Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Invasive stimulation of the 10th cranial nerve (vagus nerve) has demonstrated a beneficial effect in patients with elevated, body mass indices (BMI) and glucose intolerance/insulin resistance ( ). A review of the scientific literature provides at least two key lines of research that may explain how vagus nerve stimulation (VNS) impacts metabolic processes and control. First, VNS modulates inflammatory signaling pathways in immune cells (e.g., macrophages), which counters the inhibitory effects of inflammation products that inhibit insulin-mediated, glucose transport in other cells (e.g., muscle cells and adipocytes). Second, VNS promotes the conversion of glucose into glycogen by upregulating the activity of glycogen synthetase in hepatic stellate cells, countering the promotion of gluconeogenesis by inflammatory cytokines.
More specifically, with respect to the former research, it is widely understood that excessive white adipose tissue (WAT) mass is associated with high levels of free fatty acids, which can activate cell surface, antigen-binding receptors referred to as Toll-like receptors (TLRs). Activation of TLRs promotes an innate immune response and expression of inflammatory cytokines that results in influx and activation of immune cells. In fact, excessive WAT can be populated with significant numbers of activated macrophages ( ). These activated macrophages can potentiate and perpetuate a chronic inflammatory state ( ).
The inflammatory cascade, if left unchecked, can accelerate in a positive feedback process which can severely damage or destroy the host organism. As a result, prolonged inflammation is regulated to a chronic stable level by feedback inhibitory proteins designed to slow or block the cellular processes that produce cytokines and other aspects of the inflammatory cascade. In the event that the inflammation trigger is resolved or removed by the immune system, these feedback inhibitory proteins help to bring the inflammatory process to a close, including the self-limiting expression of the inhibitory proteins themselves.
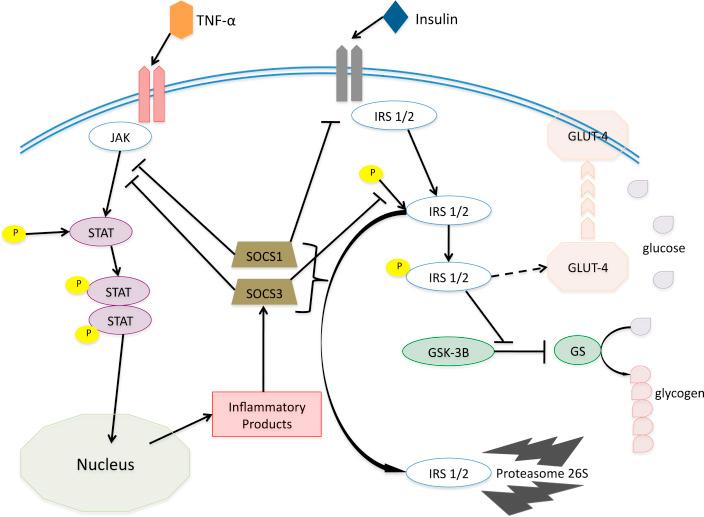
Chronic inflammation related to obesity, however, has no resolution that is mediated by the immune system. Chronic inflammation thus has been implicated in chronically high levels of expression of these feedback regulatory proteins as they attempt to prevent the positive feedback loop of inflammation from damaging the host. Among these feedback inhibitors of inflammation is the protein class known as Suppressors of Cytokine Signaling (SOCS) ( ). Several of these SOCS proteins have functions that lead directly to insulin resistance as they inhibit the signaling pathway of the insulin receptor (IR) ( ). It has been proposed that a purpose for this inhibition of insulin signaling is to reduce the competition for circulating glucose for immune cell function (which does not require insulin for glucose uptake) ( ). Insulin resistance is a hallmark of Type 2 Diabetes (T2D).
VNS has been shown to suppress inflammation, especially inflammation initiated by TLR activation ( ). Several clinical study reports provide encouraging data that appear to support the pursuit of VNS for T2D ( ). Barriers to entry for the use of VNS have centered on its need for surgery (i.e., costs and risks). The development of noninvasive VNS (nVNS) devices however, overcomes these barriers and presents the potential for nVNS to be used as a brief, daily therapy for the management of insulin resistance.
In parallel with antiinflammatory effects, parasympathetic activation has been shown to upregulate the enzyme activity of glycogen synthetase, which rapidly reduces circulating levels of glucose. By contrast, sympathetic activation has been shown to activate gluconeogenesis. It is not surprising, therefore, that the ARIC study revealed that T2D patients have low vagal tone when compared with nondiabetic patients ( ).
Glucose is the most widely used energy source for all forms of life and is implicated in the metabolic processes by organisms ranging from yeast to sequoia trees, mollusks, and humans. One reason for its early selection over other potential energy storage molecules and its conservation across millions of years of evolution may be related to its lower propensity to glycosylate proteins ( ). Glycosylation alters the structure of proteins by incorporating glycans into the tertiary and quaternary structure of polymeric organic molecules that include many proteins. This lower propensity of glucose to glycosylate proteins is, however, nonzero. For example, long-term imbalances in glucose levels in the bloodstream can be observed through measurements of the beta- N -1-deoxy fructosyl component of hemoglobin, which is the result of a nonenzymatic glycosylation of a beta protein chain ( ).
Dietary intake and hepatic synthesis are the primary sources of glucose in the bloodstream. Ensuring that the brain, spinal cord, peripheral nerves, and other cells have a sufficient supply of glucose is a critical, dynamic, control function, with insulin and glucagon secretion playing central roles ( ). However, while short, and even intermediate periods of elevated blood glucose present little risk, prolonged or chronically high levels of blood glucose can lead to a spectrum of complications that range from neurologic damage, injury to kidneys, enhanced cardiovascular risk, and even retinal damage. Sequellae from these injuries can be peripheral nerve damage leading to neuropathic pain, peripheral vascular disease, hypertension, and blindness.
After eating, blood sugar rises. Glucose is necessary for survival; however, in large concentrations it can lead to a hyperglycemic state that can disrupt protein structure and function. Insulin is, therefore, released by the beta cells, also known as Islet of Langerhans cells, of the pancreas to maintain appropriate levels of glucose, especially through uptake of glucose into the muscle (as glycogen stores) and adipocytes (as fatty acids). Insulin released by the pancreas binds to the transmembrane IR, which lies on the surface of most cells. Receptor binding leads to the activation of the intracellular, insulin receptor substrate (IRS) protein that is associated with the intracellular portion of the IR. IRS activation causes the glucose transporter protein (specifically GLUT4) to translocate to the cell membrane, and to mediate the influx of glucose. When glucose levels are low, glucagon is released to promote the production of glucose by the liver, and to a lesser extent, the intestines and kidneys, through gluconeogenesis.
The processes involved in inflammation are energy intensive processes, involving macrophages, T-cells, B-cells, and the spectrum of degranulating cells that include eosinophils, neutrophils, and mast cells. These cell types produce a wide array of signaling proteins, cytokines, and cytotoxic substances and undergo dramatic changes in morphology and activation states; therefore, the availability of energy is critical. These cells do not have the capability of storing energy for times of increased demand, and thus are entirely dependent on the glucose levels in the bloodstream for the energy necessary to function. To limit the competition for glucose, one of the functions of cytokines, released by immune cells, is to perpetuate an inflammatory cascade within nonimmune (and nonnerve) cells. As described more fully, the inflammatory processes within these cells lead to inhibition of insulin signaling, creating insulin resistance and impaired glucose uptake. That is, adipocytes and muscle cells fail to take up sufficient glucose. Not surprisingly, clinical symptoms of inflammation are fatigue and muscle weakness.
As insulin resistance rises and circulating glucose levels persist or rise, the pancreas produces higher levels of insulin (hyperinsulemia) in an attempt to enhance the pressure on cells to take up the circulating glucose. The increase in insulin levels creates a risk of damage from hyperglycemia. Simultaneously, circulating cytokines drive both the production of glucose in the liver (gluconeogenesis) ( ) and, subsequently, the production of fatty acids (lipogenesis). Lipogenesis is a hallmark of steatosis, steatohepatitis, and other forms of nonalcoholic fatty liver disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH), which can lead to cirrhosis and liver failure ( ).
A hallmark of T2D, observed by Himsworth in 1936, is insensitivity to insulin ( ). One cause of insulin insensitivity has been shown to be the failure of insulin to affect the sequence of coordinated intracellular chemical interactions (i.e., signaling pathway). Multiple potential inhibitors of insulin signaling exist, and among them are Inhibitor of Kappa Kinase-beta (IKKβ) and Jun N -terminal Kinase (JNK), which are proteins that are activated along the signaling cascade that is associated with TLR binding ( ). More particularly, these proteins reportedly alter IRS-1 and IRS-2 phosphorylation sites from tyrosine to serine. This interferes with glucose uptake by inhibiting the signal that promotes GLUT4 vesicle translocation to the cell surface ( ).
Inhibition of normal insulin signaling may also result from the action of several members of the SOCS proteins family (CIS and SOCS1-8), which are produced as part of the feedback inhibition of the inflammation cascade (involving Janus kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) protein). More specifically, SOCS1 and SOCS3 are feedback, inhibitory proteins that are generated by the binding of inflammatory cytokines that include IL-1β, TNF-α, and Interferon gamma (IFN-γ) to their associated receptors, and specifically Nuclear Factor kappa B (NF-κB) and STAT1, the nuclear transcription factors that are activated by these cytokines. SOCS1 mediates a negative feedback of TLR-mediated, inflammatory signaling via control of both Myeloid Differentiation primary response gene 88 (MyD88) dependent and MyD88-independent signaling ( ). Both SOCS1 and SOCS3 contain a kinase inhibitory region (KIR) that directly suppresses JAK tyrosine kinase activity ( ).
While SOCS1 and SOCS3 inhibit cytokine signaling cascades, these proteins appear to act on the intracellular domain of the IR to inhibit pathways that promote the normal metabolic effects of insulin ( ). They also facilitate the proteasomal degradation of IRS-1 and IRS-2 (principle, signaling proteins, phosphorylated by the IR) ( ).
More particularly, SOCS1 interacts with the catalytic domain of the IR, blocking the tyrosine phosphorylation of IRS2. SOCS3 inhibits insulin signaling by a direct binding through its Sarcoma (Src) Homology 2 (SH2) domain with the juxtamembrane phosphotyrosine 960 on the IR, thus preventing the interaction of IRS-1 and 2 with the receptor. SOCS6 also inhibits tyrosine kinase activity of the IR ( ). It has also been shown that these SOCS proteins interact with tyrosine phosphorylated IRS1 and IRS2 to cause their ubiquitination 1
1 The addition of ubiquitin to a substrate protein is called ubiquitination or ubiquitylation. Ubiquitination can affect proteins in many ways: it can signal for their degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions.
and more rapid transport to the proteasome for degradation.
It has been proposed that the evolutionary purpose of the insulin resistance–promoting activity of SOCS1 and SOCS3 in glucose storage cells is as an adaptive response to redirect energy to immune cells, which have limited, glycogen storage capacity ( ). However, this strategy becomes maladaptive in the setting of obesity, resulting in insulin resistance and T2D ( ). See Fig. 133.1 .
The inhibitory effects on IR function and glucose transport of both proinflammatory signaling proteins and proteins that serve feedback inhibitory purposes in key inflammatory pathways suggests a link between inflammation and insulin resistance. In 1993, Hotamisligil reported on expression of the potent, proinflammatory cytokine, TNF-α, in the adipose tissue of obese mice and linked its expression to insulin resistance ( ). This has been corroborated numerous times in animals and in humans with T2D ( ). As obesity was long observed to be a cofactor in the development of insulin resistance, the question that remained was how obesity might lead to an inflammatory response.
It is known that accumulation of significant adipose tissue mass has been associated with a proinflammatory state. More particularly, WAT, which is known to function as a storage of energy reserves, has been more strongly associated with this phenomenon, as opposed to brown adipose tissue (BAT). In fact, WAT has been recognized to have a much more complex function than simply storing energy reserves and is now recognized as serving as an endocrine organ with its own collection of agents (adipokines), which include Interleukin-6 (IL-6), IL-1β, TNF-α, leptin, and adiponectin ( ). Perhaps, most supportive of the assertion that WAT is a source of proinflammatory pressure comes from the fact that WAT releases chemotactic factors that lead to the infiltration of monocytes from circulation, their maturation into resident macrophages, and the recruitment of other immune cells. Reports suggest that up to 40% of the cells in accumulated WAT deposits can be macrophages ( ).
One mechanism that has been reported that explains the activation state of macrophages in the WAT involves the activation of TLRs and other cell surface, pattern recognition receptors (PRRs) by circulating free fatty acids (FFAs) ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here