Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Genetic testing involves analyzing genetic material to obtain information related to a person's health status using chromosomal (cytogenetic) analysis (see Chapter 98 ) or DNA-based testing.
Diagnostic genetic testing helps explain a set of signs and symptoms of a disease. The list of disorders for which specific genetic tests are available is extensive. The website http://www.ncbi.nlm.nih.gov/gtr/ provides a database of available tests that is provider driven, so claims are not validated by the site's host, the National Institutes of Health (NIH).
Single-gene disorders can be tested by at least 3 different approaches: linkage analysis (though this is now rarely used), array comparative genomic hybridization ( aCGH ), and direct mutation analysis, usually by DNA sequencing ( Table 94.1 ). Linkage analysis is used if the responsible gene is mapped but not yet identified, or if it is impractical to find specific mutations, usually because of the large size and larger number of different mutations in some genes. Array CGH can be used to detect large, multigene deletions or duplications ( copy number variations ). In addition, with increasing resolution, single-gene or smaller intragenic deletions or duplications can be detected by aCGH, although it is important to note that coverage of each gene may vary from different providers. Direct DNA mutation analysis is preferred and is possible with the availability of the complete human genome sequence. An emerging feature is the increasing recognition of oligogenic disease where more than one disease gene contributes to a complex, or “blended,” phenotype. The ability to sequence hundreds to thousands of genes at once has provided insight into this added layer of complexity in disease pathogenesis.
| TYPE OF MUTATION TESTING | RESOLUTION | ADVANTAGES | DISADVANTAGES | SAMPLE REQUIREMENTS |
|---|---|---|---|---|
| Linkage analysis | Depends on location of polymorphic markers near putative disease gene | Possible when specific disease-causing genetic mutation is not identifiable or found | Can give only diagnostic probability based on likelihood of genetic recombination between presumed DNA mutation and polymorphic markers | Requires multiple family members with documented mendelian pattern of inheritance within family |
| Array comparative genomic hybridization (aCGH) | Several hundred base pairs to several hundreds of kilobases | Able to detect small deletion or duplications within 1 or more genes | Can miss small deletions or insertions depending on resolution of the array used | Single patient sample sufficient, but having sample from biological parents can help with interpretation |
| Direct DNA-based testing (e.g., DNA sequencing) | Single–base-pair changes | High specificity if previously described deleterious mutation is found | Can miss deletion or duplication of a segment of gene | Single patient sample sufficient, but having sample from biological parents can help with interpretation |
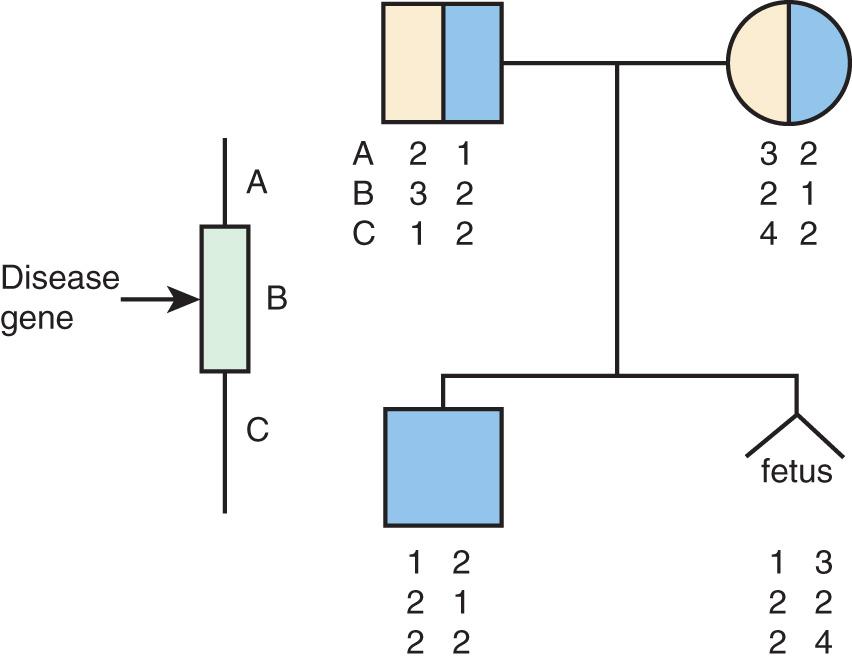
Linkage testing involves tracking a genetic trait through a family using closely linked polymorphic markers as a surrogate for the trait ( Fig. 94.1 ). It requires testing an extended family and is vulnerable to several pitfalls, such as genetic recombination, genetic heterogeneity, and incorrect diagnosis in the proband. Genetic recombination occurs between any pair of loci, the frequency being proportional to the distance between them. This problem can be ameliorated by using very closely linked markers and, if possible, using markers that flank the specific gene. Genetic heterogeneity can be problematic for a linkage-based test if there are multiple distinct genomic loci that can cause the same phenotype, resulting in the risk that the locus tested for is not the one responsible for disease in the family. Incorrect diagnosis in the proband also leads to tracking the wrong gene. Linkage testing remains useful for several genetic conditions, but it is increasingly being superseded by the availability of direct DNA sequencing of either single genes or of the whole collection of genes that encode all proteins. It is critically important that genetic counseling be provided to the family to explain the complexities of interpretation of test results.
Array comparative genomic hybridization can detect copy number variation in a patient's DNA by comparing it to a standard control DNA (see Chapter 98 ). In so doing, aCGH provides a level of genetic resolution between that available with DNA sequencing and that available with chromosome analysis. Whereas earlier technologies could only identify large deletions or duplications that might encompass multiple genes, aCGH can resolve deletions or duplications of several kilobases within 1 gene. In theory, this approach can detect deletion and duplication mutations that would be missed by either chromosome analysis or direct mutation testing by DNA sequencing. However, because the specific resolution and coverage of different aCGH platforms can vary tremendously for different gene regions, the sensitivity for detecting deletions and duplications can vary for different diseases and laboratories. The highest resolution is what would be detection of on average deletion or duplication at the single exon level.
Direct DNA-based mutation testing avoids the pitfalls of linkage testing by detecting the specific gene mutation (i.e., sequence change). The specific approach used is customized to the biology of the gene being tested. In some disorders, 1 or a few distinct mutations occur in all affected individuals. This is the case in sickle cell anemia, in which the same single-base substitution occurs in everyone with the disorder. In other conditions, many possible mutations may account for the disorder in different individuals. In cystic fibrosis, for example, >1,000 distinct mutations have been found in the CFTR gene. Mutation analysis is challenging because no single technique can detect all possible mutations. However, with the completion of the human genome sequence and high-throughput DNA sequencing technology, the approach of choice is to directly sequence DNA that is generated by polymerase chain reaction (PCR) amplification of DNA isolated from peripheral blood white blood cells. The limitation of this approach is that only DNA that is amplified is sequenced, and usually this is restricted to the coding or exonic regions of a gene. Because mutations sometimes occur in the noncoding intronic regions, failure to detect a mutation does not exclude the diagnosis. Whole genome sequencing should identify mutations in the noncoding regions. In addition, genes in a deleted region will not be detected. Although DNA sequencing can be highly specific, it is not completely sensitive because of practical limitations of what is commercially available. Gene sequencing techniques may not be able to identify diseases caused by triplet repeat sequences; specific tests are needed.
The most useful development in clinical DNA diagnosis is application of next-generation sequencing technology to testing panels of genes that target disease symptoms (e.g., seizures, ataxia syndromes) or the whole exome ( whole exome sequencing [WES]) . Soon, whole genome sequencing (WGS), where both coding and noncoding sequences are identified, will provide even more information, although initial clinical interpretation will still be limited to the coding sequences of the approximately 20,000 human genes, the so-called digital exome, as it is extracted electronically from the whole genome data. The challenge is not so much the generation of DNA sequence, but the interpretation of enormous genetic variation within a single sample. Direct sequencing of tens to hundreds of genes in next-generation sequencing panels offer a potentially higher sensitivity because the “depth” of read is higher without complicating high discovery rate of variants of unknown sequences (VUS) . WES and WGS also offer the potential for identifying new disease-gene associations as well as phenotypes caused by more than one disease gene (i.e., oligogenic phenotypes).
An important ethical consideration is the reporting of incidental findings, whether medically actionable or not medically actionable in a patient. WES and WGS may identify mutations that cause aminoglycoside-sensitive hearing loss, which would be medically actionable. At the same time, the discovery of apolipoprotein E variants in a child that increase Alzheimer disease risk susceptibility may not be medically actionable. Therefore, counseling for patients undergoing these tests is important so that only wanted results are reported back to the patient. Guidelines are currently evolving for reporting of incidental findings for WES by the American College of Medical Genetics ( www.acmg.net ). Practice varies among institutions and recommendations vary among international genetic organizations about the approach for revealing incidental findings from WES/WGS to patients; many leave the choice up to the patient and family. Most require revealing to the patient and/or family significant diseases (actionable) with a specific and successful treatment or prevention strategy ( Table 94.2 ).
| Childhood onset | Medically actionable * |
| Childhood onset | Not medically actionable † |
| Adult onset | Medically actionable * |
| Adult onset | Not medically actionable † |
* “Medically actionable” refers to a variant in a gene in which knowledge of the particular variant will affect medical decision-making, such as initiation of a treatment or family planning.
† “Not medically actionable” refers to variants that increase the individual's risk for a disease in which no treatment is proven to significantly change medical decision-making.
Genetic testing is interpreted by 3 factors: analytic validity, clinical validity, and clinical utility. Analytical validity is test accuracy: Does the test correctly detect the presence or absence of mutation? Most genetic tests have a very high analytical validity, assuming that human error, such as sample mix-up, has not occurred. Human errors are possible, and unlike most medical tests, a genetic test is unlikely to be repeated, because it is assumed that the result will not change over time. Therefore, human errors can go undetected for long periods of time. However, it may be reinterpreted over time as our knowledge base of what are disease-causing mutations and genes increases over time.
Clinical validity is the degree to which the test correctly predicts presence or absence of disease. False-positive and false-negative test results can occur. False-positive results are more likely for predictive tests than for diagnostic tests. An important contributing factor is nonpenetrance : an individual with an at-risk genotype might not clinically express the condition. Another factor is the finding of a genetic variant of unknown significance (VUS). Detection of a base sequence variation in an affected patient does not prove that it is the cause of the patient's disorder. In WES there may be more than 30,000 VUS; in WGS there may be more than 3,000,000 VUS. Various lines of evidence are used to establish pathogenicity. These include finding the variant only in affected individuals, inferring that the variant alters the function of the gene product, determining whether the amino acid altered by the mutation is conserved in evolution, and determining whether the mutation segregates with disease in the family. In some cases, it is possible to be certain whether the variant is pathogenic or incidental, but in other cases it might be impossible to definitively assign causality with 100% confidence.
False-negative results reflect an inability to detect a mutation in an affected patient. This occurs principally in disorders where genetic heterogeneity— allelic (different mutations occur in 1 causative gene) or locus (>1 gene can cause a disease) heterogeneity—is the rule. It is difficult to detect all possible mutations within a gene, because mutations can be varied in location within the gene and in the type of mutation. Direct sequencing may miss gene deletions or rearrangements (i.e. structural variants), and mutations may be found within noncoding sequences such as introns or the promoter; a negative DNA test does not necessarily exclude a diagnosis.
Clinical utility is the degree to which the results of a test guide clinical management. For genetic testing, clinical utility includes establishing a diagnosis that obviates the need for additional workup or guiding surveillance or treatment. Test results may also be used as a basis for genetic counseling. For some disorders, genetic testing is possible, but the test results do not add to the clinical assessment. If the diagnosis and genetic implications are already clear, it might not be necessary to pursue genetic testing.
Predictive genetic testing involves performing a test in a person who is at risk for developing a genetic disorder ( presymptomatic ), usually on the basis of family history, yet who does not manifest signs or symptoms. This is usually done for disorders that display age-dependent penetrance; the likelihood of manifesting signs and symptoms increases with age, as in cancer or Huntington disease.
A major caution with predictive testing is that the presence of a gene mutation does not necessarily mean that the disease will develop. Many of the disorders with age-dependent penetrance display incomplete penetrance . A person who inherits a mutation might never develop signs of the disorder. There is concern that a positive DNA test could result in stigmatization of the person and might not provide information that will guide medical management. Stigmatization might include psychological stress, but it could also include discrimination, including denial of health, life, or disability insurance, or employment (see Chapter 95 ).
It is generally agreed that predictive genetic tests should be performed for children only if the results of the test will benefit the medical management of the child. Otherwise, the test should be deferred until the child has an understanding of the risks and benefits of testing and can provide informed consent. Individual states offer varying degrees of protection from discrimination on the basis of genetic testing. A major milestone in the prevention of genetic discrimination was the passage of the Genetic Information Nondiscrimination Act (GINA) in 2008, which is a U.S. federal law that prohibits discrimination in health coverage or employment based on genetic information; it does not protect against refusal of life insurance.
It is expected that genetic tests will become available that will predict risk of disease. Common disorders are multifactorial in etiology; many different genes may contribute to risk of any specific condition (see Chapter 99 ). Most of the genetic variants that have been found to correlate with risk of a common disease add small increments of relative risk, probably in most cases too little to guide management. It is possible that further discovery of genes that contribute to common disorders will reveal examples of variants that convey more significant levels of risk. It is also possible that testing several genes together will provide more information about risk than any individual gene variant would confer.
The rationale for predispositional testing is that the results would lead to strategies aimed at risk reduction as part of a personalized approach to healthcare maintenance. This might include avoidance of environmental exposures that would increase risk of disease (cigarette smoking and α 1 -antitrypsin deficiency), medical surveillance (familial breast cancer and mammography), or in some cases, pharmacologic treatment (statins and hypercholesterolemia). The value of predispositional testing will need to be critically appraised through outcomes studies as these tests are developed.
Polymorphisms in drug metabolism genes can result in distinctive patterns of drug absorption, metabolism, excretion, or effectiveness (see Chapters 72 and 99 ). Knowledge of individual genotypes will guide pharmacologic therapy, allowing customization of choice of drug and dosage to avoid toxicity and provide a therapeutic response. An example is testing for polymorphisms within the methylenetetrahydrofolate reductase ( MTHFR ) gene for susceptibility of potentially increased toxicity to methotrexate antimetabolite therapy for acute lymphoblastic leukemia.
Genetic counseling is a communication process in which the genetic contribution to health, specific risks of transmission of a trait, and options to manage the condition are explained to individuals and their family members ( Table 94.3 ). Genetic counselors are specialized healthcare providers trained in the psychosocial aspects of counseling and the science of medical genetics who may serve as members of medical teams in many different specialties. The genetic counselor is expected to present information in a neutral, nondirective manner while providing resources and psychosocial support to the individual and family to cope with decisions that are made.
Advanced parental age
Maternal age ≥35 yr
Paternal age ≥40 yr
Previous child with or family history of:
Congenital abnormality
Dysmorphology
Intellectual disability
Isolated birth defect
Metabolic disorder
Chromosome abnormality
Single-gene disorder
Adult-onset genetic disease (presymptomatic testing)
Cancer
Huntington disease
Pharmacogenomics
Consanguinity
Teratogen exposure (occupational, abuse)
Repeated pregnancy loss or infertility
Pregnancy screening abnormality
Maternal serum α-fetoprotein
Maternal 1st-trimester screen
Maternal triple or quad screen or variant of this test
Fetal ultrasonography
Noninvasive prenatal testing (NIPT)
Fetal karyotype
Heterozygote screening based on ethnic risk
Sickle cell anemia
Tay-Sachs, Canavan, and Gaucher diseases
Thalassemias
Universal carrier screening panels
Follow-up to abnormal neonatal genetic testing
Prior to whole genome or exome sequencing
Prior to preimplantation genetic testing
Genetic counseling has evolved from a model of care that was developed in the context of prenatal diagnosis and pediatrics into a multidisciplinary approach to medicine that factors into all aspects of healthcare ( Table 94.3 ). In the prenatal setting, a common indication for genetic counseling is to assess risk of occurrence or recurrence of having a child with a genetic condition and to discuss management or treatment options that might be available before, during, or after the pregnancy, such as preimplantation genetic diagnosis, prenatal diagnosis or fetal intervention, and perinatal management. In pediatrics and adult genetics practices, the goals of genetic counseling are to help establish a diagnosis in an individual, provide longitudinal care and psychosocial support to the family, and discuss the genetic basis and inheritance of the condition as it relates to immediate and distant family members.
The genetic counseling role has expanded, particularly with advances in understanding the genetics of adult-onset or common and rare disease therapeutics. In the former context, genetic counseling has a major role in risk assessment for cancer, especially breast, ovarian, or colon cancer, for which well-defined risk models and genetic tests are available to assess risk to an individual. In the latter, the genetic counselor may discuss developments in rare disease therapeutics and make appropriate referral for medical therapies.
The type of information provided to a family depends on the urgency of the situation, the need to make decisions, and the need to collect additional information. There are 4 situations in which genetic counseling plays a particularly important role in this process.
The 1st situation is the prenatal diagnosis of a congenital anomaly or genetic disease. The need for information is urgent because a family must often make time-sensitive decisions about treatment and management options, such as fetal intervention or continuation of a pregnancy in the context of fetal anomalies. Risks to the mother must also be considered. The 2nd type of situation occurs when a child is born with a life-threatening congenital anomaly or suspected genetic disease . Decisions must be made immediately with regard to how much support should be provided for the child and whether certain types of therapy should be attempted. The 3rd situation arises when there are concerns about a genetic condition affecting one later in life. For example, this may occur in an adolescent or young adult with a family history of an adult-onset genetic disorder (e.g., Huntington disease, hereditary breast/ovarian cancer), in an individual with a suspected yet undiagnosed genetic condition, or if a couple with a personal or family history of a genetic condition (or a carrier) is planning a family. In these situations it is often necessary to have several meetings with a family to discuss possible testing, screening, and management options. Urgency is not as much of an issue as being sure that they have as much information and as many options as are available. The 4th situation is genetic counseling before genome sequencing, where the family is given options of what they want reported back to them (actionable/nonactionable incidental findings vs a specific diagnosis).
Providing accurate information to families requires the following:
Taking a careful family history and constructing a pedigree that lists the patient's relatives (including miscarriages, abortions, stillbirths, deceased persons) with their sex, age, ethnicity, and state of health, up to and including third-degree relatives.
Gathering information from hospital records about the affected individual and, in some cases, about other family members.
Documenting prenatal, pregnancy, and delivery histories.
Reviewing the latest available medical, laboratory, and genetic information concerning the disorder.
Performing a careful physical examination of the affected individual (photographs, measurements) and of apparently unaffected individuals in the family (this is usually performed by a physician rather than a genetic counselor).
Establishing or confirming the diagnosis by the diagnostic tests available.
Giving the family information about support groups and local and national resources.
Providing new information to the family as it becomes available (a mechanism for updating needs to be established).
Counseling sessions must include the specific condition, knowledge of the diagnosis of the particular condition, natural history of the condition, genetic aspects of the condition and risk of recurrence, prenatal diagnosis and reproductive options, therapies and referrals, support groups, and nondirective counseling.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here