Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Indications
Anatomic considerations
Technical considerations
From Holcomb GW, Murphy JP: Ashcraft's Pediatric Surgery 5th edition (Saunders 2010)
Inguinal hernia repairs are one of the most common operations performed by pediatric surgeons, and consultations for inguinal hernia are among the most frequent reasons for pediatric surgical referral. An inguinal hernia in a child usually refers to an indirect inguinal hernia but may include a femoral hernia and, rarely, a direct inguinal hernia. The diagnosis and management of inguinal hernias and hydroceles in infants and children and the attendant complications and controversies are discussed in this chapter.
Inguinal hernias were first described in the Ebers Papyrus in 1550 bc . Celsus is thought to have performed hernia repairs in ad 50. Galen, in ad 150, described the processus vaginalis, defined hernias as a rupture of the peritoneum, and advised surgical repair. Ambrose Paré advocated repair of inguinal hernias in childhood in the 16th century. There was a flurry of progress in the 1800s, with Cooper's 1807 identification of the transversalis fascia and the ligament associated with his name and Cloquet's 1817 observation that the processus vaginalis is often patent at birth as well as his description of femoral hernias. In 1877, von Czerny first described narrowing the inguinal canal and tightening the external inguinal ring, followed by Bassini's description of internal inguinal ring tightening and reinforcement of the posterior canal in 1887. Bassini's interest in the subject of inguinal anatomy was personal because he had sustained a groin wound with a cecal-cutaneous fistula in 1867. Gross reported a 0.45% recurrence rate in a large series of hernia repairs (3874 children) in 1953.
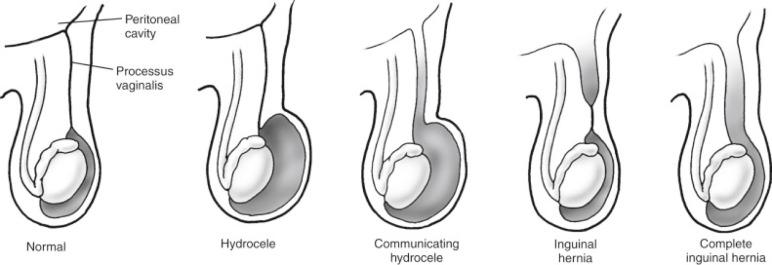
The processus vaginalis is a peritoneal diverticulum extending through the internal inguinal ring into the canal. It can be seen by 3 months of fetal life. The somatic base of this diverticulum is the transversalis portion of the endoabdominal fascia. The gonads form on the anteromedial nephrogenic ridges in the retroperitoneum during the 5th week of gestation. The gonads are attached to the scrotum by the gubernaculum in the male and to the labia via the round ligament in the female. Gonadal descent begins by 3 months' gestation, and the testis reaches the internal inguinal ring by about 7 months. Descent of the testis is initiated and directed by release of calcitonin gene-related peptide (CGRP) from the genitofemoral nerve (via fetal androgen release). CGRP mediates closure of the patent processus vaginalis (PPV), although this process is not completely understood. The testis begins to descend down the canal by the 7th month of fetal life preceded and guided by the processus vaginalis. The processus, which is located anterior to the cord structures, gradually obliterates, and the scrotal portion forms the tunica vaginalis. The female anlage of the PPV is the canal of Nuck, a structure that leads to the labia majora. This also closes by about 7 months of fetal life, and ovarian descent is arrested in the pelvis. The precise incidence of PPV in newborns is unknown and depends on gender and gestational age. The incidence has been estimated to be 40% to 60% but may be lower. However, at autopsy, only 5% of adults have a PPV. PPVs at birth can still close, but this becomes less likely with increasing age. It is failure of the PPV to close that results in an indirect inguinal hernia. As mentioned, the factors driving PPV closure are incompletely understood. Intra-abdominal pressure probably plays a role because disorders with increased abdominal pressure/fluid (e.g., ventriculoperitoneal shunts) are associated with an increased incidence of indirect inguinal hernia and with increased bilaterality. Indirect inguinal hernias are more common on the right. The various clinical findings related to the processus vaginalis are illustrated in Figure 85-1-1 .
The layers of the abdominal wall contribute to the layers of the testis and spermatic cord as the gonad descends. The internal spermatic fascia is a continuation of the transversalis fascia, the cremaster muscle derives from the internal oblique, and the external spermatic fascia originates from the external oblique aponeurosis. The processus vaginalis envelops the testis as the visceral and parietal layers of the tunica vaginalis.
Approximately 0.8% to 4.4% of all children will develop an inguinal hernia, with a positive family history in about 11.5%. In reviewing cases at our hospital over the past 4 years, there were 15,321 general surgical operations. Within this group, there were 1991 (13%) inguinal hernia repairs. Fifteen percent were performed in infants younger than 6 months of age, 54% of patients were between 6 months and 5 years of age, and 31% were 5 years of age or older. In another series of 6361 pediatric herniorrhaphies performed by a single surgeon, the male-to-female ratio was 5 : 1. Right-sided hernias were twice as common as those on the left. The mean age in this series was 3.3 years.
The incidence of inguinal hernia varies directly with the degree of prematurity. The overall incidence of inguinal hernia in premature infants is estimated to be 10% to 30%, whereas term newborns have a rate of 3% to 5%. Co-morbidities such as chronic lung disease associated with prematurity may play a substantial role in the development of an inguinal hernia in this population.
Other entities associated with an increased incidence of inguinal hernia ( Table 85-1-1 ) include cryptorchidism, abdominal wall defects, connective tissue disorders (Ehlers-Danlos syndrome), mucopolysaccharidoses such as Hunter's or Hurler's syndrome, cystic fibrosis, ascites, peritoneal dialysis, ventriculoperitoneal shunts, congenital hip dislocation, and meningomyelocele.
|
Patients with cystic fibrosis have an increased risk (eightfold in one report) of inguinal hernia, with an incidence as high as 15%. This heightened risk may be due to elevated intra-abdominal pressure from respiratory problems, but developmental/embryologic factors may also play a role because the risk of hernia is increased in unaffected siblings and parents. Ventriculoperitoneal shunts are associated with an increased incidence of inguinal hernia as well as increased bilaterality, increased incarceration risk, and increased recurrence. In a series of 430 children who underwent placement of a ventriculoperitoneal shunt, 15% developed hernias and hydroceles occurred in another 6%. Injury to the shunt and shunt infection are other problems specific to these children.
Most hernias are asymptomatic except for inguinal bulging with straining. They are often found by the parents or by the pediatrician on routine physical examination. The diagnosis is clinical and rests squarely on the history and physical examination. Maneuvers such as having the child raise the head while supine or “blowing up a balloon” with a thumb in the mouth may be helpful in small children. Standing the child upright may also help demonstrate the hernia. The differential diagnosis includes a retractile testis, lymphadenopathy, hydrocele, and prepubertal fat. In older children, neoplasia must be considered.
A common occurrence is a normal examination in combination with a suggestive history. Some surgeons have the child return for a second examination in 2 to 3 weeks, whereas others accept a good history as an indication for operation. Although subjective and dependent on the experience of the surgeon, our preference is for the latter course. False-negative explorations should be rare. In a series of 6361 hernia repairs by a single surgeon (definitive inguinal hernia on examination was the indication for operation), there was only one false-negative exploration (0.02%).
Ancillary findings such as a “silk glove sign” (feeling the thickened peritoneum of the patent processus as the cord is palpated) are of variable reliability. Radiologic diagnostic aids are not generally necessary or helpful. Herniograms are of historic interest. Ultrasonography can be used to identify a PPV indirectly via widening of the internal inguinal ring (more than 4 to 5 mm is positive), but the technique is highly operator dependent and not in widespread use. It is not generally necessary to restrict an asymptomatic child's activities until repair is scheduled, but prompt repair may decrease interim incarceration.
Another question that has arisen in the laparoscopic era is what to do with an incidentally discovered PPV in a child undergoing operation for an unrelated problem. A common scenario is that of unilateral or bilateral PPV discovered during the course of a laparoscopic appendectomy. A hernia repair should not be performed concomitantly in that setting. The child and the family should be informed of the findings and instructed to watch for symptoms.
There are no good data comparing regional to general anesthesia for pediatric inguinal hernia repair. A 2003 Cochrane meta-analysis of available data regarding this issue in premature infants concluded: “There is no reliable evidence from the trials reviewed concerning the effect of spinal as compared to general anaesthesia on the incidence of post-operative apnoea, bradycardia, or oxygen desaturation in ex-preterm infants undergoing herniorrhaphy.”
Overnight stay is not necessary after inguinal hernia repair for healthy children or term infants. However, the risk of postoperative apnea and bradycardia is increased in premature infants and overnight monitoring is necessary. The postconceptual age (gestational age + chronologic age) is commonly used to decide which infants require admission. Several studies have addressed this issue. Sixty weeks postconceptual age is used at our hospital, because a less than 1% risk of postoperative apnea was found in former premature infants of more than 56 weeks' postconceptual age in a comprehensive analysis of eight prospective studies.
Because premature infants have an increased incidence of inguinal hernia, this is a common diagnosis in the neonatal intensive care unit. The incidence of bowel incarceration in premature infants is significantly increased (threefold in one large series). Many institutions use 2 kg as a lower limit for repair in asymptomatic and otherwise relatively healthy newborns. We usually repair the hernia before discharge to avoid the need for readmission to repair the hernia and to decrease the risk of incarceration. However, this decision is surgeon-dependent and often depends on other co-morbidities.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here