Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The small intestine and large intestine have many similarities in structure and function. In some cases, different regions of the intestinal tract carry out certain functions in much the same manner. In other cases, however, substantial heterogeneity exists between different intestinal segments (e.g., ileum versus jejunum) or between different mucosal areas (e.g., villus versus crypt) in one intestinal segment.
As discussed in Chapter 41 , the basic structure of the intestine is a hollow cylinder with columnar epithelial cells lining the lumen, with circular and longitudinal layers of smooth muscle in the wall, and with endocrine and neural elements (see Fig. 41-2 ). Enteric neurons, as well as endocrine and paracrine agonists, regulate both epithelial transport and motor activity during both the interdigestive and the postprandial periods. As a result, the intestines propel their contents in a caudad direction while either removing fluid and electrolytes from the intestinal lumen (i.e., absorption) or adding these substances to the lumen (i.e., secretion).
Among mammals, absorption of dietary nutrients is an exclusive function of the small intestine. Only during the neonatal period does significant nutrient absorption take place in the large intestine. The small intestine absorbs nonelectrolytes after extensive digestion of dietary nutrients by both luminal and brush-border enzymes, as discussed in Chapter 45 . In contrast, both the small intestine and the large intestine absorb fluid and electrolytes by several different cellular transport processes, which may differ between the small intestine and large intestine and are the subject of this chapter.
Another vitally important function of the intestinal epithelium is the secretion of intestinal fluid and electrolytes. Teleologically, fluid secretion may be considered an adaptive mechanism of the intestinal tract that protects from noxious agents, such as bacteria and bacterial toxins. In general, the cellular mechanisms of intestinal electrolyte secretion in the small intestine and colon are similar, if not identical. Frequently, the adaptive signal that induces the secretory response also induces a motor response from the intestinal muscle, resulting in a propagated propulsive response that promotes dilution and elimination of the offending toxin.
Both the small intestine and the large intestine have a specialized epithelial structure that correlates well with epithelial transport function.
The small intestine ( Fig. 44-1 A ) consists of finger-like projections— villi — surrounded by the openings of glandular structures called crypts of Lieberkühn, or simply crypts. Both villi and crypts are covered by columnar epithelial cells. The cells lining the villi are considered to be the primary cells responsible for both nutrient and electrolyte absorption, whereas the crypt cells primarily participate in secretion.
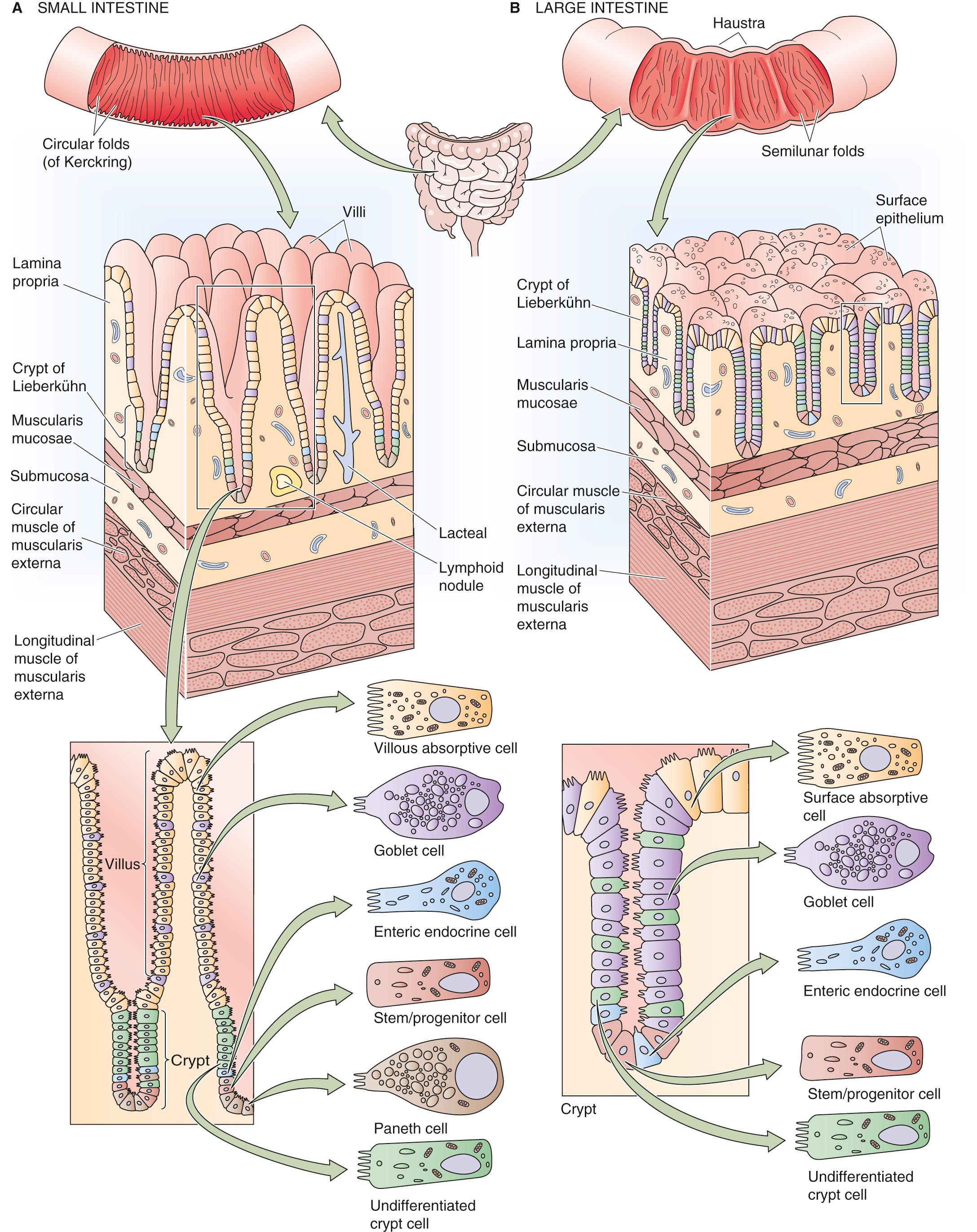
The colon (see Fig. 44-1 B ) does not have villi. Instead, the cells lining the large intestine are surface epithelial cells, and interspersed over the colonic surface are numerous apertures of colonic crypts (or glands) that are similar in function and structure to the small-intestinal crypts. Not surprisingly, the surface epithelial cells of the colon are the primary cells responsible for colonic electrolyte absorption, whereas colonic gland cells are generally believed to mediate ion secretion.
The intestinal mucosa is a dynamic organ with continuous cell proliferation and migration. The zone of cell proliferation is at the base of the crypt in both the small and large intestine, and the program of events is similar in both organs. The progenitor cell is a stem cell that differentiates into several specialized cells (e.g., vacuolated, goblet, and Paneth cells) that line the villi and crypts in the small intestine and the surface and glands in the colon. The vacuolated cell migrates along the crypt-villus axis and becomes a villous absorptive cell after undergoing substantial changes in its morphological and functional characteristics. In the small intestine, these villous cells migrate until they reach the tips of the villi, undergo apoptosis (see p. 1241 ), and then slough into the lumen of the intestine. The overall period from the initiation of cell proliferation to sloughing is ~48 to 96 hours. The overall rate of cell migration may increase or decrease: decreased cell turnover occurs during starvation, whereas increased cell turnover occurs during feeding and lactation, as well as after intestinal resection. The compensatory response that follows intestinal resection involves both luminal and hormonal factors.
An additional hallmark of both the small and large intestine is the presence of structures that amplify function by increasing the luminal surface area. These structures exist at three levels. In the small intestine, the first level consists of the macroscopic folds of Kerckring. The second level consists of the microscopic villi and crypts that we have already discussed. The third level is the submicroscopic microvilli on the apical surfaces of the epithelial cells. Thus, if the small intestine is thought of as a hollow cylinder, the net increase in total surface area of the small intestine (versus that of a smooth cylinder) is 600-fold. The total surface area of the human small intestine is ~200 m 2 , or the surface area of a doubles tennis court ( Table 44-1 ). The colonic surface area is also amplified, but to a more limited extent. Because the colon lacks villi, amplification is a result of only the presence of colonic folds, crypts, and microvilli. Amplification is an effective means of increasing the surface area that is available for intestinal absorption, the primary function of the small and large intestine.
| SMALL INTESTINE | LARGE INTESTINE | |
|---|---|---|
| Length (m) | 6 | 2.4 |
| Area of apical plasma membrane (m 2 ) | ~200 | ~25 |
| Folds | Yes | Yes |
| Villi | Yes | No |
| Crypts or glands | Yes | Yes |
| Microvilli | Yes | Yes |
| Nutrient absorption | Yes | No |
| Active Na + absorption | Yes | Yes |
| Active K + secretion | No | Yes |
The fluid content of the average diet is typically 1.5 to 2.5 L/day. However, the fluid load to the small intestine is considerably greater—8 to 9 L/day. The difference between these two sets of figures is accounted for by salivary, gastric, pancreatic, and biliary secretions, as well as the secretions of the small intestine itself ( Fig. 44-2 ). Similarly, the total quantity of electrolytes (Na + , K + , Cl − , and  ) that enters the lumen of the small intestine also comes from dietary sources in addition to endogenous secretions from the salivary glands, stomach, pancreas, liver, and small intestine.
) that enters the lumen of the small intestine also comes from dietary sources in addition to endogenous secretions from the salivary glands, stomach, pancreas, liver, and small intestine.
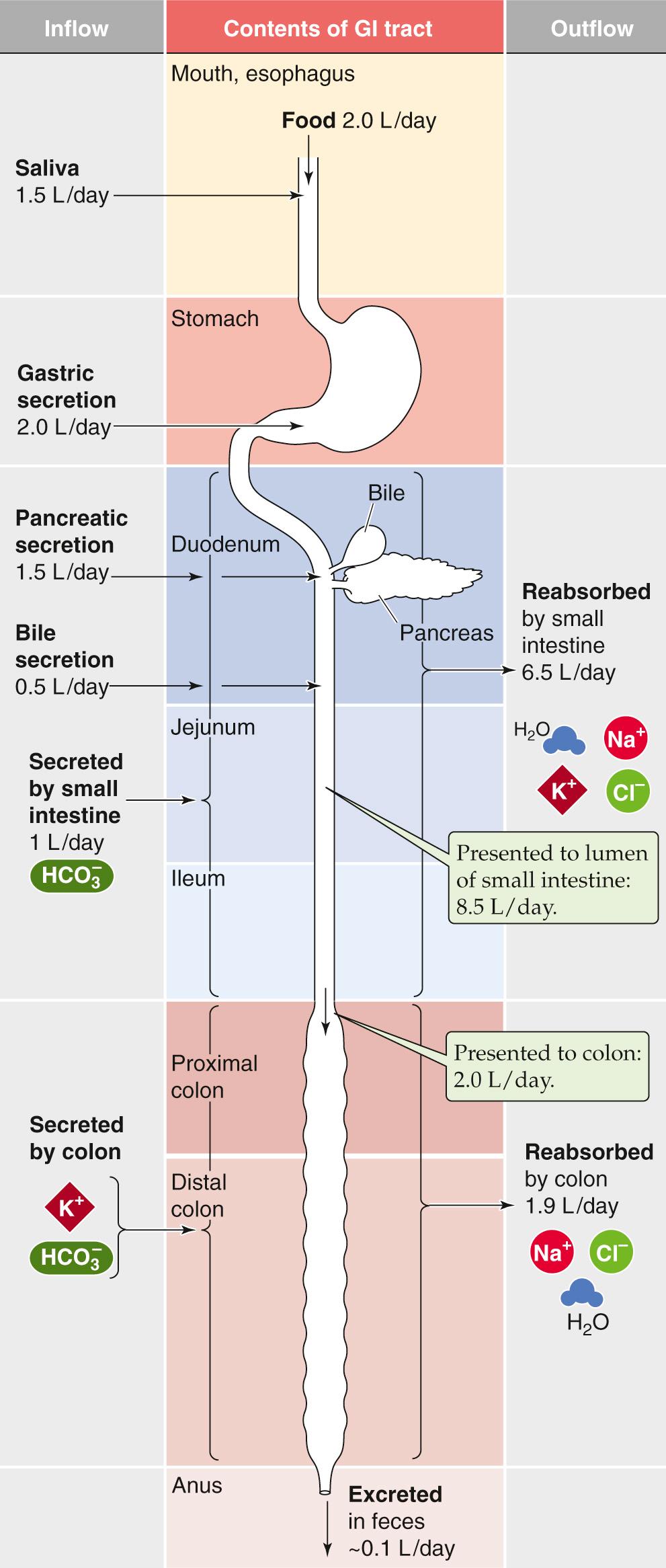
We can calculate the absorption of water and electrolytes from the small intestine by comparing the total load that is presented to the lumen of the small intestine (i.e., ~7.5 L/day entering from other organs + ~1.0 L/day secreted by the small intestine = ~8.5 L/day) with that leaving the small intestine (i.e., ileocecal flow). The latter is ~2.0 L/day in normal subjects. Thus, overall small-intestinal water absorption is about 8.5 – 2.0, or ~6.5 L/day. Na + absorption is ~600 mmol/day. Maximal small-intestinal fluid absorption has not been directly determined but has been estimated to be as great as 15 to 20 L/day.
Colonic fluid absorption is the difference between ileocecal flow (~2.0 L/day) and stool water, which is usually <0.2 L/day (~0.1 L/day). Thus, colonic water absorption is about 2.0 – 0.1, or ~1.9 L/day. In contrast, the maximal colonic water absorptive capacity is between 4 and 5 L/day. As a result, a significant increase in ileocecal flow (e.g., up to perhaps 5 L/day, as occurs with a decrease in small-intestinal fluid absorption) will not exceed the absorptive capacity of the large intestine. Thus, a compensatory increase in colonic fluid absorption can prevent an increase in stool water (i.e., diarrhea) despite substantial decreases in fluid absorption by the small intestine.
 , whereas the colon absorbs net amounts of water, Na + , and Cl − and secretes both K + and
, whereas the colon absorbs net amounts of water, Na + , and Cl − and secretes both K + and 
Net ion movement represents the summation of several events. At the level of the entire small or large intestine, substantial movement of ions occurs from the intestinal lumen into the blood and from the blood into the lumen. The net ion movement across the entire epithelium is the difference between these two unidirectional fluxes.
Fluid and electrolyte transport in the intestine varies considerably in two different axes, both along the length of the intestines (segmental heterogeneity) and from the bottom of a crypt to the top of a villus or to the surface cells ( crypt-villus/surface heterogeneity). A comparison of two different segments of intestine (e.g., duodenum versus ileum) shows that they differ substantially in function. These differences in function reflect segmental heterogeneity of ion transport processes along the longitudinal axis of the intestine in different macroscopic regions of both the small and the large intestine; these differences are both qualitative and quantitative. For example,  stimulation of Na + absorption occurs only in the proximal part of the small intestine. In contrast, the so-called electrogenic Na + absorption (i.e., absorption associated with the development of a transepithelial potential difference) is restricted to the rectosigmoid segment of the colon.
stimulation of Na + absorption occurs only in the proximal part of the small intestine. In contrast, the so-called electrogenic Na + absorption (i.e., absorption associated with the development of a transepithelial potential difference) is restricted to the rectosigmoid segment of the colon.
Within an intestinal segment (e.g., a piece of ileum), crypt-villus/surface heterogeneity leads to differences in transport function along the radial axis of the intestine wall. For example, it is generally believed that absorptive function is located in villous cells in the small intestine (and surface epithelial cells in the large intestine), whereas secretory processes reside in the crypt cells. Finally, at a certain level within a single villus or crypt—or within a very small area of the colonic surface epithelium—individual cells may demonstrate further heterogeneity (cellular heterogeneity), with specific transport mechanisms restricted to different cells.
Overall ion movement in any segment of the intestine represents the summation of these various absorptive and secretory events. These events may be paracellular or transcellular, may occur in the villus or crypt, and may be mediated by a goblet cell or an absorptive cell.
Despite the segmental heterogeneity of small-intestinal electrolyte transport, overall water and ion movement in the proximal and distal portions of the small intestine is similar: in health, the small intestine is a net absorber of water, Na + , Cl − , and K + , but is a net secretor of  (see Fig. 44-2 ). Fluid absorption is isosmotic in the small intestine, similar to that observed in the renal proximal tubule (see pp. 758–759 ). In general, absorptive processes in the small intestine are enhanced in the postprandial state. The human colon carries out net absorption of water, Na + , and Cl − with few exceptions, but it carries out net secretion of K + and
(see Fig. 44-2 ). Fluid absorption is isosmotic in the small intestine, similar to that observed in the renal proximal tubule (see pp. 758–759 ). In general, absorptive processes in the small intestine are enhanced in the postprandial state. The human colon carries out net absorption of water, Na + , and Cl − with few exceptions, but it carries out net secretion of K + and  .
.
As discussed on pages 136–140 , intestinal epithelial cells are polar; that is, they have two very different membranes—an apical membrane and a basolateral membrane—separated from one another by tight junctions. The transport processes present in the small and large intestine are quite similar to those present in other epithelia, such as the renal tubules, with only some organ-specific specialization to distinguish them. The transepithelial movement of a solute across the entire epithelium can be either absorptive or secretory. In each case, the movement can be either transcellular or paracellular. In transcellular movement, the solute must cross the two cell membranes in series. In general, movement of the solute across at least one of these membranes must be active (i.e., against an electrochemical gradient). In paracellular movement, the solute moves passively between adjacent epithelial cells through the tight junctions.
All transcellular Na + absorption is mediated by the Na-K pump (i.e., Na,K-ATPase) located at the basolateral membrane. This enzyme is responsible for Na + extrusion across the basolateral membrane and results in a relatively low [Na + ] i (~15 mM) and an intracellular-negative membrane potential. This Na + gradient serves as the driving force, in large part, for Na + entry into the epithelial cell across the luminal (apical) membrane, a process mediated either by Na + channels or by Na + -coupled transporters (e.g., Na/glucose cotransport, Na-H exchange). The epithelial cell may also use this Na + gradient to energize other transport processes at the apical or basolateral membrane.
Fluid movement is always coupled to active solute movement. ![]() N44-1 The model of the osmotic coupling of fluid movement to solute movement in the intestine is similar to that in all or most epithelial cells (see p. 139 ). It is likely that the water movement occurs predominantly by a paracellular route
N44-1 The model of the osmotic coupling of fluid movement to solute movement in the intestine is similar to that in all or most epithelial cells (see p. 139 ). It is likely that the water movement occurs predominantly by a paracellular route ![]() N44-2 rather than by a transcellular route.
N44-2 rather than by a transcellular route. ![]() N44-3
N44-3
The classical view, presented in the text, is that water transport always osmotically follows solute transport—that is, there is no such thing as a water pump (see pp. 127–136 ). However, Loo, Wright, and Zeuthen have suggested that the Na/glucose cotransporter SGLT1 can transport >200 water molecules for every two Na + ions and one glucose molecule that it transports. ![]() N44-5 In the specific case of the small intestine, these authors propose that SGLT1 can cotransport enough water to account for ~50% of the total water absorption across the brush border of the human small intestine. On the other hand, Lapointe and colleagues have argued that the observed water movement is in fact secondary to local osmotic gradients that drive water movement via the classical pathway.
N44-5 In the specific case of the small intestine, these authors propose that SGLT1 can cotransport enough water to account for ~50% of the total water absorption across the brush border of the human small intestine. On the other hand, Lapointe and colleagues have argued that the observed water movement is in fact secondary to local osmotic gradients that drive water movement via the classical pathway.
Loo and colleagues have proposed that the Na/glucose cotransporter SGLT1 in the human small intestine cotransports not only Na + and glucose, but water as well. In other words, with each cycle, SGLT1 would move 2 Na + ions, 1 glucose molecule, and >200 water molecules. The authors envisage that the Na + ions and glucose molecule—along with the water molecules—would diffuse from the extracellular fluid into a pore within the cotransporter protein. The cotransporter would then undergo a conformational change that would close an outer gate and thereby occlude these ions and molecules from the extracellular fluid. By opening an inner gate, the cotransporter would deocclude these particles and allow the 2 Na + ions, the glucose molecule, and the 200+ water molecules to enter the cytoplasm of the intestinal cell (i.e., enterocyte). There is no controversy that this general model—minus the water—explains how SGLT1 works. The question is whether each cycle of the cotransporter also moves a fixed number of water molecules through the membrane protein along with the Na + and glucose. Loo and colleagues suggest that the water pumped by SGLT1 would account for about half of the water taken up by the small intestine.
On the other hand, Lapointe and colleagues have challenged the conclusion of Loo and colleagues, suggesting that the data of Loo and colleagues can more easily be explained by the classical model. That is, as SGLT1 cotransports Na + and glucose from the extracellular to the intracellular fluid, water would follow osmotically.
Pappenheimer and Reiss have estimated that about 50% of the fluid absorption in the small intestine occurs by a paracellular route. Moreover, they concluded that solvent drag—the entrainment of solutes by the paracellular flow of water—is a major pathway for the absorption of glucose and amino acids in the small intestine.
In his 1988 review, Pappenheimer proposed the following model: Na + -coupled glucose uptake into the intestinal cell—followed by the deposition of solute in the lateral intercellular spaces between epithelial cells—provides the osmotic driving force for paracellular H 2 O movement, and hence solvent drag. Moreover, he speculated that Na + -coupled glucose transport somehow causes contraction of a ring of actomyosin just beneath the apical membrane, which pulls the tight junction apart slightly and increases the paracellular conductance to H 2 O.
As far as the transcellular component of H 2 O movement is concerned, the aquaporins AQP7 and AQP8 are present in the apical membranes of the small intestine and may play a role in transcellular H 2 O movement. Aquaporins are present in the colon, although their role there is not established.
The aquaporins AQP7 and AQP8 are present in the apical membranes of the small intestine and may play a role in transcellular H 2 O movement. Although aquaporins are present in the colon, their role is not established.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here