Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute viral infections can produce a variety of clinical manifestations and differing degrees of severity. The vast majority of viral infections range from asymptomatic to mild, occur in the community, and are self-limited. Rarely, acute viral infections cause severe disease, which necessitates critical care management. Common viral upper respiratory tract infections can cause serious acute exacerbations of underlying lung disease. Certain viral agents can become life-threatening when they infect vulnerable hosts or if novel pathogens emerge with an absence of immunologic experience in humans. Systemic viral infections, particularly seasonal and pandemic influenza, will be covered in this chapter. However, the increasing availability of rapid genomic diagnostics, immunotherapies, and antiviral chemotherapies now necessitates that intensive care unit (ICU) clinicians maintain some working familiarity with other viral pathogens that can cause serious illness. A summary of clinical presentations, major pathologies, mechanisms of spread, and treatment strategies for a variety of human viral pathogens will be reviewed.
Influenza is an acute febrile respiratory illness of varying severity causing seasonal epidemics during the winter months in temperate climates and year-round endemic infections in tropical climates. This viral zoonosis is indigenous to migratory wild birds, with periodic introduction into domesticated poultry, swine, marine mammals, and humans. The consequences of viral pathogen transfer from the avian reservoir or animal vectors to humans can be devastating, with substantial mortality rates, rapid transmission, and potential for global pandemics. The fate of influenza virus infection in human populations depends on the viral virulence properties, antigenic differences from previous influenza outbreaks, fitness of viral replication, dissemination within humans, and status of the host immune defenses.
For seasonal cases, severe disease may occur in individuals with vulnerabilities in host defenses, including the very young, the very old, and those with immunodeficiency or chronic cardiopulmonary disease. However, healthy and young individuals can be seriously affected, particularly in pandemic years when novel viruses emerge. The incidence rates of epidemics depend on yearly variation in viral transmissibility, infectivity, and host susceptibility. Influenza pandemics typically have much higher incidence rates as the virus circulates throughout the entire susceptible global population. In 2009 the H1N1 pandemic swept through a huge number of patients susceptible to the novel swine influenza A (H1N1)pdm09. Other historic pandemics included the Spanish flu of 1918 (H1N1), the Asian flu of 1957 (H2N2), and the Hong Kong flu of 1968 (H3N2). The Spanish flu was particularly catastrophic, infecting a third of the world’s population and killing an estimated 50–100 million people.
Even in a typical nonpandemic year, influenza accounts for hundreds of thousands of deaths worldwide and exacts billions of dollars in terms of morbidity and lost productivity. Estimates from the United States indicate that each year, at least 610,660 life-years are lost, with between 9 and 45 million illnesses, up to 31.4 million outpatient visits, 12,000–61,000 deaths, and ∼$10.4 billion in direct medical costs from influenza alone. , The staggering amount expended for influenza care is $16.3 billion in projected lost earnings and an estimated total cost burden (including lost life-years) amounting to $87.1 billion. The estimated global mortality varies from 291,000 to 645,000 deaths per year, and global costs during a pandemic year such as 2009 have been estimated to be upwards of 374 billion dollars. The costs of intensive care services required for managing the most severely ill influenza victims alone are enormous.
Influenza viruses are single-stranded RNA viruses of the family Orthomyxoviridae that are classified by their matrix protein composition. , Three influenza types can infect humans: influenza A, influenza B, and influenza C. The influenza A virus is divided into specific subtypes based upon two major antigenic proteins on its surface: hemagglutinin (HA) and neuraminidase (NA). Influenza A can replicate in the gastrointestinal (GI) tract of some avian species without causing symptoms, but infection and symptoms may occur in other bird species, mammals, and humans. It is the only subtype with pandemic potential. Influenza B almost exclusively infects humans. The lack of animal reservoir leads to less genetic variability, making pandemics with influenza B not possible. However smaller-scale mutations cause seasonal epidemics with potential serious repercussions. Influenza C affects humans, dogs, and pigs but seldom causes severe illness or epidemics in humans.
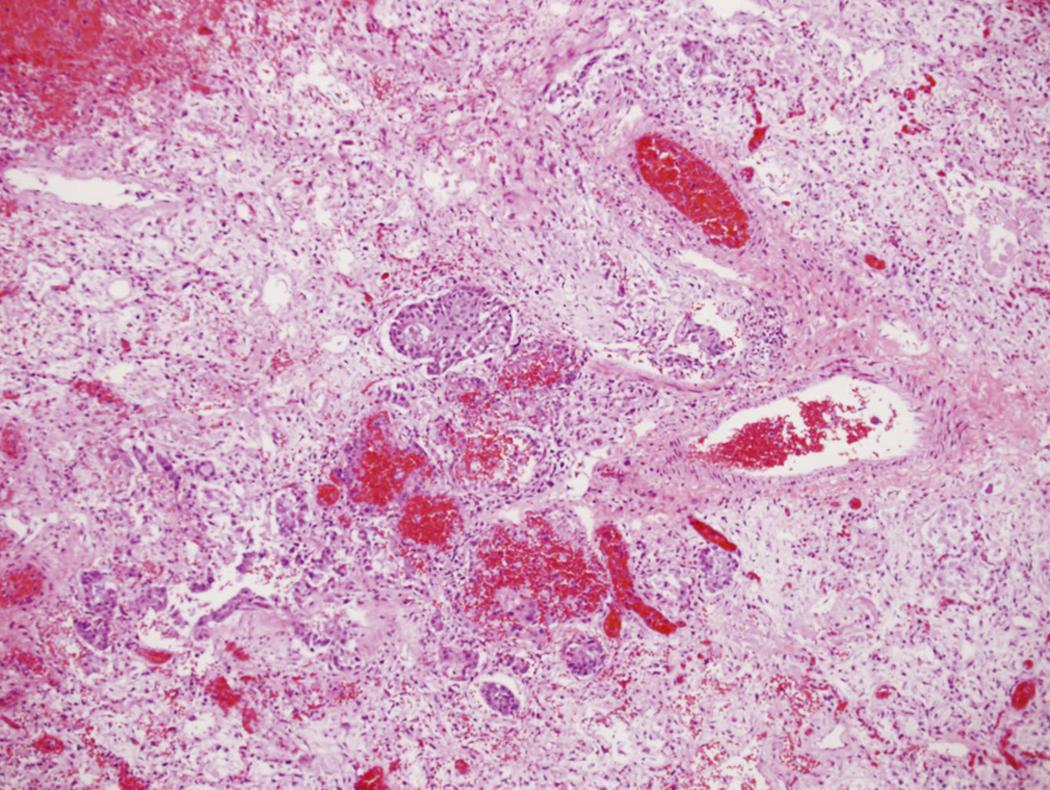
Influenza virus has a particular predilection for respiratory epithelial cells. Infected cells undergo abrupt cessation of protein synthesis and subsequent apoptosis. Viral particles are released infecting nearby cells. Local immune cells are also infected, causing measurable deficiencies in immune function, increasing the likelihood of secondary complications. Lung histopathology of fatally infected patients reveals a diffuse alveolar filling process, with early hyaline membrane formation, and occasionally focal areas of hemorrhage. The alveolar lining is thickened, with lymphocytic infiltrates and, occasionally, early fibrosis. A typical lung tissue section is seen in Fig. 119.1 .
The genome of influenza A viruses consists of eight separate single-stranded RNA segments, each encoding a major viral protein. RNA replication provides a high background mutation rate resulting in antigenic “drift”—point mutations that change the major surface antigens. The resulting variation is sufficient to evade host immune responses and cause seasonal epidemics. However, influenza pandemics typically occur after an antigenic “shift”—when animal vectors are coinfected simultaneously by more than one influenza A subtype, large-scale reassortment of viral genome segments can occur, resulting in entirely new hybrid viruses with new antigenic constituents. As an example, the swine-origin influenza A/Mexico City/4/2009 (H1N1) pandemic strain was a quadruple-reassorted virus derived from gene segments originating from ducks, Eurasian swine, North American swine, and human-adapted influenza viruses.
Avian-origin influenza viruses can occasionally be transmitted to mammals, causing outbreaks in animals and potentially giving rise to human pandemics. Swine are important “mixing vessels” in shuttling avian influenza viruses to humans. Their mucous membranes express a diversity of sialic acid–coated glycopeptides in a favorable conformation to bind both avian and human viruses via the HA glycoprotein. Avian species primarily express α2,3-linked sialic acids, human upper airways primarily express α2,6-linked sialic acids, and swine express both receptors. This biochemical arrangement in swine facilitates simultaneous dual infections with avian- and human-adapted viruses and the attendant risk of hybrid viruses. , ,
Seasonal influenza strains in humans bind readily to α2,6 linkages found in the upper airways. This usually leads to high transmission frequency by airborne droplets deposited upon the upper airways, but a relatively low risk of primary influenza pneumonia. However, the lower airways and alveolar pneumocytes of humans express α2,3-linked sialic acids, and avian viruses that bind efficiently to α2,3 linkages can cause severe lower tract disease if deposited into the distal airways. The avian strain of H5N1 preferentially binds to α2,3 linkages in the distal airways, and therefore is poorly transmissible but can cause severe pneumonia. Poultry workers, particularly those in close proximity to infected livestock, can receive large enough inoculums into the distal airways to cause severe pneumonia, with mortality rates ranging from 50%–70%. ,
Notably, the Spanish flu of 1918 (subtype of H1N1) expressed an HA that could bind with high affinity to both α2,6- and α2,3-linked sialic acids. , This resulted in high transmissibility and spread within the upper airways in addition to severe pneumonia. Disturbingly, the recent 2009 pandemic subtype of H1N1 also bound with high affinity to both α2,6- and α2,3- linkages, resulting in potential for high transmissibility and severe devastation. However, much lower case-fatality rates were seen (<0.1%), likely attributed to fewer viral virulence factors ( Table 119.1 ). Other mitigating factors included infection control strategies, large-scale vaccination, improved supportive care, and effective antivirals. Additionally, populations born before the early 1950s had a degree of clinical protection from preexisting immunity induced by historical circulation of other H1N1 viruses that evolved from the 1918 H1N1 pandemic virus.
| Viral Trait | Mechanism of Virulence | Comments |
|---|---|---|
| HA and NA – epitope variations | Immune escape from recognition by preexisting antibodies within the population from previous virus exposure | Antigenic drift (point mutations) leads to epidemics; antigenic shift (reassorted genomes) leads to pandemics |
| HA – cleavability | HA undergoes proteolysis by host-derived proteases before receptor binding | Readily cleaved HA is associated with avid binding and disease severity |
| HA – binding preference | α2,3-linked sialic acid receptor in alveoli; α2,6 linkage in upper airways | Viruses that bind to the α2,3 linkage or both α2,3 and α2,6 are more virulent; cause lower tract disease and increased transmissibility |
| HA:NA ratio | NA cleaves sialic acid glycopeptides on host epithelium (binding site for HA) | Optimal ratio of NA and HA activity needed for high replication and viral shedding |
| NS-1 | This viral nonstructural protein inhibits host innate and adaptive immune response; inhibits IFN expression; blocks T-cell activation | Some subtypes have truncated variants of NS-1, associated with lower virulence |
| PB1-F2 | This viral peptide targets host mitochondria, induces apoptosis in CD8 T cells and alveolar macrophages, increases severity of viral pneumonia, increases risk of secondary bacterial infection | Many subtypes do not encode full-length PB1-F2; truncated forms of PB1-F2 are associated with lower virulence; absence of full-length PB1-F2 in 2009 H1N1 pandemic strain led to lower pathogenicity |
| NA inhibitor resistance | H275Y mutation in the viral NA gene, alters NA inhibitor binding site, leads to oseltamivir resistance | Previously common mutation in seasonal H1N1, but rarely seen in the 2009 H1N1 pandemic strain and in circulating subtypes since then |
| M2 inhibitor resistance | S31N mutation in viral M2 gene, alters M2 inhibitor binding site, leads to amantadine and rimantadine resistance | Common in both H3N2 and H1N1 circulating subtypes |
| PB2 temperature range | Viral polymerase preferentially replicates at lower temperatures in mammalian respiratory tract and at higher temperatures in avian GI tract | Mutant polymerases can effectively replicate at broad temperature range, aiding transfer from birds to humans |
The major subtypes of influenza A currently circulating in humans include several distinct clades of H1N1 and H3N2. The avian flu (H5N1) continues to cause sporadic disease in humans and circulate in birds. Although this is often cited as the most recent pandemic threat, it has yet to demonstrate sustained person-to-person or community-level transmission. Other avian influenza subtypes (H7N2, H7N3, H7N7) cause sporadic outbreaks of mild severity, but more alarming was a novel avian flu (H7N9) that emerged in China in 2013, causing severe pneumonia. Thankfully, inefficient person-to-person transmission has limited spread of this serious subtype. Several other influenza A subtypes are circulating sporadically in humans and animals, but of the 198 possible subtype combinations, only 131 have been detected in nature. The rapid and dramatic evolution of influenza viruses in animals and humans continues to impose a threat to our global population of potential catastrophic proportions. For current updated information on local influenza activity and subtype distribution, the reader is directed to http://www.cdc.gov/flu .
Classical seasonal influenza in adults is typified by a 4- to 5-day period of sudden-onset fever, chills, upper respiratory tract symptoms, headache, muscle pain, and weakness. High fever is characteristic and correlates with severity of initial symptoms. Fevers typically last for 3 days but may persist for up to 8 days. Diarrhea is more common with influenza than with most other viral upper respiratory tract infections. Other distinguishing features include the predominance of systemic symptoms, often overshadowing the respiratory symptoms (dry cough, pharyngeal pain, nasal discharge and obstruction).
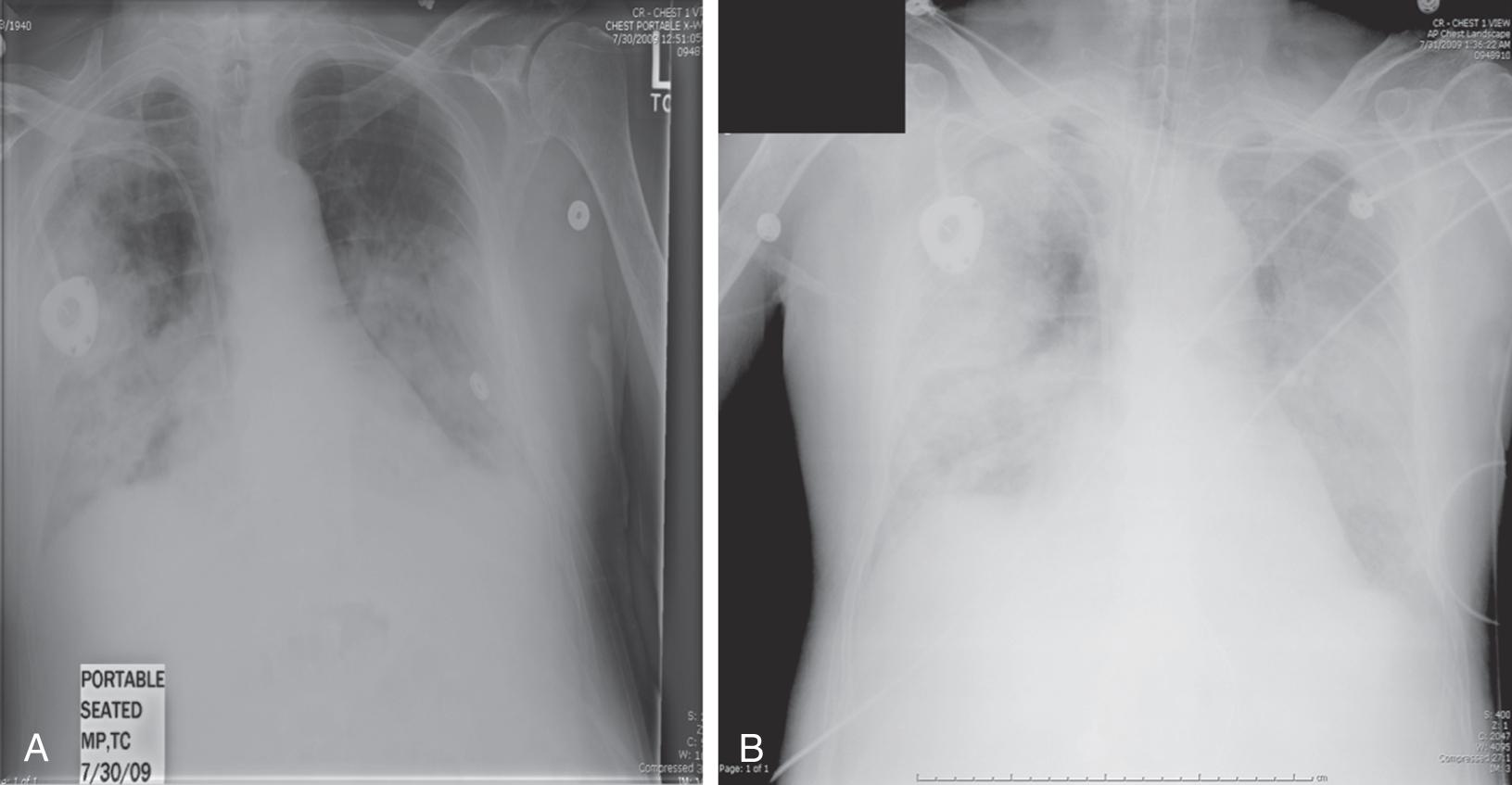
Primary influenza pneumonia is the most common serious complication and occurs in up to 29% of patients hospitalized for influenza. Early symptoms are indistinguishable from typical influenza aside from a rapid and fulminant progression of hypoxemia and widespread bilateral interstitial pneumonitis on chest radiograph ( Fig. 119.2 ). These patients generally present to the hospital within 4 days of symptom onset with shortness of breath and require ICU admission within <1 day of hospital presentation. Influenza pneumonitis frequently progresses to hypoxemic respiratory failure, with over 80% of patients requiring mechanical ventilation. Very few patients can successfully be managed with noninvasive ventilation strategies alone. The mean admission PaO 2 /FiO 2 in the ICU is <150 mm Hg, and advanced ventilatory/oxygenation strategies are often needed for refractory cases.
Secondary bacterial pneumonia and influenza-bacterial coinfection are frequently severe, and also common, affecting between 11% and 35% of hospitalized cases. Secondary bacterial pneumonia is generally attributable to damaged airways and poor mucociliary clearance after severe influenza pneumonia. Patients frequently experience a period of improvement after the classical influenza illness before recrudescence of fever, cough, purulent sputum, and consolidation on chest radiograph. Influenza-bacterial coinfection is more complex, with bacterial and viral synergism. Viral PB1-F2 proteins induce pneumocyte apoptosis, which facilitates Streptococcus pneumoniae growth in lung tissue. Sialic acids that have been cleaved by NA allow more efficient S. pneumoniae binding to epithelial surfaces and increase lethality in experimental models. ,
The most common pathogens involved in secondary bacterial pneumonia and influenza-bacterial coinfection include S. pneumoniae (up to 35%) and Staphylococcus aureus (up to 28%), but others may be implicated, including Streptococcus pyogenes, Haemophilus influenzae, Moraxella catarrhalis, and gram-negative bacilli. , Given the frequency of secondary bacterial infection, clinicians should have a low threshold for considering antibacterial agents against these commonly observed pathogens.
In children with severe influenza infection, the median age of hospitalized patients is 5.0 years (range 1 month to 17 years). , , , One or more chronic comorbid illnesses are typically observed (70.2%): lung disease (44%), neurologic diseases (19%), immune suppression or immunodeficiency (16%), history of prematurity (9%), and congenital heart disease (7%). Mechanical ventilation is used in approximately 68% of children admitted to the ICU, and the median duration of ventilation is 6 days (range 0–67).
Influenza may cause serious or life-threatening complications in other organ systems, including cardiac, renal, hepatic, and neurologic disease. , Myocarditis is a common complication of influenza illness and may occur in up to 10% of hospitalized patients. The diagnosis is made on the basis of presenting symptoms, elevated cardiac enzymes, and echocardiographic findings. Importantly, myocarditis may be present with or without serious pulmonary involvement. Occasionally, myocarditis leads to serious complications in the ICU, including atrial and ventricular arrythmias, atrioventricular (AV) conduction block, pericardial effusions with or without tamponade, acute myocardial infarctions, and reduced ejection fraction with heart failure. , Reduced ejection fraction may occur in up to 80% of patients with influenza myocarditis, leading to serious repercussions in patients with critical illness and shock.
Septic shock may occur in up to 15%–30% of critically ill patients with influenza and is more common in the setting of bacterial coinfection. In addition to the effects of myocarditis, a severe surge of cytokines and capillary leak causes reduced systemic vascular resistance, reduced perfusion pressure, microcirculatory coagulopathy, reduced end-organ perfusion, and cellular toxicity. The presence of shock is associated with approximately 30% mortality, and studies have shown that S. aureus coinfection is an independent predictor for mortality. Acute kidney injury (AKI) may ensue as a result of pre-renal and tubular injury secondary to sepsis and/or rhabdomyolysis. Many patients also receive nonsteroidal antiinflammatory drugs (NSAIDs) because of myalgias and other systemic symptoms, adding potential nephrotoxicity.
Neurologic complications often accompany severe influenza infection, including encephalitis, encephalopathy, and seizures. Computed tomography (CT) and magnetic resonance imaging (MRI) studies of the brain are often abnormal, but findings are nonspecific. Guillain-Barré syndrome (GBS) incidence increases in the months during and after seasonal influenza. It has been suggested that influenza may trigger this serious neurologic complication.
Patients at highest risk for severe infection and influenza complications requiring ICU care are those with a history of chronic lung disease and/or severe immunosuppression. Underlying lung disease occurs in approximately 20% of patients with influenza in the ICU, most commonly asthma and chronic obstructive pulmonary disease (COPD). Other special populations at increased risk of severe disease and mortality are elderly patients (>65 years of age), women who are pregnant or recently postpartum, residents of long-term care facilities, those with obesity, and Indigenous populations. , Additional risk factors include patients with other chronic medical conditions, including neurologic diseases (12%), hematologic or oncologic conditions (9.9%), and cardiac conditions (4.6%). However, approximately half of hospitalized patients during pandemic years might otherwise be healthy. , ,
Whereas most patients with influenza do not develop critical illness, 11%–19% of patients hospitalized with laboratory-confirmed influenza require treatment in the ICU. , Those who develop critical illness often deteriorate rapidly, and severe illness may be protracted. For patients requiring mechanical ventilation, the mean duration is approximately 5–12 days. Many patients are difficult to oxygenate, and patients often require ancillary therapies such as extracorporeal membrane oxygenation (ECMO) and nitric oxide therapy, leading to increased length of ICU stay and excess mortality. , ,
Similar to prior pandemics, the primary cause of death remains complications from secondary bacterial infection, including multisystem organ failure and cardiovascular collapse. , However, mortality rates have significantly improved in recent years (∼17%) as a result of better oxygen delivery, supportive ICU care, vaccination, antibacterial agents, and antiviral medications. Unfortunately, sophisticated ICU care remains out of reach for many patients in developing countries, and case-fatality rates remain regrettably high.
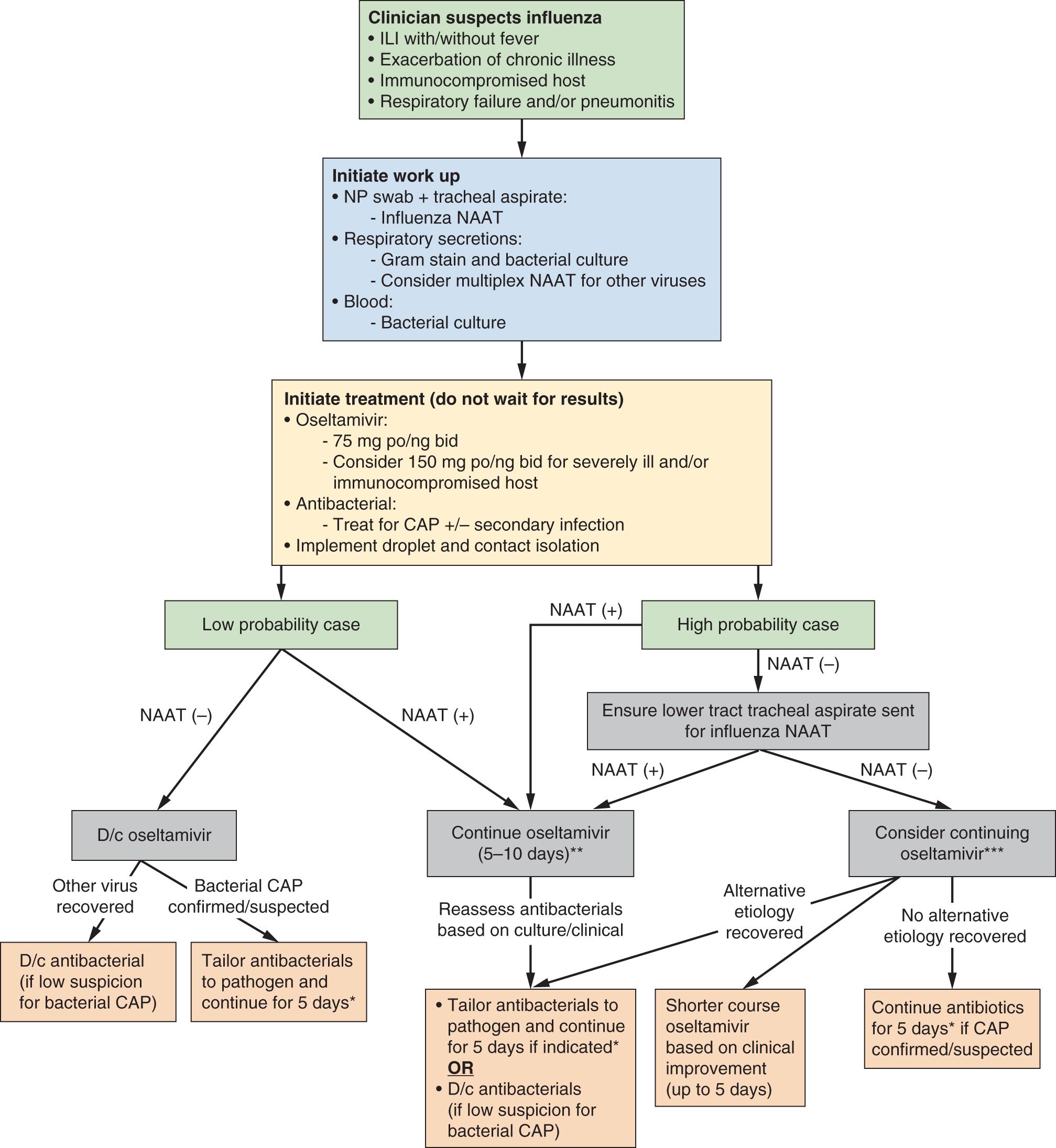
Clinicians should suspect and test for influenza in all patients who present to the hospital during influenza season with an acute respiratory illness, acute decompensation of chronic medical conditions, or any immunocompromised patients. Importantly, a history of current-season influenza vaccination does not exclude the diagnosis of influenza because of variable vaccine effectiveness from year to year. Uncomplicated disease does not typically result in significant respiratory compromise, but shortness of breath should raise concern for severe disease. Other clinical signs indicating severe infection include hemoptysis, frothy pink sputum, and purulent sputum with diffuse lung crackles. Percutaneous oximetric assessment of oxygenation or arterial blood gas evaluation of PO 2 should be performed when assessing a patient with suspected severe influenza. Relative hypoxia should trigger further assessment, including a chest radiograph. Laboratory findings commonly found at presentation with severe disease typically include normal to low leukocyte count and elevated creatine kinase. , , A leukocyte shift to immature forms is unusual in the absence of bacterial coinfection. An approach to the diagnosis and treatment of influenza is presented in Fig. 119.3 .
Early laboratory diagnosis of influenza infection is greatly facilitated by the use of rapid reverse transcriptase–polymerase chain reaction (RT-PCR) testing. Clinicians should collect nasopharyngeal specimens using flocked swabs. In patients who require mechanical ventilation, endotracheal aspirates should be collected in addition to the nasopharyngeal specimens in order to maximize diagnostic yield. , , Virus may only be detected in lower tract samples in patients with severe pneumonitis. For critically ill patients, the use of multiplex nucleic acid amplification tests to detect a panel of other respiratory viruses is recommended. The use of immunofluorescent techniques, enzyme-linked immunoassays, and other rapid diagnostic tests is discouraged because of lack of diagnostic sensitivity. , , Viral cultures require up to 1 week for processing and should only be considered to provide isolates for further characterization.
Starting March 2009, a novel strain of a human-adapted influenza virus, influenza A(H1N1)pdm09, spread from an initial large outbreak in Mexico to virtually all countries of the world. By September 27, 2009, there were over 340,000 cases with 4100 deaths worldwide. , Over the period of June to September 2009, there were dramatic spikes in Australia, New Zealand, and South America that breached the capacity for ICU care in some regions. Ultimately, 18,500 lab-confirmed influenza deaths occurred worldwide, but modelling data estimate that >300,000 respiratory or cardiovascular deaths attributed to influenza occurred globally, predominantly in patients under 65 years of age and in low- to middle-income countries. This led to lessons that have advanced our current understanding of influenza and control of other emerging global pandemics. Public health officials were not prepared to quickly assess the transmissibility, severity, and impact of disease. Implementation of risk management plans and global communications were delayed. Despite blunting of the North American spread by widespread deployment of an effective, inactivated, monovalent vaccine program, as many as 61 million Americans were infected, with 274,000 hospitalizations and approximately 12,500 deaths between April 2009 and April 2010.
The events that transpired in Canada were illustrative of the influenza situation in much of the Northern Hemisphere. Among critically ill Canadian patients with influenza A(H1N1)pdm09, the mean age was 32 years, with a possible predilection for more severe disease in women (67% of patients). Pregnant women, obese patients, and Indigenous Canadians were overrepresented and suffered from a disproportionate high level of disease severity, but only 30% suffered from serious comorbid illness. , , , Similar clinical findings were reported in other regions of the world. , , , Nosocomial transmission occurred in approximately 10% of patients. Transmission to healthcare workers occurred early in the outbreak, but this was minimized once the pandemic was recognized and appropriate infection-control safeguards were instituted. A summary of clinical risk factors, comorbidities, and severe complications associated with the latest influenza pandemic is found in Table 119.2 .
| Risk Factors and Comorbidities | Comments |
|---|---|
| ** Age <5 years | Especially children <2 years; those with chronic cardiopulmonary disease; <6 months of age associated with higher hospitalization and mortality |
| Age >65 years | Poor vaccine response; reduced host response to influenza infection |
| ** Chronic cardiopulmonary diseases | Chronic pulmonary disease at highest risk; includes COPD, asthma, cystic fibrosis; cardiac disease includes congestive heart failure, coronary artery disease |
| Metabolic disease and chronic liver disease | Diabetes mellitus; cirrhosis |
| Chronic neurologic illness | Includes disorders of the brain, spinal cord, and peripheral nerves; seizures, epilepsy; stroke; developmental delay and institutionalization |
| Pregnancy | Includes all stages of pregnancy, up to 2 weeks postpartum; women in the third trimester at particular increased risk |
| Obesity | Particularly with extreme obesity; BMI >35 kg/m 2 |
| Hemoglobinopathy | Sickle cell disease |
| ** Immunosuppression | Glucocorticoids, chemotherapy, malignancy, HIV, organ transplant recipients; lung transplant and advanced HIV at highest risk |
| Indigenous populations | Increased prevalence of some chronic health conditions; social determinants of health; healthcare access |
| Access to healthcare services | Nursing home and long-term care residents; hospitalized patients |
| Secondary bacterial pneumonia | Longer ICU and hospital stays; more nosocomial complications; greater mortality rate |
** Comorbidities associated with highest risk of influenza complications, including hospitalization and mortality.
The most common specific symptoms with influenza A(H1N1)pdm09 included high fever and respiratory symptoms in greater than 90% of patients. Weakness and myalgias were less common. A variety of severe clinical syndromes necessitating ICU care were observed, including:
Rapidly progressive, diffuse pneumonitis associated with severe, refractory hypoxemia in relatively healthy teens or adults
Secondary bacterial pneumonia, or influenza-bacterial coinfection, frequently with gram-positive pathogens including S. pneumoniae and S. aureus
Acute and prolonged exacerbation of asthma or COPD in those with preexisting disease
Life-threatening decompensation of chronic underlying disease in those patients with serious comorbidities, including congestive heart failure, chronic renal failure, end-stage liver disease, poorly controlled diabetes, or immune compromise
Bronchiolitis and croup in infants and young children, which frequently required hospitalization, but not ICU care
During pandemic periods, relatively young patients with few serious comorbidities can be affected because of widespread lack of immunity to novel circulating viruses and vigorous intrinsic inflammatory responses. Additionally, vulnerable populations with usual risk factors can be greatly affected, similar to nonpandemic years. The impact on hospital care and costs can be dramatic and necessitates thoughtful critical care resource management in modern ICUs that have limited surge capacity to meet sudden demand for a future pandemic. , , Establishing a pandemic preparedness plan is imperative to ensure institutions have the capacity to respond to emerging pandemics and mitigate health impact.
Almost all patients with severe influenza in the ICU will have deficits in oxygenation, leading to acute respiratory distress syndrome (ARDS) and requiring ventilatory support. , Shock and renal failure are also common, often exacerbated by efforts to optimize oxygenation through diuresis, coupled with high intrathoracic pressures and limited venous return. , ,
Despite ARDS standard treatment with lung-protective low tidal volume ventilation and open lung strategy with high positive end-expiratory pressure (PEEP), primary influenza pneumonia often results in a relative insensitivity to usual measures of oxygenation and markedly abnormal lung compliance. Control mode ventilation with appropriate sedation and use of neuromuscular blockade to avoid ventilatory dysynchrony and high airway pressures are often needed. Avoidance of volume overload (and judicious diuresis) may also reduce duration of ventilation and length of stay in the ICU for most patients with ARDS. , Because some patients worsen despite these standard measures, early consideration of transfer to a referral center with experience in rescue therapies should be considered, particularly if severe hypoxemia is encountered.
Ancillary strategies may be required to improve refractory hypoxemia, minimize ventilator-induced lung injury, and improve cardiothoracic dynamics. Strategies include prone ventilation, lung recruitment maneuvers, inhaled nitric oxide, and ECMO. These strategies require considerable resources and expertise and offer variable impacts on clinical outcomes.
Early prone ventilation has been demonstrated to be an effective strategy to improve oxygenation and mortality for most causes of ARDS. , Although little is known about influenza-specific outcomes with prone ventilation, it is recommended for those with severe ARDS in the appropriate experienced facility and in the absence of contraindications. Lung recruitment maneuvers may be used to recruit collapsed alveoli, but because of a lack of convincing benefit, it should not be done routinely. Use of inhaled nitric oxide has demonstrated improvements in oxygenation; however, studies have failed to show improvement in morbidity or mortality, and some studies suggest there may be an increased risk of renal injury. Inhaled nitric oxide should not be considered standard therapy. Since the 2009 pandemic, there has been a considerable rise in the use of venovenous ECMO in patients with refractory ARDS from influenza. Although data are limited, pooled analyses suggest that this modality may reduce mortality and should be considered for severe influenza where other options do not exist. , ,
Septic shock is common in critically ill patients with influenza and typically results in refractory hypotension and need for moderate to high doses of vasopressors. Optimal fluid resuscitation strategies remain unknown in this setting and must be balanced with fluid-restrictive strategies that aim to improve oxygenation. It is recommended that all patients with severe influenza, ARDS, and septic shock be treated in accordance with international guidelines and receive at least 30 mL/kg of crystalloid via bolus dosing. The development of AKI often necessitates renal replacement therapy to correct bicarbonate deficiencies to allow for increased tolerability of hypercapnia when poor lung compliance prohibits effective ventilation.
For patients with influenza-associated critical illness, clinical improvement is slow and ICU length of stay is typically prolonged. Clinicians should focus on minimizing the complications of critical illness, including ICU-acquired weakness, delirium, and psychosocial disturbances. Long-term ventilation weaning is often needed, and tracheostomy may be required.
In severely ill patients with suspected influenza, early initiation of antiviral therapy is predicated on clinical presenting features and epidemiologic data. Therapy must not be delayed pending laboratory confirmation, as initiation within the first 48 hours of illness is most likely to provide benefit. , For critically ill individuals, early initiation of antiviral therapy may improve survival, but antivirals should be administered for all those with severe illness, regardless of the duration of symptoms before hospitalization. The choice of antiviral therapy is dependent on circulating influenza subtypes and local surveillance data examining the risk of oseltamivir resistance.
The NA inhibitor oseltamivir is the preferred agent for severe influenza. Oseltamivir works by selectively binding to the influenza envelope protein NA, inhibiting its enzymatic activity and preventing spread to other uninfected cells. It reduces the duration and severity of symptoms and reduces viral shedding. , It may also reduce the risk of secondary bacterial infection and reduce mortality in critically ill patients, though data are limited to pooled analyses of studies that included less severe cases. , The typical dosing for oseltamivir is 75 mg orally twice daily, which must be adjusted for renal dysfunction. Doubling or tripling the dose (150–225 mg orally twice daily) is typically well tolerated, but unnecessary in hospitalized patients without severe illness , ; however, this strategy may benefit severely ill patients, particularly with immunocompromise or patients infected with H5N1 avian influenza. In patients with primary influenza pneumonia, viral shedding is often prolonged, with viral clearance occurring after a median of 11 days while on oseltamivir. In these severely ill patients, an extended duration beyond 5 days may be warranted, particularly for those with ARDS or immunocompromise. Current recommendations suggest continued use until adequate clinical response or infection resolution, but formal studies on optimal duration are lacking. Oseltamivir is very well tolerated, but occasionally associated with nausea and vomiting, which rarely requires discontinuation of therapy.
Rare oseltamivir-resistant viruses were isolated during the 2009 H1N1 pandemic but remain very uncommon in current circulating subtypes. The majority of influenza B and influenza A (H3N2) remain susceptible to NA inhibitors. The resistance mutation H275Y, which renders oseltamivir and peramivir ineffective, has rarely been detected (<1.0%) in influenza A (H1N1), but sensitivity to zanamivir is preserved. , This is in contrast to the seasonal H1N1 subtypes circulating before the 2009 pandemic year, which were often resistant to oseltamivir.
Alternative NA inhibitors may be available, including peramivir and zanamivir. These agents are not currently recommended because of limited data in patients with severe lower respiratory tract disease. Peramivir is given by intravenous (IV) route at a typical single adult dose of 600 mg, but duration may be extended to once daily for 5 days in critically ill patients. In studies of ambulatory patients with lab-confirmed influenza, peramivir was shown to reduce time to symptom resolution compared with placebo and to be noninferior to oseltamivir. , In one study of critically ill patients, similar mortality and ICU length of stay were observed when peramivir was compared with oseltamivir; therefore IV peramivir may be a reasonable alternative to oseltamivir in patients who are unable to tolerate enteral agents. , Although GI absorption of oseltamivir among critically ill patients had been questioned, comparable blood concentrations have clearly been demonstrated in critically ill influenza patients compared with healthy volunteers. , Zanamivir by inhalation is not recommended in critically ill patients because of the risk of bronchospasm, lack of efficacy data, and lack of systemic absorption; however, it may be an alternative option for ambulatory patients without asthma or COPD to reduce the duration of symptoms.
Baloxavir marboxil is a newer antiviral that selectively inhibits cap-dependent endonuclease, preventing initiation of influenza messenger RNA (mRNA) synthesis and blocking viral proliferation. It is effective in reducing duration of symptoms in acute uncomplicated influenza, but its role in severe infection, in critically ill patients, or in combination therapy with oseltamivir has not been studied. The adamantane family of antivirals, including amantadine and rimantadine, are no longer routinely recommended because of their limited spectrum of activity (only effective against influenza A) and high levels of circulating resistance.
The most important adjunctive pharmacologic treatment in critically ill patients with influenza is empiric broad-spectrum antibiotics to cover possible secondary bacterial pneumonia or influenza-bacterial coinfection. Antibiotics should be selected according to local antibiograms to cover the most common pathogens involved, namely S. pneumoniae, S. aureus (including methicillin-resistant S. aureus [MRSA]), S. pyogenes, and occasionally, gram-negative bacteria. De-escalation of antibiotics is appropriate as lower tract respiratory cultures are finalized. If secondary decompensation occurs after a period of initial clinical improvement, additional antibiotic treatment may be warranted while investigating for newly acquired bacterial or fungal nosocomial infections, examining for empyema, lung abscess, sepsis, or other causes of deterioration.
Many other potential adjunctive therapies for the treatment of severe influenza have been studied. Use of IV immunoglobulins, convalescent serum or plasma, and hyperimmune globulin derived from donors who have recovered from influenza have demonstrated mixed results. Meta-analyses suggest that early administration of such products may be associated with improved outcomes, but the effect on survival remains uncertain. , In addition, case series suggest that similar therapies may be of use in severe influenza A (H5N1) infection, , but more data are needed before recommending these therapies.
Glucocorticoids have been used to reduce inflammatory responses in influenza, septic shock, and a variety of other infectious conditions. Although few randomized trials have been performed, meta-analyses of largely observational studies demonstrated prolonged viral replication, more frequent superinfections with bacterial and fungal pathogens, and increased mortality. Because of the very low quality of evidence, potential benefits of glucocorticoid treatment remain uncertain. But extreme caution is needed, and their use in primary influenza pneumonia should be avoided in the absence of a clear alternative indication.
The effect of statins in reducing inflammation and improving illness severity with influenza has been studied in small observational studies. Although there is a suggestion that these may improve outcomes, further trials are needed to assess the risks and benefits before recommending their use. One small open-label trial examined the role of clarithromycin and naproxen in combination with oseltamivir for the treatment of hospitalized patients with influenza. Although this trial demonstrated a reduction in mortality, patients who were already critically ill were not included. This combination merits further investigation.
Influenza is transmitted from person-to-person primarily through large-particle respiratory droplets; thus patients with suspected influenza should be instructed to use appropriate hand hygiene, cough etiquette, and be given a face mask to wear upon entry into healthcare facilities. Patients with suspected influenza should be in single-patient rooms, if available, during the initial phase of hospital admission. If clinical demand exceeds the availability of single rooms, cohorting patients with influenza into common rooms may be necessary. Patients who must be transported outside of the room should wear a mask if tolerated, or when necessary, an oxygen delivery system that limits the spread of aerosols. Influenza vaccination is the best way to prevent or mitigate the severity of influenza illness, and in the absence of contraindications, is recommended annually for the entire population, particularly those with risks for severe disease. Vaccination and postexposure chemoprophylaxis are recommended for close contacts who are at very high risk of influenza complications.
Healthcare personnel (HCP) should apply droplet precautions by wearing standard surgical tie masks and use appropriate hand hygiene. Studies have found N95 masks offer no additional protection compared with surgical masks, yet many still advocate their use during cough-inducing procedures. HCPs should also consider contact precautions with gloves when anticipating contact with bodily fluids or touch with contaminated surfaces. Protective eyewear is recommended when providing direct care in close proximity to the patient. The annual influenza vaccine should be mandatory for all HCPs unless specific contraindications exist. Ill HCPs with fever and respiratory symptoms should be instructed not to work until fever has dissipated for >24 hours. For additional information regarding infection prevention strategies in healthcare settings, the reader is directed to updated recommendations from the Centers for Disease Control and Prevention (CDC) at https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm .
Coronaviruses are ubiquitous around the world and frequently cause mild to moderate upper respiratory tract infections in humans. These infections seldom cause critical illness; however, three notable severe syndromes were noted with coronaviruses emerging in the twenty-first century: severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease-19 (COVID-19). SARS is caused by the SARS-associated coronavirus (SARS-CoV), which emerged in November 2002. The first cases were discovered in China, with subsequent clusters occurring in countries around the world. Its somewhat unusual lengthy prodrome of fever and constitutional symptoms typically lasted 3–7 days without respiratory symptoms. Subsequently, dry cough would ensue with potential progression to ARDS and case fatality rates of 9.6%. Ultimately, SARS occurred in localized epidemics in several countries, infecting approximately 8000 people and 774 deaths globally. Because of intensive infection-control strategies, it disappeared as quickly as it emerged by July 2003.
MERS has become an endemic viral pathogen in the Middle East, first appearing in June 2012. Over 2400 lab-confirmed cases have been reported, predominantly from the Arabian Peninsula. Although transmission specifics are not fully understood, person-to-person transmission has occurred, and contact with camels appears to be an important risk factor. MERS predominantly causes a severe, acute, febrile, respiratory syndrome that frequently is accompanied by shock and AKI. The vast majority of patients require ICU care and mechanical ventilation. Hospital mortality appears to be the highest of the coronaviruses, approaching 25% in hospitalized patients and 50% in those who require ICU care.
In December 2019, a novel coronavirus emerged, causing a cluster of pneumonia cases in Wuhan, China. This novel coronavirus, named severe acute respiratory coronavirus 2 (SARS-CoV-2), is genetically similar to that of SARS-CoV. At the time of this writing, the emerging epidemic is rapidly spreading throughout Southeast Asia, and emerging in many other countries, threatening a global pandemic. Genetic sequencing indicates that the virus probably “jumped” animal species from snakes to bats and then to human hosts. Person-to-person spread of SARS-CoV-2 has been demonstrated from epidemiologic studies, and healthcare workers have been disproportionately affected, similar to prior outbreaks of serious viral respiratory illnesses.
The incubation period of SARS-CoV-2 is thought to last up to 14 days, but most symptoms appear within 5 days of exposure. Clinical manifestations of COVID-19 vary from asymptomatic to severe respiratory compromise. Most patients experience an abrupt onset of fever and respiratory symptoms. Approximately 15% develop severe disease with lower respiratory tract involvement, 5% develop critical illness, and 2.3% of patients die. Mortality rates are much higher in elderly adults and approach 50% for patients in the ICU. Strict isolation precautions and extreme infection control measures have been important in attempts to limit the spread of this outbreak. Work on vaccines, immunotherapy, and antiviral chemotherapies are underway, but currently treatment is simply supportive.
Although a highly effective vaccine has dramatically reduced the incidence of measles in the past decades, recent social and political factors have contributed to low vaccination rates and resurgence of disease in some areas. The World Health Organization (WHO) recently announced a staggering statistic that measles accounted for more than 140,000 deaths worldwide in 2018. The mortality rates are particularly high in children under 5 years of age. Measles typically causes a relatively mild childhood exanthem in otherwise healthy children. This highly contagious virus causes rapid spread of fever, rash, cough, conjunctivitis, and coryza. Regrettably, unvaccinated, malnourished children in developing countries, often with other concomitant respiratory illnesses, fare much worse and can die from measles-induced pneumonia or encephalitis or suffer from permanent blindness and/or deafness.
Pneumonia is the most common life-threatening complication of severe measles infection, often resulting in death, particularly in patients <5 years or >20 years of age. Additionally, measles may cause persistent morbidity and mortality after naturally acquired infection secondary to immunosuppression and risk for secondary infection. Rarely, measles can be complicated by encephalitis, acute demyelinating encephalomyelitis, or, years later, with subacute sclerosing panencephalitis (SSPE). Treatment is largely supportive; however, vitamin A may reduce morbidity and mortality, and ribavirin may be given for life-threatening infection.
Herpes simplex virus (HSV), varicella-zoster virus (VZV), and herpes B virus are large, enveloped DNA viruses that exhibit lifelong latent infection. , They all cause vesicular skin lesions but may cause other systemic manifestations. In the ICU, these viruses may be encountered as part of a life-threatening clinical syndrome with primary infection or as a result of secondary reactivation of latent infection, typically causing mild breakouts of skin lesions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here