Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter, the student should be able to answer the following questions:
What is the basic layering pattern of the neocortex, and how do cortical inputs and outputs align with this layering pattern? What is the functional significance of the variation in the layering pattern between cortical areas?
What are the major functions of each of the lobes of the cerebrum?
How does the electroencephalogram (EEG) reflect cortical activity? What are evoked potentials?
How does cerebral dominance correlate with language and hand preference?
What is aphasia, and what is compromised in the different types of aphasia?
How do synaptic and cellular processes support learning and memory? How is memory distributed in the brain?
What role does plasticity play in neural development and in response to damage of the nervous system?
In earlier chapters, the interaction of the nervous system with the body and the outside world was discussed in terms of the transduction and analysis of sensory events, the organization of motor function, and relatively simple central processes that link them, such as reflexes (e.g., the stretch reflex and the vestibulo-ocular reflex). The nervous system has other capabilities, so-called integrative or higher cognitive function, that are less directly tied to specific sensory modalities or motor behavior. These functions require interactions between different parts of the cerebral cortex and between the cerebral cortex and other parts of the brain. The neural basis for some of these higher functions is discussed in this chapter. Because these functions (as well as sensory perception and voluntary motor function) are so highly dependent on the cerebral cortex, its basic organization is described first.
The human cerebral cortex occupies a volume of about 600 cm 3 and has a surface area of 2500 cm 2 . The surface of the cortex is highly convoluted and folded into ridges known as gyri. Gyri are separated by grooves called sulci (if shallow) or fissures (if deep; see Fig. 4.7 ). This folding greatly increases the surface area of cortex that can be fit into the limited and fixed volume within the skull. Indeed, most of the cortex cannot be seen from the brain surface because of this folding.
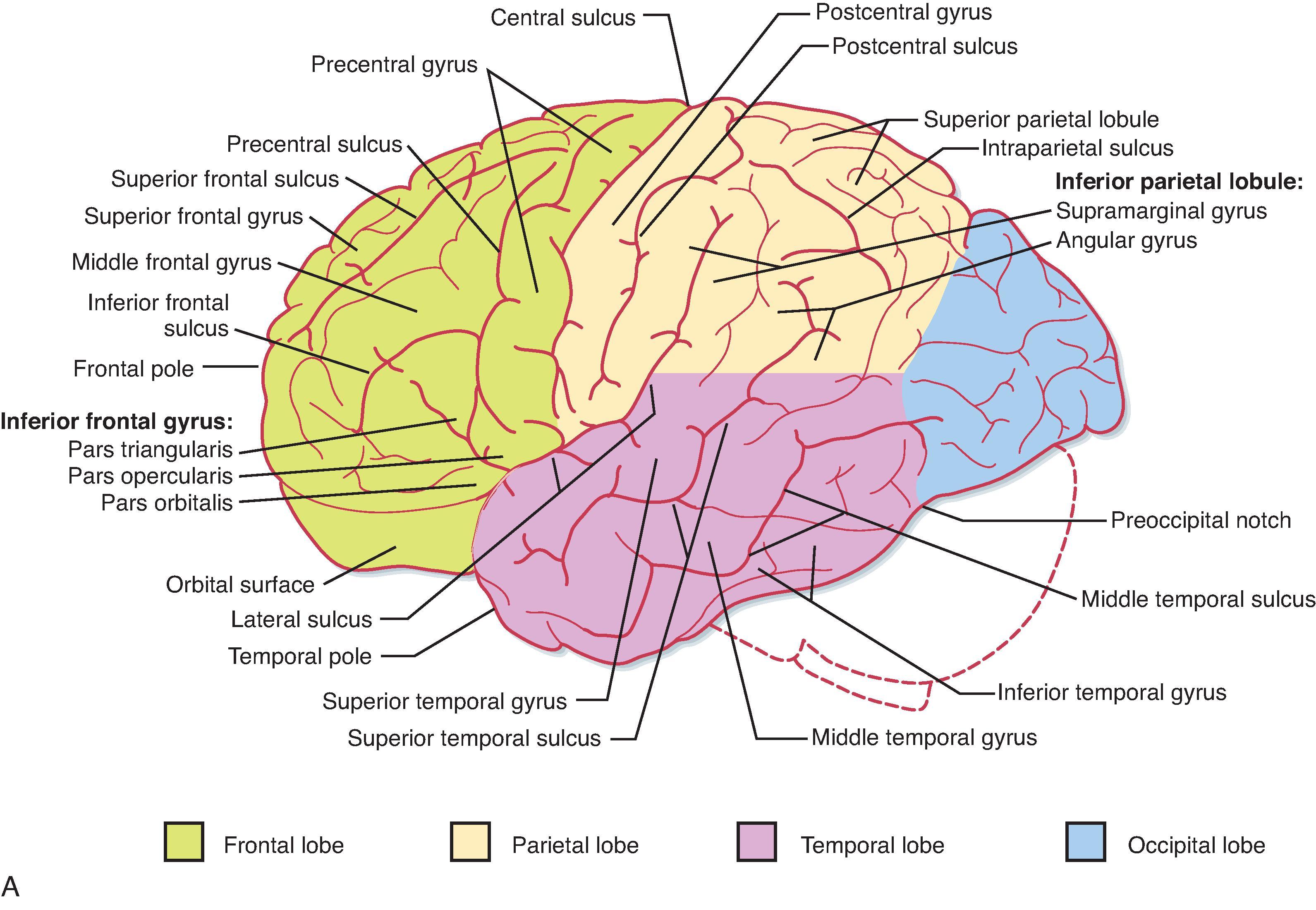
The cerebral cortex can be divided into the left and right hemispheres and subdivided into a number of lobes ( Fig. 10.1 ; see also Fig. 4.7 ), including the frontal, parietal, temporal, and occipital lobes. The frontal and parietal lobes are separated by the central sulcus; both are separated from the temporal lobe by the lateral fissure. The occipital and parietal lobes are separated (on the medial surface of the hemisphere) by the parieto-occipital fissure (see Fig. 10.1 ). Buried within the lateral fissure is another lobe, the insula (see Fig. 4.6 B ). A group of structures that make up the limbic lobe is on the medial aspect of the hemisphere, and its largest part, the hippocampal formation, is folded into the parahippocampal gyrus of the temporal lobe and cannot be seen from the surface of the brain.
Activity in the two hemispheres of the cerebral cortex is coordinated by interconnections through the cerebral commissures. The bulk of the cortex is connected through the massive corpus callosum (see Figs. 4.9 , 10.1 ), and parts of the temporal lobes connect through the anterior commissure.
There are three types of cerebral cortex: neocortex, archicortex, and paleocortex. The neocortex has six cortical layers ( Fig. 10.2 ), the archicortex has three layers, and the paleocortex has four to five layers. In humans, approximately 90% of the cerebral cortex is neocortex.
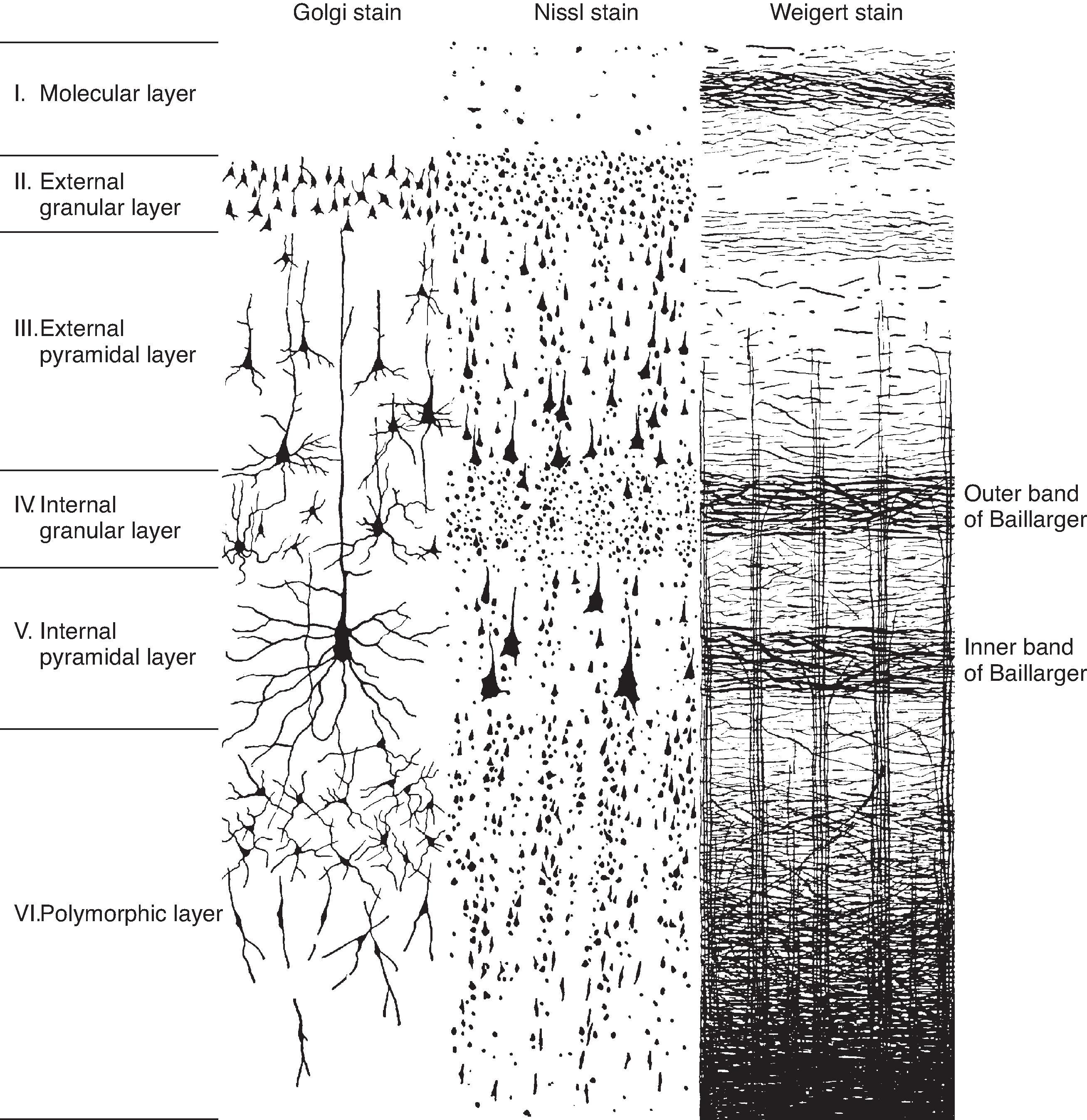
A number of different neuronal cell types in the neocortex have been described (see Fig. 10.2 ). Pyramidal neurons are the most abundant cell type and account for approximately 75% of neocortical neurons. Various other types of nonpyramidal neurons make up the balance, including stellate cells and GABAergic interneurons. Pyramidal cells have a large triangular cell body, a long apical dendrite directed toward the cortical surface, and several basal dendrites. The cell’s axon emerges from the body opposite to the apical dendrite, and those from the larger pyramidal cells project into the subcortical white matter. The axon may give off collateral branches as it descends through the cortex. Pyramidal neurons release the excitatory amino acid glutamate. Inhibitory interneurons release GABA, have various sizes and shapes with short axonal projections within a cortical area. Stellate cells in the cortex have a small soma, numerous branched dendrites, are abundant in layer IV, project locally, but can be either glutamatergic or GABAergic (described in the next section).
Each of the six layers of the neocortex has a characteristic cellular content (see Fig. 10.2 ). Layer I (molecular layer) has few neuronal cell bodies and contains mostly axon terminals synapsing on apical dendrites. Layer II (external granular layer) contains mostly stellate cells. Layer III (external pyramidal layer) consists mostly of small pyramidal neurons. Layer IV (internal granular layer) contains mostly stellate cells and a dense matrix of axons. Layer V (internal pyramidal layer) is dominated by large pyramidal neurons, the main source of cortical efferents to most subcortical regions. Layer VI (multiform layer) contains pyramidal, fusiform, and other types of cells.
Most input to the cortex from other regions of the central nervous system (CNS) is relayed by neurons in the thalamus, as described in earlier chapters for sensory and motor pathways. The projections from the thalamus to the cortex are a significant component of cortical organization that is observed clearly in the layering pattern. Thalamocortical fibers from thalamic nuclei that have specific (topographically mapped) cortical projections end chiefly in layer IV but also in layers III and VI. Neurons in other thalamic nuclei (particularly those relaying input from the brainstem reticular formation) project diffusely and terminate in layers I and VI to modulate cortical activity globally, perhaps in conjunction with changes in state (e.g., sleep or waking).
In addition to subcortical inputs, every region of the cortex receives input from other cortical regions. There are some large fiber bundles that connect widely separated cortical regions, and commissural fibers connect corresponding regions in each hemisphere (these projections terminate in layers I and VI), but in relative terms, the largest source of synapses in a cortical region is local, either from within the region itself or from its neighbors.
The cortical efferent axons originate from pyramidal neurons. The smaller pyramidal cells of layers II and III mostly project to adjacent cortical areas directly and to contralateral regions via the corpus callosum. The larger pyramidal cells of layer V project in many pathways to the spinal cord, brainstem, striatum, and thalamus. The pyramidal neurons of layer VI form corticothalamic projections that target the same thalamic nuclei that provide their afferent input, thus creating circuits of reciprocal thalamocortical and corticothalamic connections. The specific patterns of input from the thalamus have another influence on cortical organization. As discussed in the sensory and motor systems, the topographic mapping of cortical input defines a columnar organization. A column is a narrow, vertically oriented (from the white matter to the cortical surface) region in which the neurons have correlated activity because of shared input from the thalamus. Within a column, there is a great richness in vertical interconnections and fewer lateral interconnections (to cells in neighboring columns), which enable columns to act as a functional unit of the cortex. Despite their relative paucity, however, the lateral interconnections can exert powerful actions, as shown by inhibitory interconnection between regions within motor cortex (see Fig. 9.16 ). Interestingly, the columnar organization can be greatly influenced by functional interactions, as well as by genetics. (See the section “Neural Plasticity.”)
The architecture of the neocortex varies regionally, which presumably reflects the functional specialization of cortical areas. Different aspects of this variation are the bases of several methods for subdividing the cortex into discrete areas. The most widely used strategy is cytoarchitectonics, in which variations in cell density and structure are considered. Myeloarchitectonics (variations in axon density and size) and chemoarchitectonics (expression of molecular markers) are also used to classify cortical areas. Although several cytoarchitectonic maps of the cortex have been devised, the one by Korbinian Brodmann is most commonly used. In this map, the cortex is divided into 52 discrete areas ( Fig.10.3 ), numbered in the order that Brodmann studied them. Areas commonly referred to include Brodmann areas 3, 1, and 2 (the primary somatosensory cortex located on the postcentral gyrus); area 4 (the primary motor cortex located on the precentral gyrus); area 6 (the premotor and supplementary motor cortex); areas 41 and 42 (the primary auditory cortex on the superior temporal gyrus); and area 17 (the primary visual cortex, mostly on the medial surface of the occipital lobe). Subsequent studies confirmed that Brodmann areas are distinctive with regard to their cytoarchitecture, interconnections, and functions, but more recent work has shown that there is some plasticity both in the size of the areas and in their internal organization (see the section “Neural Plasticity”).

Although cytoarchitectonic maps, like Brodmann’s, give the impression of sharp boundaries between contiguous areas, the variation between many of the defined cortical areas is actually fairly subtle and, rather than sharing a well-defined border, most neighboring regions may gradually transition into one another. Nevertheless, some areas have quite distinct cortical characteristics, particularly the primary sensory and motor cortices. For example, the primary and premotor areas are referred to as agranular cortex, because no clear layer IV is present in these areas. Moreover, among the motor areas, the primary motor cortex is distinguished by the presence of large layer V pyramidal cells, the largest of which are called Betz cells. These enormous cells have axons that contribute to the corticospinal tracts and whose soma size (diameter > 150 µm) is necessary for the metabolic maintenance of so much axoplasm. Note that despite being the histological criterion for identifying primary motor cortex, Betz cell axons account for less than 5% of all corticospinal fibers.
In contrast to the motor areas, the primary sensory cortices (e.g., somatosensory, auditory, and visual) typically have a very prominent layer IV (internal granular layer), which is dominated by stellate cells (see Fig. 10.2 ), and therefore they are classified as granular cortices. Indeed, the primary visual cortex is also known as the striate cortex because of a particularly prominent horizontal sheet of myelinated axons in layer IV known as the stripe of Gennari. In a sense, the terms granular and agranular are inaccurate because all cortical areas have similar percentages of pyramidal calls (≈75%) and nonpyramidal cells (25%). Nevertheless, the key idea is that the grouping of the cell types into layers varies dramatically between the frontal motor areas, where the nonpyramidal neurons do not form a distinct internal granular layer, and the primary sensory cortices, where they do.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here