Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Influenza is an acute febrile respiratory viral illness that usually occurs in annual outbreaks of varying severity and occasionally causes worldwide epidemics (pandemics). Increasingly, sporadic zoonotic infections, sometimes associated with limited human-to-human transmission, are being reported. Influenza viruses infect the respiratory tract, are highly contagious, and classically produce prominent systemic symptoms early in the illness. Influenza infection causes various clinical syndromes in adults, including nonfebrile common colds ( Chapter 329 ), pharyngitis ( Chapters 269 and 397 ), tracheobronchitis ( Chapter 84 ), pneumonia ( Chapter 85 ), and a range of nonrespiratory complications. Conversely, infections with other respiratory viruses, such as respiratory syncytial virus (RSV [ Chapter 330 ]) or adenovirus ( Chapter 333 ), may produce influenza-like illness. Influenza A viruses have caused five pandemics of varying severity within the past 120 years ( E-Table 332-1 ). The 1918–1919 pandemic caused at least 500,000 deaths in the United States and more than 40 million worldwide, whereas the 2009 H1N1 pandemic was associated with substantially less mortality. Seasonal epidemics can cause enormous morbidity, economic loss, and often substantial mortality. Each year in the United States, seasonal influenza epidemics are associated with an estimated 4.3 to 16.7 million medical visits, 140,000 to 710,000 hospitalizations, and 12,000 to 56,000 respiratory and circulatory deaths. During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic ( Chapter 336 ), masking and social isolation led to a decline in diagnosed cases of influenza.
Influenza viruses belong to the family Orthomyxoviridae and are divided into four types (A, B, C, and D) based on their protein composition and other properties ( E-Table 332-2 ). The virion ( E-Fig. 332-1 ) is a medium-sized enveloped pleomorphic particle covered with two types of surface glycoprotein spikes: the trimeric hemagglutinin (H or HA) and the tetrameric mushroom-shaped neuraminidase (N or NA). The envelope is composed of a lipid bilayer overlying the matrix (M1) protein that surrounds the viral genome, which consists of eight segments of single-stranded negative-sense RNA in influenza A and B viruses. Influenza C viruses have seven segments and only a single surface glycoprotein. Genomic replication occurs in the nucleus of infected cells, and multiple cellular proteins and pathways are involved during the infection of host cells.
| YEAR ONSET | INTERVAL (YR) | SUBTYPE DESIGNATION | EXTENT OF ANTIGENIC CHANGE IN INDICATED SURFACE PROTEIN † | SEVERITY OF PANDEMIC (MORTALITY) ∗ |
|---|---|---|---|---|
| 1889 | 42-59 | H3N? | H+++N? | Severe |
| 1918 | 29 | H1N1 ‡ | H+++N+++ | Very severe |
| 1957 | 39 | H2N2 | H+++N+++ | Severe |
| 1968 | 11 | H3N2 | H+++N− | Moderate § |
| 1977 | 9 | H1N1 | H+++N+++ | Negligible || |
| 2009 | 32 | H1N1 | H++N++ | Mild-moderate || |
∗ Severity is defined as the global impact on mortality.
† Compared with antecedent or cocirculating virus.
‡ Formerly designated H0N1 (swine virus prototype) or Hsw1N1.
§ The population had some immunity to N2 neuraminidase.
|| The older population was largely immune because of previous infection with earlier circulating, antigenically identical (1977) or related (2009) viruses; those born after 1957 were primarily affected. The impact of the 2009 pandemic virus, based on estimated years of life lost, was comparable to that observed in the 1968 pandemic in the United States.
| DESIGNATION | LOCATION (APPROXIMATE SIZE) | FUNCTION | OTHER |
|---|---|---|---|
| Hemagglutinin (HA) | Surface, transmembrane (566 aa) | Cell attachment via sialic acid–bearing receptors and penetration; fusion of host cell and viral membranes | Type-, subtype-, and strain-specific antigens; key antigen in inactivated vaccines |
| Neuraminidase (NA) | Surface, transmembrane (454 aa) | Virus release and spread; enzymatic activity causes removal of sialic acid residues from receptors | Type-, subtype-, and strain-specific antigens; activity linked to risk for pneumonia; site of action of neuraminidase inhibitors |
| M1 or matrix | Internal (252 aa) | Major structural envelope protein; virus assembly | Type-specific antigen; conserved T-cell epitopes |
| M2 | Surface, transmembrane (97 aa) | Ion channel; virus uncoating and budding | Influenza A only; site of action of amantadine/rimantadine; ectodomain as possible vaccine candidate |
| Nucleoprotein (NP) | Internal (498 aa) | Major ribonucleoprotein complex component; associated with RNA and polymerase proteins | Type-specific antigen; conserved T-cell epitopes |
| Polymerase proteins (PB1, PB2, PA) | Internal (PB1-757 aa, PB2-759 aa, PA-716 aa) | Viral RNA replication and mRNA transcription (RNA-dependent RNA polymerase, cap-binding, endonuclease) | Determinant of replication efficiency; specific PB2 mutations associated with mammalian adaptation; site of action of polymerase inhibitors |
| NS1 | Nonstructural (230 aa) | Multifunctional; regulation of viral RNA and protein synthesis; host protein interactions | Interferon antagonist; inhibition of innate immune signaling |
| NEP (NS2) | Internal (121 aa) | Nuclear export factor | From splicing of NS1 mRNA |
| PB1-F2 | Nonstructural (87 aa) | Pro-apoptotic factor; pro-inflammatory effects; interferon antagonist | Expressed from 1+ reading frame of PB1 of certain viruses |
| PA-X | Nonstructural (41-61 aa) | Endonuclease activity; inhibition of host protein synthesis | Modulation of the host response to infection; expressed by 1+ ribosomal frame-shifting |
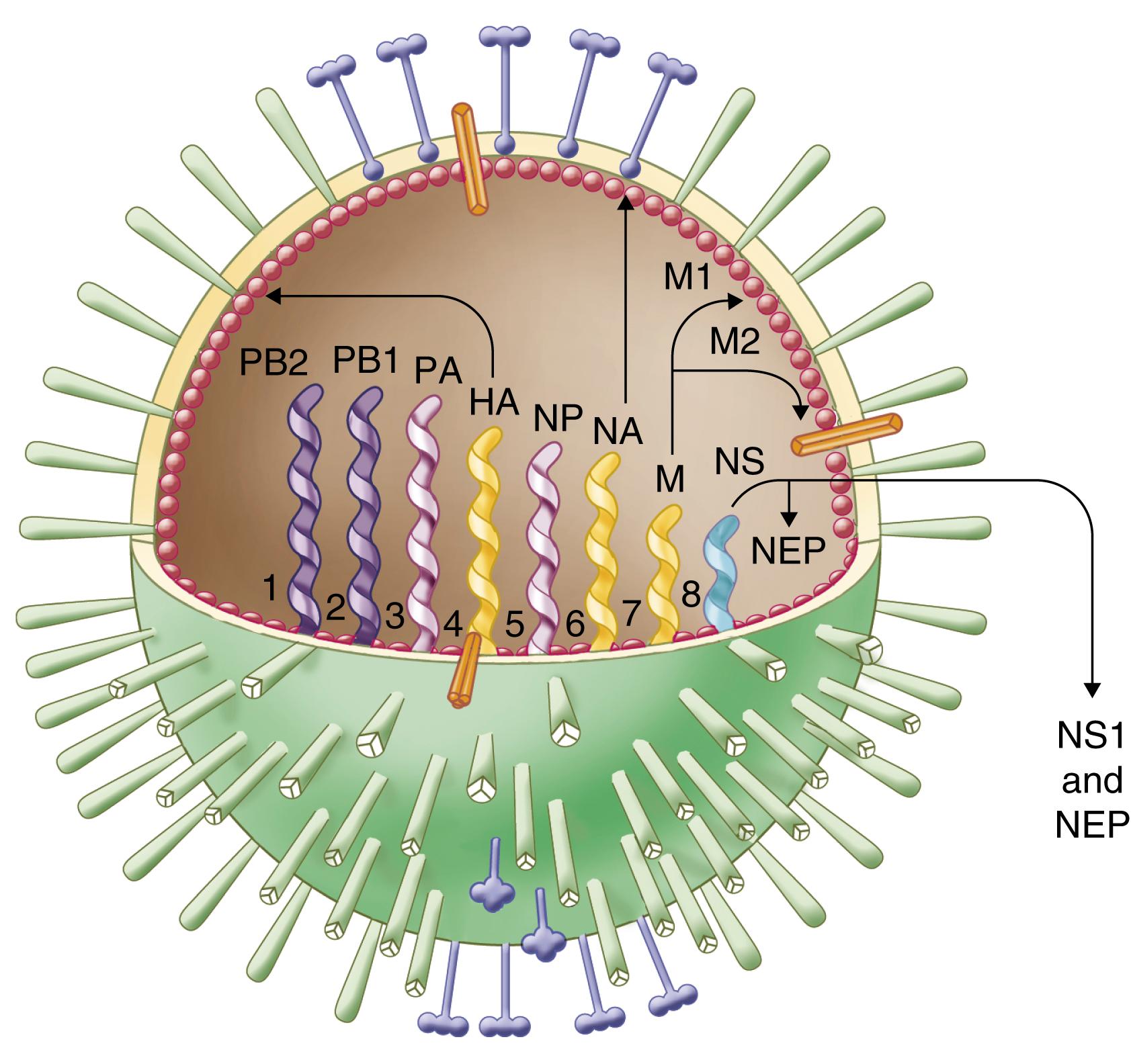
Whereas influenza B and C viruses are principally human pathogens, influenza A viruses primarily infect aquatic birds and sometimes establish lineages that circulate in other animal hosts, including other avians, swine, horses, marine mammals, and dogs. Influenza D appears to be pathogenic in cattle and perhaps other domestic animals. Influenza A viruses are further classified into subtypes on the basis of their HA and NA glycoproteins. Sixteen HA and nine NA subtypes are currently recognized in avians, and two others are recognized in bats. Only three HAs (H1, H2, and H3) and two NAs (N1 and N2) have been documented thus far in epidemic and pandemic human influenza A viruses, although other HAs (e.g., H5, H6, H7, H9, H10) and NAs (e.g., N4, N7, N8, N9) have been found in zoonotic infections. Each strain is identified by type, subtype if influenza A, location of isolate, sample number, and year of isolation (e.g., A/California/04/2009 (H1N1)).
Influenza viruses are unique among the respiratory viruses with regard to their extent of genetic and antigenic variation, epidemic behavior, and frequent association with excess mortality during community outbreaks—characteristics and pathogenicity that exemplify human influenza virus’s efficient host-to-host transmissibility. The changing antigenicity of the surface glycoproteins largely accounts for repeated influenza epidemics. Antibody to HA can neutralize viral infectivity and is thus the major determinant of immunity. Current vaccines are based largely on inducing hemagglutination-inhibition (HAI) or neutralizing antibodies to HA. Anti-NA antibody limits viral replication and probably reduces the severity of infection. Variation involves either relatively minor (antigenic drift) or major (antigenic shift) changes in antigenicity. Significant antigenic variation is much less frequent with influenza B or C than with influenza A.
Antigenic drift results from point mutations in the HA gene segment that cause amino acid substitutions in at least one of five key antigenic sites on HA. Drift can also occur in NA and in T-cell epitopes on internal proteins. Antigenic variants emerge frequently (every year or every few years) within an influenza A or B virus. For example, the original H3N2 variant, A/Aichi/68, has undergone successive drifts resulting in epidemic strains that include the recent circulation of A/Kansas/14/2017 (H3N2)-like virus. Immunologic selection favors transmission of the new variant over the old because of the less frequent presence of antibody to the new virus in the population.
Antigenic shift results from the appearance of a novel influenza A virus with HA, NA, or both HA and NA glycoproteins that are new to humans or that reappear after decades of absence. Because of the lack of population immunity, a new strain that is efficiently transmitted from person to person can cause pandemic disease. The origins of new pandemic strains and the basis for their subsequent seasonal circulation remain incompletely understood. Avian influenza viruses have most frequently served as the reservoir of new genes for pandemic viruses. Reassortment of gene segments may occur when two influenza viruses simultaneously infect a single cell and in reassortment events in which human viruses acquired avian genes led to both the 1957 and 1968 pandemic viruses. Because swine can support replication of both human and avian viruses, they have been postulated to serve as a mixing vessel for the generation of new strains or as the host in which avian viruses can adapt to mammals. The 2009 H1N1 pandemic virus arose as a quadruple reassortant that derived gene segments from Asian and North American swine lineage viruses that harbored genes derived from swine, avian, and human viruses.
Multiple reassortment events may occur over a period of years before a pandemic virus emerges. For example, the 1918 pandemic virus appears to have been composed primarily of avian genes that underwent adaptation in a mammalian host before causing the pandemic. Frequent intra-subtypic reassortment also occurs among seasonal human influenza A viruses and sometimes leads to new antigenic variants and sometimes to altered virulence.
An epidemic is an outbreak of influenza confined to one geographic location. In temperate climates, community epidemics of influenza A virus infection often have a characteristic pattern, typically reaching a sharp peak in 2 or 3 weeks after initial recognition and persisting for 6 to 10 weeks. Increased numbers of schoolchildren with febrile respiratory illness are often the first indication of influenza in a community, soon followed by illnesses in adults and, 1 to 2 weeks later, by increased hospital admission of patients with influenza-related complications. Hospitalization rates in high-risk persons increase two- to five-fold during major epidemics. School and employment absenteeism increases, as does mortality from pneumonia and underlying conditions, especially in older adults during A/H3N2 epidemics. Epidemics occur almost exclusively during the late autumn and winter months in temperate areas, but activity may extend into spring months. Because of the seasonal differences between northern and southern hemispheres, seasons cycle between the hemispheres. Influenza activity may occur year-round in the tropics or display other patterns. The reasons for the distinct seasonality of influenza in temperate climates are uncertain but may include the school calendar, increased close indoor contact, and low absolute humidity, which affects airborne transmissibility. Winter holidays delay the peaks of seasonal epidemics and shift the risk of infection toward adults. Outbreaks sometimes occur in tour groups (land or ship) and in chronic care facilities during summer months, particularly after the appearance of a drift variant. Regional differences in the timing, magnitude, and causative viruses of influenza outbreaks are common. During epidemics, overall attack rates typically range from 5 to 20% in adults. Attack rates of 40 to 50% may occur in semiclosed populations, such as on hospital floors and chronic care facilities, as well as in highly susceptible age groups such as children. Influenza A and B viruses, two different influenza A subtypes, two different influenza B lineages, or two different strains within a single subtype may co-circulate or occur sequentially during one season in a given location. In addition, simultaneous outbreaks of influenza A virus and RSV or other respiratory viruses occur. Strains circulating at the end of one season’s epidemic are sometimes responsible for the next season’s outbreak (the so-called herald wave phenomenon).
Pneumonia- and influenza-related deaths fluctuate annually, with peaks in the winter months in temperate climates. When such deaths exceed the expected threshold, the cause is typically influenza A, but influenza B virus or RSV can occasionally be responsible. Influenza A/H3N2-dominant seasons are associated with two to three times higher mortality rates than are nonpandemic H1N1- and B-dominant seasons. Although mortality is usually greatest during pandemics, substantial mortality occurs with epidemics. During seasonal influenza, more than 85% of pneumonia- and influenza-related deaths occur in persons aged 65 years and older. Mortality risk is especially high in those aged 85 years and older. Cardiovascular events (e.g., myocardial infarction) and worsening of other chronic diseases (e.g., asthma, chronic obstructive pulmonary disease) contribute substantially to the increased mortality during influenza epidemics.
Pandemics of influenza A result from the emergence of a new virus capable of sustained person-to-person transmission and to which the population contains no or limited immunity. The virus spreads worldwide, often circulating outside of the usual influenza season, and usually infects persons of all ages. Preexisting immunity due to prior infection with antigenically related viruses can provide partial protection, as occurred in older adults during the 2009 H1N1 pandemic. Pandemics are associated with high morbidity rates, especially in children, and sometimes notably increased mortality rates in pregnant women and young and middle-aged adults. For example, more than 90% of deaths in the 1918 and 2009 pandemics occurred in persons younger than 65 years. In the 2009 pandemic, adults hospitalized with H1N1 were more likely to have severe pneumonia, shock, sepsis, and organ failure and to require intensive care unit (ICU) care than were patients with seasonal influenza.
The interval between pandemics is variable (10 to 40 years) and unpredictable. The most severe pandemics have been associated with major antigenic alterations in both major surface glycoproteins. Depending on population susceptibility and perhaps changes in the virus, one or more waves, sometimes with increased severity, may follow the initial one. As the level of immunity in the population increases, antigenic drift within the subtype may cause repeated epidemics in subsequent years, and excess mortality in persons younger than age 65 may continue for some years. For example, the pandemic 2009 H1N1 virus has continued to cause fatal infections at least 10 years after its initial emergence.
Zoonotic infections may be acquired from swine, poultry, or rarely other animals. Although most avian influenza viruses do not cause infections directly in humans, zoonotic infections due to avian H5, H7, H9, and rarely other subtypes continue to cause outbreaks in regions where human and poultry live in close proximity (e.g., chicken farming, wet markets), thereby representing potential pandemic threats. Initially recognized by a cluster of human cases in Hong Kong in 1997, an epizootic of avian H5N1 infections has affected poultry in many areas of Asia, the Middle East, Europe, and Africa and continues to cause sporadic human illnesses with high mortality and occasional instances of nonsustained human-to-human transmission. From 2013 to 2017, repeated waves of zoonotic avian H7N9 infections have occurred during winter and early spring in China, with case-fatality rates of approximately 35% among hospitalized patients. This virus continues to evolve genetically, thereby leading to variants with altered antigenicity and increased virulence for birds and possibly humans.
In the United States infrequent zoonotic infections have been recognized for decades in people exposed to swine. Since 2012, reassortant swine H3N2 viruses that acquired the M gene and sometimes other genes from the pandemic 2009 H1N1 virus (designated variant H3N2, or H3N2v) have caused hundreds of sporadic infections, particularly in the context of agricultural fairs, sometimes followed by limited human-to-human transmission and serious infections. Other variant swine-origin viruses (H1N1v, H1N2v) have also caused zoonotic infections.
Influenza virus infection is transmitted from person to person by virus-containing respiratory secretions. Large-droplet and small-particle aerosols traveling over a short range (generally within 2 meters) appear to be the predominant route of transmission; longer-range airborne transmission may also occur rarely under unique conditions (e.g., aerosol-generating medical procedures). Other routes, including hand contamination from secretion-laden fomites followed by self-inoculation into the eye or nose, may also contribute. Infection by avian viruses can occur after direct contact with infected birds or their excreta, exposure to contaminated environments and infectious aerosols, ingestion of inadequately cooked food, and sometimes by inoculation into the conjunctiva.
The cellular receptor binding patterns and tissue tropism of influenza viruses are key determinants in transmissibility and pathogenesis. Efficient virus transmission between humans depends largely on virus attachment to and replication in cells bearing α-2,6-linked sialosaccharides in the upper respiratory tract and tracheobronchial tree. By comparison, the α-2,3-linked sialosaccharides, which are higher-affinity binding receptors for avian viruses, are distributed mostly in the distal bronchioles, alveoli, and conjunctiva. Once the virus initiates infection of the respiratory tract epithelium, successive cycles of viral replication infect large numbers of cells and result in destruction of respiratory epithelium and sometimes pneumocytes through direct cytopathic effects or apoptosis.
The incubation period averages 2 days and varies from about 1 to 4 days for seasonal influenza but may be up to 1 week and possibly longer in infections caused by avian viruses. The quantity and duration of virus replication in the respiratory tract generally correlate with the severity of illness and levels of host pro-inflammatory cytokine-chemokine responses. Rapid innate cellular responses are induced through toll-like receptors and retinoic acid inducible gene I ( RIG-1 ) that detect viral RNA and lead to the production of cytokines and interferons (IFNs). Elevated levels of mediators (e.g., IFN-α, interleukin [IL]-6, IL-8, monocyte chemoattractant protein-1 [MCP-1], macrophage inflammatory protein [MIP]–1β, and tumor necrosis factor [TNF]–α), which are frequently detected in blood and respiratory secretions, contribute to systemic symptoms, including fever, and tissue injury. Deficient IFN responses have been associated with severe influenza disease.
The duration of viral replication depends on age, immune status, underlying conditions, and the viral strain. In seasonal influenza, viral RNA generally can be detected in the upper respiratory tract for 3 to 5 days in adults but for longer (7 to 10 days) in the elderly and hospitalized patients, and viral RNA may persist for weeks to months in immunocompromised hosts. By comparison, viral detection by culture, which may better reflect infectivity, is shorter. Patients who suffer from influenza pneumonia appear to have higher viral levels and more prolonged viral replication in their lower respiratory tracts. Viremia or extrapulmonary dissemination (e.g., CNS, GI) may occur in serious avian or pandemic virus infections. Detection of viral RNA in the blood is associated with a poorer prognosis in immunocompromised patients.
Nasal and bronchial biopsy specimens from persons with uncomplicated influenza reveal desquamation of the ciliated columnar epithelium and loss of cilia. The lungs in fatal influenza may show necrotizing bronchitis, diffuse alveolar damage with epithelial necrosis, alveolar edema and hemorrhage, and hyaline membrane formation, followed later by squamous metaplasia and fibrosis. Secondary bacterial infections (e.g., Staphylococcus aureus, Streptococcus pneumoniae [ Chapter 268 ], Streptococcus pyogenes [ Chapter 269 ]) develop as a result of altered bacterial flora, damage to bronchial epithelium with depressed mucociliary clearance, decreased polymorphonuclear and alveolar macrophage functions, accumulation of alveolar fluid, and suppression of other host immune responses.
Adaptive humoral and cell-mediated responses are important for immunity to influenza. Humoral immunity to natural influenza virus infections appears to be strain specific and largely subtype dependent. A child’s first influenza A infection results in immune memory for that viral strain and its related HA subtypes within the same group (“immune imprinting”); subsequent infections or immunizations reinforce the antibody responses, which could contribute to protection against related subtypes (e.g., initial H3N2 infection reducing the risk of H7N9 illness, which is also a group 2 virus). Vaccine-induced immunity is highly strain-specific and typically short-lived (about 6 months), thereby explaining the need for annual update and administration. Protective immunity is mediated by neutralizing antibodies; the generally accepted correlates are serum-neutralizing antibody titers of 1:8 or greater, nasal-neutralizing antibody titers of 1:4 or greater, or an associated increase in the serum HAI titers of 1:40 or greater. However, considerable variability can be seen with the viral strain, in part based on the patient’s age, and immune status. Type-specific cell-mediated immunity, which involves CD4+ and CD8+ T-lymphocytic responses, is important for the termination of viral replication and limitation of the infection. Increasing evidence suggests that human genetic factors, such as polymorphism of genes that regulate immune responses, play a role in determining the severity of influenza infection, especially in the immunologically naïve hosts. Examples include polymorphisms in the IFN-induced transmembrane 3 ( IFITM3 ) protein, TMPRSS2 protease, CD55 and C1QBP complement-pathway, and SFTPA2 surfactant protein genes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here