic products in a dynamic chain of metabolic reactions. Studies have shown that the colon contains a biomass of 500 to 800 mL, depending on the residues of the diet, with a daily dynamic turnover of 100 to 200 mL, which is the volume of fecal output ( Fig. 17.4 ). Gas production increases as soon as fermentable residues enter the colon, and the activity declines gradually, lasting for hours as long as substrates remain available, so that the residue loads of consecutive meals contribute to gas production ( Fig. 17.5 ). Therefore, basal gas production during fasting depends on the content of the colon, which is derived from the diet on previous days. The volume of gas produced depends on the amount of unabsorbed, fermentable residues present in the diet, although knowledge about the specific foodstuffs and products in the diet that contribute to intestinal gas production is limited and largely empiric.
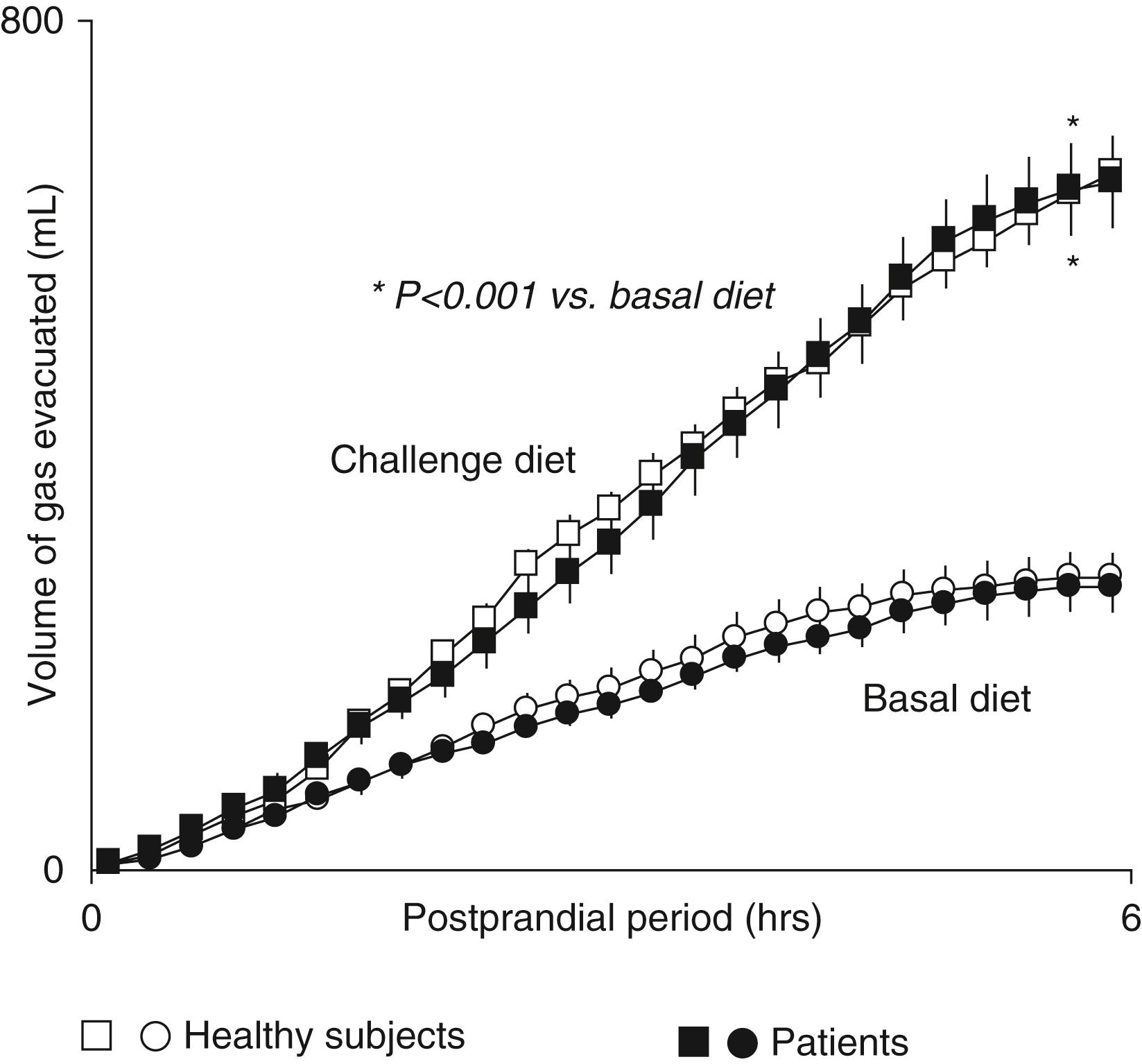
Some colonic microorganisms consume intraluminal gases (H 2 , CO 2 , and O 2 ), and this catabolism accounts for a proportion of intraluminal gas disposal. Three types of microorganisms consume H 2 : acetogens, sulfate-reducing organisms, and methanogens. Acetogens consume H 2 and CO 2 to synthesize short-chain fatty acids. Sulfate-reducing microorganisms use H 2 to reduce sulfate to sulfide. Methanogens use H 2 to reduce CO 2 to CH 4 . Only about 40% of adults have sufficient concentrations of methanogens to yield detectable breath CH 4 concentrations.
Methanogens oxidize H 2 more rapidly than other H 2 -consuming microorganisms. The inability of some individuals to increase breath H 2 excretion probably reflects extremely efficient consumption of H 2 by methanogens rather than a failure to produce H 2 . The degree of methanogenesis has been reported to be high in persons with constipation. Proposed explanations for this association are that slow colonic transit in constipated persons enhances the proliferation of methanogens and, conversely, that CH 4 slows intestinal transit.
The composition of the colonic microbiota (and therefore, the amount of gas produced on a given diet) varies considerably among individuals, depending on early environmental conditions as well as factors encountered later in life, such as diet and antibiotic exposures. Even in the same individuals, dietary habits influence microbiota composition: whereas fiber-rich diets increase its diversity, diets low in fermentable residues induce the opposite effect One study showed that a 3-day diet rich in flatulogenic residues increased the relative abundance of methanogens in healthy subjects. Chronic ingestion of high doses of an intestinally malabsorbed disaccharide (e.g., lactulose by patients with constipation or lactose by persons with intestinal lactase deficiency) results in diminished breath H 2 excretion following a challenge dose of the same disaccharide. This phenomenon may result from colonic proliferation of organisms such as Bifidobacterium spp. that ferment lactose or lactulose via non-H 2 releasing pathways or of gas-consuming microorganisms. Similarly, oral administration of a galacto-oligosaccharide with prebiotic properties has been shown to increase the volume of gas produced within the intestine; the volume then declines to baseline over the subsequent 3 weeks of administration. Conceivably, the initial increase in gas production is due to the fermentation of the prebiotic, and the subsequent decline is due to adaptation by the microbiota. Indeed, changes in the microbiota composition are detected by the end of the administration period. Another study indicated that adaptation of microbiota to regular prebiotic consumption involves a shift in microbiota metabolism toward low-gas‒producing pathways, with a nonsignificant upregulation of gas-consuming activity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here