Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infectious agents are second only to tobacco use as a potentially preventable cause of cancer in humans. Estimates vary between 15% and 30% as to the percentage of cancers worldwide that are associated with an infectious etiology. The burden is greater in the developing world, but the impact even in the United States and other developed countries is significant. Specific viruses, parasites, and bacteria are now associated with specific human cancers. These are discussed in some detail in this chapter.
There are three major mechanisms by which an infectious agent can cause a cancer, and these may involve the initiation as well as the promotion of carcinogenesis. The first is perhaps the most common, resulting from the infectious agent causing a persistent infection with chronic inflammation. This can result in the formation of reactive oxygen and nitrogen species by macrophages at the site of the infection. These reactive molecules can damage DNA and proteins as well as membranes and thus contribute to carcinogenesis. Chronic inflammation due to the persistent infection can then lead to repeated cycles of cell damage and cellular proliferation. Cells that are cycling in the presence of reactive molecules are more likely to acquire genetic mutations that could contribute to the initiation as well as the promotion of cancer. A second mechanism involves the direct participation of the infectious agent in the transformation of the cell through the activation of a cellular oncogene pathway or the inactivation of a tumor suppressor gene. A third mechanism, relevant to the human immunodeficiency virus (HIV), is that the infection may result in immunosuppression and the decreased recognition of infected or transformed cells by the host immune system. Indeed, many of the cancers observed in immunosuppressed patients, such as those infected with HIV, are those that have been associated with other viruses.
The recognition of an infectious etiology for specific cancers provides the opportunities to prevent those cancers by preventing or controlling the infections. Depending on the infectious agent, this could involve public health measures or changes in cultural practices. It could also involve the development of vaccines to prevent the initial infections, as has now been achieved for hepatitis B virus (HBV) and the genital-tract human papillomaviruses (HPVs). It could also involve the treatment of the infections with specific therapeutics or the development of novel therapies for those agents for which there are not yet specific or effective drugs.
Viral oncology has its beginnings as a discipline from observations made during the early part of the 20th century: in 1908, when the transmissibility of avian leukemia was first described by Ellermann in Denmark, and in 1911, when the transmissibility of an avian sarcoma in chickens was described by Rous. The importance of these findings was not appreciated at the time, and the full impact on virology and medicine was not recognized until the 1950s. Indeed, the work of Peyton Rous showing that cell-free extracts containing a filterable agent from a sarcoma in chickens could induce tumors in injected chickens within a few weeks was finally recognized with a Nobel Prize in 1966. Rous’s original work pointed out that a filterable agent (the working definition of a virus at that time) not only was capable of inducing tumors, but also was responsible for determining the phenotypic characteristics of the tumor. Because these studies were carried out in birds and not in mammals, however, this early work was consigned to the rank of avian curiosities.
In the 1930s, Richard Shope published a series of papers demonstrating the cell-free transmission of tumors in rabbits. The first studies involved fibromatous tumors found in the footpads of wild cottontail rabbits that could be transmitted by injecting cell-free extracts in either wild or domestic rabbits; a virus referred to as the Shope fibroma virus is now known to be a pox virus. Other studies carried out by Shope demonstrated that cutaneous papillomatosis in wild cottontail rabbits could also be transmitted by cell-free extracts. In a number of cases, these benign papillomas would progress spontaneously into squamous cell carcinomas in infected domestic rabbits or in the infected cottontail rabbits. In general, however, the field of viral oncology lay dormant until the early 1950s, with the discovery of the murine leukemia viruses by Ludwig Gross and of the mouse polyomavirus by Gross, Stewart, and Eddy. The identification of tumor viruses in mice opened the field of experimental viral oncology. Researchers had the hope that these initial observations in mammals could be extended to humans and that a fair proportion of human tumors might also be found to have a viral etiology. The Special Viral Cancer Program in the 1960s at the National Cancer Institute grew from this intense interest in viral oncology and the belief that human tumor viruses would be identified.
Many of the most important developments in modern molecular biology derive from studies in viral oncology from the 1960s and 1970s. The discovery of reverse transcriptase, the development of recombinant DNA technology, the discovery of messenger RNA splicing, and the discovery of oncogenes and, more recently, tumor suppressor genes all have been developments that emerged directly from studies in viral oncology. Oncogenes were first recognized as cellular genes that had been acquired by retroviruses through recombination processes to convert them into acute transforming RNA tumor viruses. It is now recognized that oncogenes participate in many different types of tumors and can be involved at different stages of tumorigenesis and viral oncology. This has contributed significantly to our concepts in nonviral carcinogenesis. It is likely that the direct-transforming, oncogene-transducing retroviruses do not play a major causative role in naturally occurring cancers in animals or in humans, but rather represent laboratory-generated recombinants. A list of human viruses that are now associated with human cancer is presented in Table 6-1 . Also included on this list are viruses such as the transforming adenoviruses that, although capable of transforming normal cells into malignant cells in the laboratory, have not been associated with any known human tumors.
| Virus Family | Type | Human Tumor | Cofactors | Comments |
|---|---|---|---|---|
| Adenovirus | Types 2, 5, 12 | None | N/A | Important experimental model |
| Hepadnavirus | Hepatitis B virus (HBV) | Hepatocellular carcinoma (HCC) | Aflatoxin, alcohol, smoking | Causative |
| Herpesvirus | Epstein-Barr virus (EBV) | Burkitt’s lymphoma | Malaria | EBV |
| EBV-associated malignancies in immunosuppressed individuals | Immunodeficiency | Causative | ||
| Nasopharyngeal carcinoma | Nitrosamines, genetic | Causative | ||
| Hodgkin’s lymphoma | ? | Variable association | ||
| Gastric carcinoma | ? | Variable association | ||
| KSHV (HSV8) | Kaposi’s sarcoma | AIDS | Causative | |
| Castleman’s disease | ? | Causative | ||
| Primary effusion lymphomas | ? | Causative | ||
| Flavivirus | Hepatitis C virus (HCV) | Hepatocellular carcinoma | Aflatoxin | Causative |
| Papillomaviruses | HPV16, -18, -31, -33, -35, -39, and others | Anogenital cancers, some upper airway cancers | Smoking, oral contraceptives, ?other factors | Causative |
| HPV5, -8, -17, -20, -47 | Skin cancer | Genetic disorder (EV), UV, immunosuppression | Unclear if causative | |
| Polyomavirus | MCV | Merkel cell cancer | UV, immunosuppression | Likely causative |
| BK | ?Prostate preneoplastic lesions | ? | Unclear if causative | |
| JC | ?Brain tumors | ? | Unclear if causative | |
| SV40 ∗ | ?Mesotheliomas, brain tumors, etc. | ? | Unlikely | |
| Retroviruses | HTLV-1 | ATL | ?Genetic | Causative |
| HTLV-2 | None | N/A | Not associated with human malignancy |
∗ SV40 is simian virus 40, a nonhuman primate virus closely related to the human polyomaviruses BK and JC.
Also listed in Table 6-1 are cofactors that are believed to be important in the carcinogenic processes associated with each of these viruses. It is clear that none of these viruses by themselves is sufficient for the induction of the specific neoplasias with which they have been associated. Each of the viruses associated with these human cancers is thought to be involved at an early step in carcinogenesis. Subsequent cellular genetic events such as somatic mutations are thought to be important at the subsequent steps involved in the multistep process of malignant progression.
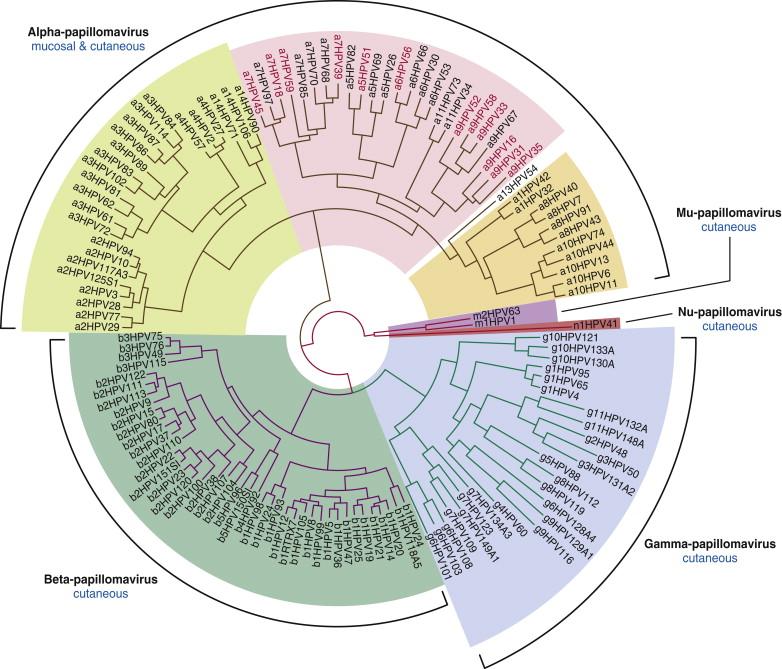
The human papillomaviruses cause warts and papillomas and are associated with some specific human cancers. The papillomaviruses have been found exclusively in higher vertebrates, in species ranging from birds to man. More than 140 different types of HPVs are now recognized, and new types are still being recognized. Because serologic reagents are not available for all types, some HPVs have been typed by their DNA sequence. Many of the HPVs have now been fully or partially sequenced, and these DNA sequence data now lead their phylogenetic organization ( Figure 6-1 ). Some of these viruses as well as the clinical syndromes with which they are associated are presented in Table 6-2 .
| A. Cutaneous Lesions and HPVs | ||
| Clinical association viral types | ||
| Plantar wart | HPV1 | |
| Common wart | HPV2, 4 | |
| Mosaic wart | HPV2 | |
| Multiple flat warts | HPV3, 10, 28, 41 | |
| Macular plaques in EV | HPV5, 8, and other beta HPV types | |
| Butcher’s warts | HPV7 | |
| B. Genital Tract HPVs | ||
| Condyloma acuminata (exophytic) | HPV6, 11 | |
| Giant condyloma (Bushke-Lowenstein tumor) | HPV6, 11 | |
| Subclinical infection | All genital tract HPV types | |
| Squamous intraepithelial lesions | HPV16, 18, 31, 33, etc. | |
| Bowenoid papulosis | HPV16, 18, etc. | |
| Cervical cancer | Strong association, “high risk” | HPV16, 18, 31, 45 |
| Moderate association | HPV33, 35, 39, 51, 52, 56, 58, 59, 68 | |
| Weak or no association, “low risk” | HPV6, 11, 26, 42, 43, 44, 51, 53, 54, 55, 66 | |
| Other anogenital cancers (vulvar, penile, etc.) | HPV16 and other “high-risk” HPV types | |
| Respiratory papillomas | HPV6, 11 | |
| Conjunctival papillomas | HPV6, 11 | |
| Focal epithelial hyperplasia (oral cavity) | HPV13, 32 | |
| Oropharyngeal cancer | HPV16 | |
The papillomaviruses have a specific tropism for squamous epithelial cells (keratinocytes). The functions of the papillomaviruses necessary for the production of infectious virions, which include vegetative viral DNA replication and the synthesis of the capsid proteins, occur only in the fully differentiated squamous epithelial cells of a papilloma. Viral capsid protein synthesis and virion assembly occur only in the terminally differentiated cells of the upper layers of the epithelium. The viral genome is present in the epithelial cells of all layers of the epithelium, including the basal layer. It is generally believed that the expression of specific viral genes in the basal layer and in the lower layers of the epidermis stimulates cellular proliferation and alters the keratinocyte differentiation profile, characteristic of a wart. As squamous epithelial cells migrate upward through the layers and differentiate, the pattern of viral gene expression changes, resulting in the expression of the late genes (L1 and L2) that encode the capsid proteins.
The genomic organization of all the papillomaviruses is quite similar. All of the open reading frames (ORFs) that could serve to encode proteins for these viruses are located on only one of the two viral DNA strands, and only one strand is transcribed. The HPV genome can be divided into three distinct regions: (1) an “early” region that encodes the viral proteins (E1, E2, etc.) involved in viral DNA replication, transcriptional regulation, and cellular transformation, (2) a “late” region that encodes the viral major (L1) and minor (L2) capsid proteins, and (3) a region called the “long control region” (LCR) or alternatively, the upstream regulatory region (URR) that does not contain any ORFs, but does contain cis-regulatory elements, including the origin of DNA replication and important transcription factor binding sites. A diagram of the organization of the HPV16 genome, which is typical of all the HPVs, is shown in Figure 6-2 .
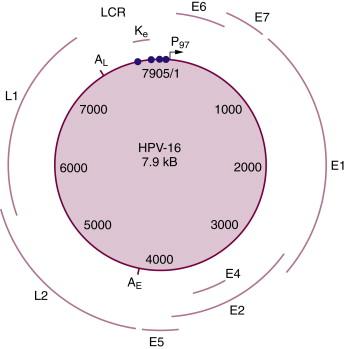
The late genes (L1 and L2) are expressed only in the more differentiated cells of the epithelium, whereas the early (E) region genes are expressed throughout the epithelium. A more detailed description of the biology and molecular biology of the papillomaviruses can be found in Fields Virology .
Only some papillomaviruses are associated with cancer. These include several animal PVs as well as a subset of the HPVs. From an experimental standpoint, the cottontail rabbit papillomavirus (CRPV) that was first identified by Richard Shope has been extensively studied as a model for papillomavirus-induced carcinogenesis. One of the principal features of carcinogenic progression associated with PVs is the synergy often observed between a specific virus and other carcinogenic factors ( Table 6-3 ). In the case of CRPV, carcinomas develop at an increased frequency in papillomas that are painted with coal tar or with methylcholanthrene. These CRPV-associated carcinomas contain viral DNA that is transcriptionally active, and the carcinogenic properties are believed to map to specific viral genes. There are additional instances where animal papillomaviruses have been associated with naturally occurring cancers, including the bovine papillomavirus type 4 (BPV-4) that causes esophageal papillomatosis and is associated with squamous cell carcinomas of upper alimentary tract. Major interest today, however, is in the role of specific HPVs with the human cancers with which they have been associated.
| Species | Cancers | Viruses | Other Factors |
|---|---|---|---|
| Human | Anogenital cancers | HPV16, -18, -31, etc. | Tobacco |
| Oropharyngeal cancers | HPV16 | ||
| Malignant progression of respiratory papillomas | HPV6, -11 | X-irradiation, smoking | |
| Nonmelanoma skin cancer | HPV5, -8, -17, and other beta genus HPVs | Genetic (EV), UV light, and immunosuppression | |
| Rabbit | Skin cancer | CRPV | Methylcholanthrene and coal tar (experimental) |
| Cattle | Alimentary tract cancers | BPV-4 | Bracken fern |
| Conjunctival cancers | Not characterized | UV light | |
| Sheep | Skin cancer | Not characterized | UV light |
Cervical cancer is the third most common malignancy among women worldwide, with approximately 530,000 newly diagnosed cases each year and about 275,000 deaths annually. About 80% of cervical cancer occurs in developing countries, where it is frequently the most common cancer of women, accounting for as many as one quarter of female cancers. It occurs less frequently in developed countries. In the United States, there are about 12,000 newly diagnosed cases annually, and about one third of these women will die of their malignant disease. The incidence of cervical cancer in the United States varies considerably among ethnic and socioeconomic groups, with the rate among African American women being about twice that of White women.
Most cervical cancers develop in the transformation zone, the region of the cervix where the columnar cells of the endocervix adjoin with the stratified squamous epithelium of the exocervix. About 85% of cervical cancers are squamous cell cancers, the remainder being adenocarcinomas and small-cell neuroendocrine tumors. The progression of normal cervical epithelial cells to malignant squamous cell carcinomas typically occurs through a series of dysplastic changes over a time span of many years, a process that is the basis of the Pap smear screening program. The histologic classifications of cervical intraepithelial neoplasia (CIN) grades 1, 2, and 3 correspond, respectively, to mild dysplasia, moderate dysplasia, and severe dysplasia or carcinoma in situ. Because of the long interval for the progression of cervical dysplasia to invasive cancer, Pap smear screening programs can identify the vast majority of premalignant lesions for appropriate treatment, thereby preventing the development of most cases of cervical cancer in countries with screening programs. Most CIN lesions do not progress to cancer but resolve; the lesser the degree of dysplasia, the more likely the lesion is to resolve.
Cervical cancer had been recognized for decades as linked to a sexually transmitted agent, long before sexually transmitted HPV infection was implicated in its pathogenesis. Venereal transmission of a carcinogenic factor with a long latency had been suggested by the early epidemiologic studies. Sexual promiscuity, an early age of onset of sexual activity, and poor sexual hygiene conditions were identified by these studies as risk factors in women for cervical carcinoma. The counterpart to cervical cancer in the male is penile cancer, because there is a correlation between the incidence rates of these two cancers in different geographic areas. Compelling evidence linking an HPV infection with cervical carcinoma followed the observation that some of the morphologic changes characteristic of cervical dysplasia seen on Pap smears were due to a papillomavirus infection. The cell with its characteristic perinuclear clearing and abnormally shaped nucleus that is diagnostic for a cervical papillomavirus infection is the koilocyte. The presence of papillomavirus particles, papillomavirus-specific capsid antigens, and HPV DNA within the cervical preneoplastic lesions provides confirmation of the HPV etiology of cervical dysplasia.
Harald zur Hausen and his colleagues identified the first papillomavirus DNAs, HPV16 and HPV18, in cervical cancer tissues in the 1980s. Using HPV DNAs as probes under conditions of reduced hybridization stringency, most cervical carcinomas were shown to harbor these or related HPV DNAs. Subsequent studies led to the identification of approximately 40 different HPVs, mostly from the alpha genus, associated with genital tract lesions, a subset of which are associated with human cervical cancer. In addition to HPV16 and HPV18, which account for approximately 70% of human cervical cancers, HPV types 31, 33, 35, 45, 52 and 58 account for a total of 95% of HPV-positive cancers. DNAs from these same HPV types are found in other human genital carcinomas, including penile carcinomas, some vulvar carcinomas, and some perianal carcinomas, as well as in the precancerous intraepithelial lesions of each of theses sites (PIN, VIN, and PAIN).
The genital-tract–associated HPVs have been classified as either high risk or low risk based on whether the lesions with which they are associated are at significant risk for malignant progression. The low-risk viruses such as HPV6 and HPV11 are associated with venereal warts, lesions that only rarely progress to cancer. The high-risk viruses such as HPV16 and HPV18 are associated with CIN and cervical cancer. The other high-risk viruses include HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV68, and HPV82. Virtually all cases of CIN3 and cervical cancer contain a high-risk HPV DNA. HPV-positive cervical cancers and cell lines derived from HPV-positive cervical cancer tissues often, but not always, contain integrated viral DNA. In those cancers in which the viral DNA is integrated, the pattern of integration is clonal, indicating that the integration event preceded the clonal outgrowth of the tumor. Integration of the viral DNA does not occur at specific sites in the host chromosome, although in some cancers the HPV DNA has integrated in the vicinity of known oncogenes. For instance, in the HeLa cell line (which is an HPV18-positive cervical carcinoma cell line), integration of the HPV18 genome is within approximately 50 kilobases of the c-myc locus on human chromosome 8. It is possible that such an integration event might provide a selective growth advantage to the cell and thus might contribute to neoplastic progression.
In cervical cancers, only a subset of the viral genes is expressed, and there is no production of virus by the cancer cells. The integration of the viral genome appears to play an important role leading to the deregulated expression of the viral E6 and E7 genes. The E6 and E7 genes are invariably expressed in HPV-positive cervical cancers. Integration of the HPV genome into the host chromosome in the cancers often results in the disruption of the viral E1 or E2 genes. Because HPV E2 is a viral regulatory factor that negatively regulates expression of the E6 and E7 genes, the disruption of E2 results in the derepression of the E6/E7 promoter, leading to deregulated expression of E6 and E7. Indeed, the introduction of E2 into cervical cancer cell lines results in the induction of cellular senescence by repressing E6 and E7 expression.
The E6 and E7 genes of the high-risk genital-tract–associated HPVs function as oncogenes. Expression of E6 and E7 together is sufficient for the efficient immortalization of primary human cells, most notably primary human keratinocytes, the normal host cell for the human papillomaviruses. In contrast to the immortalization properties of the HPV16 and HPV18 E6 and E7 proteins, the E6 and E7 proteins encoded by the low-risk viruses are either inactive or only weakly active in the same assays.
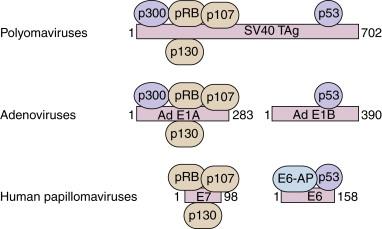
The major cellular targets for E6 and E7 are the tumor suppressor proteins p53 and pRB, respectively. E6 and E7 are, however, polyfunctional proteins and have many other biochemical activities and biologic properties that may be relevant to their activities in cervical carcinogenesis. A common theme among the small DNA tumor viruses (i.e., the polyomaviruses, the adenoviruses, and the cancer-associated HPVs) is that the immortalization and transformation properties of their encoded oncoproteins are in part due to their interactions with critical cellular regulatory proteins ( Figure 6-3 ). The HPV E7 proteins share some amino acid sequence similarity to adenovirus E1A and with portions of the SV40 large T antigen, in regions that are critical for the transformation activities of these oncoproteins. These regions of amino acid sequence similarity shared by these viral oncoproteins specify the binding to the product of the retinoblastoma tumor suppressor gene, pRB, and the related pocket proteins p107 and p130. Studies have established that a major component of the transformation activities of these viral oncoproteins is due to their respective abilities to complex and functionally inactivate pRB and the related pocket proteins. The binding of these viral oncoproteins to pRB, p107, and p130 leads to cellular proliferation rough the activation of genes under the control of the E2F family of transcription factors. The transcriptional activities of the E2F family of transcription factors are modulated by pRB and the other pocket proteins. When complexed with E2F proteins, they act as transcriptional repressors, and when dissociated from the pocket proteins by E7, E1A, or SV40 T-antigen, the E2F proteins function to activate transcription of their target genes. In the normal life cycle of the papillomaviruses, the binding of E7 to pRB is essential for the activation of the cell-cycle DNA replication machinery in differentiated keratinocytes that had otherwise exited the cell cycle. The small DNA tumor viruses, including HPV, depend on the host cell DNA replication machinery for the replication of their viral genomes. Because this machinery is only expressed in the S phase of the cell cycle, these viruses must stimulate cellular proliferation and drive the cell into the S phase in order to replicate the viral genomes. In the case of HPV, this occurs through E7 binding pRB, freeing up the E2F family of transcription factors.
A number of genetic studies indicate that E7 binding to pRB and its related pocket proteins is not sufficient to account for its immortalization and transforming functions, indicating that there are additional cellular targets and activities of E7 that are relevant to cellular transformation. Indeed, a large number of putative cellular targets for E7 have been identified using a variety of biochemical approaches and proteomic approaches. The physiologic relevance of many of these interactions is not yet clear, but some of these targets appear to be relevant to cancer. For instance, E7 can interact with cyclin-dependent kinase inhibitors. Like Ad E1A, HPV16 E7 interacts with and abrogates the inhibitory activity of p21(cip1) and p27(kip1) and thus has effects on cell cycle progression and keratinocyte differentiation. In addition, high-risk HPV E7 can cause genomic instability in normal human cells. HPV16 E7 induces G1/S and mitotic cell cycle checkpoint defects and uncouples synthesis of centrosomes from the cell division cycle. This causes formation of abnormal multipolar mitoses, leading to chromosome missegregation and aneuploidy. Moreover, there is an increased incidence of double-strand DNA breaks and anaphase bridges, suggesting that in addition to numerical abnormalities, high-risk E7 proteins also induce structural chromosome aberrations. Abnormal centrosome duplication rapidly results in genomic instability and aneuploidy, one of the hallmarks of a cancer cell. This activity is therefore likely to be functionally relevant to the contribution of high-risk HPVs to malignant progression.
The immortalization/transformation properties of the E6 protein were first revealed by studies using primary human genital squamous epithelial cells. Efficient immortalization of primary human cells by HPV16 or HPV18 requires both the E6 and E7 genes. Like SV40 large T antigen and the 55-kDa protein encoded by adenovirus E1B, the E6 proteins of the high-risk HPVs can complex with p53. The interaction of E6 with p53 is not direct but is mediated by a cellular protein, called the E6 - a ssociated p rotein (E6AP). E6AP is a ubiquitin protein ligase and, in the presence of E6, directly participates in the ubiquitylation of p53. Multiubiquitylated p53 is then recognized and degraded by the 26S proteasome. Consequently, the half-life and level of p53 are low in E6-immortalized cell lines and in HPV-positive cancers. Through its ubiquitylation of p53, HPV 16 E6 can abrogate the transcriptional activation and repression properties of p53 and disrupt the ability of wt p53 to mediate cell-cycle arrest in response to DNA damage. The p53 protein can sense DNA damage and prevent the replication of mutated DNA through its transcriptional activation of the p21 cyclin-dependent kinase inhibitor. Thus, the functional abrogation of p53 by high-risk HPV E6 results in decreased genomic stability and accumulation of DNA abnormalities in high-risk HPV E6–expressing cells. Hence, E6 can be directly implicated in the establishment and propagation of genomic instability, a hallmark in the pathology of malignant progression of cervical lesions.
The development of centrosome abnormalities and aneuploidy, two important related pathologic processes, appears to be initiated before viral DNA integration and may contribute to this process. High-risk HPV can induce abnormal centrosome duplication, which can result in genomic instability and aneuploidy. The deregulation of this mitotic event appears to depend on both E6 and E7, with the latter protein being most responsible for the effect. Indeed, the deregulated viral oncogene expression may result in chromosomal instability and aneuploidy, enhancing the likelihood of viral DNA integration.
A number of additional cellular targets have now been identified for the high-risk E6 proteins in an attempt to define additional p53-independent cellular targets. The reader is referred to the current edition of Fields Virology for a more comprehensive discussion of these additional activities, some of which may be relevant to the role of E6 in cervical carcinogenesis. Two activities are of particular importance, however, and are discussed here. The first is the binding to cellular PDZ domain–containing proteins. Interestingly, the high-risk E6 oncoproteins contain an X-(S/T)-X-(V/I/L)-COOH motif at the extreme C terminus that can mediate the binding to cellular PDZ domain–containing proteins. This motif is unique in the high-risk HPV E6 proteins and is not present in the E6 proteins of the low-risk HPV types. E6 serves as a molecular bridge between these PDZ domain proteins and E6AP, facilitating their ubiquitylation and mediating their proteolysis. Among the PDZ domain proteins implicated as E6 targets are hDlg, the human homologue of the Drosophila melanogaster Discs large tumor suppressor, and hScrib, the human homologue of the Drosophila Scribble tumor suppressor. Additional PDZ domain proteins have also been shown to be capable of binding to E6. Several PDZ-containing proteins have been shown to be involved in negatively regulating cellular proliferation. Therefore, some of the p53-independent transforming activities of the high-risk E6 oncoproteins may be linked to their ability to bind and degrade some of these PDZ motif–containing proteins.
A second important p53-independent activity of HPV16 E6 is its ability to activate telomerase in keratinocytes through the transcriptional upregulation of the rate-limiting catalytic subunit of human telomerase (hTERT). Maintenance of telomere length is an important step in cancer and can occur through the transcriptional activation of hTERT expression or through the activation of the ALT recombination pathway. Activation of hTERT is observed in most human cancers, including HPV-positive cervical cancers. The mechanism by which E6 activates the hTERT promoter has not been yet fully elucidated but could involve the direct activation of a cellular transcription factor by E6 or perhaps the E6AP-dependent degradation of a negative regulator of the hTERT promoter.
Infection by a high-risk HPV does not always cause cancer. Indeed, cancer is a rare outcome of an HPV infection, even for HPV16 and HPV18. Expression of the E6 and E7 oncogenes is not sufficient for malignant progression. The time period between infection by a high-risk HPV and the development of invasive cancer can be several decades. Thus, infection with a high-risk HPV constitutes only the initial step in cervical carcinogenesis; the genetic information carried by the virus per se is not sufficient to cause cancer. Epidemiologic studies have suggested that smoking is a risk factor for developing cervical carcinoma. The recognition that other factors are involved in the progression to cervical carcinomas suggests that papillomavirus infections may work synergistically with these other factors.
Tumor progression is, however, a complex process that involves multiple additional genetic loci. Specific chromosomal abnormalities have been detected in cervical cancer, including the loss of heterozygosity on the short arm of chromosome 3 (3p). This locus contains the FHIT (fragile histidine triad) gene, and its expression is inversely correlated with the severity of the lesion and prognosis. In addition, loss of 11q23 may involve the tumor suppressor of lung cancer gene (TSLC1), which is implicated in cell adhesion. An additional possibility is that cellular mutations or epigenetic changes could be involved in downregulating HLA antigen class I alleles and the ability of an HPV-positive cancer cell to be recognized by the host cellular immune response.
The high-risk genital tract HPV types can infect other genital areas that contain stratified squamous epithelium and cause intraepithelial neoplasias and cancer. HPV DNA, usually HPV16, can be found in a subset of cancers of the vulva, vagina, and penis. Giant condyloma acuminata, also called the Buschke-Lowenstein tumor, is a low-grade, locally invasive squamous cell carcinoma that involves the external genitalia and is associated with low-risk HPV types, usually 6 or 11.
Anal cancer is also associated with high-risk HPV infection, and the rate of anal HPV infection appears to be similar to that of cervical infection, although anal HPV infection has been studied less systematically than cervical infection. As with cervical cancer, high-risk HPV can be found in most anal cancers, usually HPV16; most anal cancers arise in the transition zone between columnar and squamous epithelium. The risk of anal cancer in the general population appears to be much lower than for cervical cancer, and the incidence of anal cancer in women is less than one tenth that of cervical cancer. The risk of anal cancer among individuals who are human immunodeficiency virus (HIV)-positive is much greater than in the general population, with especially high rates for HIV-positive male homosexuals.
HPV is linked to some head and neck cancers, although not to the majority of the cancers in this region. HPV16 accounts for about 90% of the HPV-positive tumors. Most of these HPV-associated cancers are located in the oropharynx, which includes the tonsils, tonsillar fossa, base of the tongue, and soft palate. It is not understood why the HPV-positive tumors preferentially develop in the oropharynx. In the United Sates, the incidence of these oropharyngeal cancers, which usually develop at a younger age than the HPV-negative cancers, increased more than threefold between 1988 and 2004. Genital-oral sex may be a risk factor for these tumors, and the risk of HPV infection and cigarette smoking may be more than additive. The HPV-positive tumors tend to have a characteristic basaloid pathology and share many molecular features with those of HPV-positive anogenital tumors. The tumors usually have integrated HPV DNA expressing E6 and E7. Their p53 and pRB genes are wild type, and the vast majority of them expresses p16, in contrast to the HPV-negative tumors, which tend to have mutant p53 and to be p16-negative. There is thus far, however, no clearly identifiable premalignant oropharyngeal lesion for HPV-positive tumors. HPV-positive oropharyngeal cancers carry a better prognosis than the HPV-negative ones.
Esophageal carcinomas in humans have also been reported to have some association with HPVs; however, the data as yet are not as convincing as they are with the anogenital cancers and with oropharyngeal cancers. The esophagus is lined by a squamous epithelium, and squamous cell papillomas of the esophagus have been described in humans. Additional studies seem warranted to investigate a possible role of HPV in human esophageal cancers. There have also been sporadic reports associating occasional human tumors, including colon cancer, ovarian cancer, prostate cancer, and even melanomas, with the presence of HPV DNA in the literature. In general it seems prudent to be skeptical of such reports until systematic and well-carried-out studies are confirmed in multiple laboratories.
EV is a very rare disorder in which affected individuals have a unique susceptibility to cutaneous HPV infection. The warts usually develop in childhood, become widespread, do not tend to regress, and in approximately 30% of patients may progress to squamous cell cancers. Several types of lesions may occur in the same patient. Some lesions are typical flat warts (usually caused by HPV3 or HPV10), whereas others are flat, scaly, red-brown macules. The scaly lesions are associated with a range of beta-HPV types, most frequently HPV5 and HPV8. Patients with EV are often infected by multiple HPV types.
In approximately one half of affected patients, EV occurs as an inherited disorder. Inheritance appears to have an autosomal recessive pattern in most affected families, although one family with apparent X-linked recessive inheritance has been reported. Cases with autosomal recessive inheritance appear to be genetically heterogeneous, because the condition in different families has been mapped to two distinct chromosomal loci, and two adjacent novel genes (EVER1 and EVER2) have now been molecularly identified at one of these loci (17q25).
EV patients do not have an increased susceptibility to clinical infection with other microbial agents, including other HPVs. In addition, the EV-specific HPV types have now been found in normal skin of many individuals, so the EV patients are unusual in that these HPV types produce clinically apparent lesions. However, clinical lesions associated with EV-specific HPV types have been described in other immunosuppressed individuals, such as renal transplant patients. Patients with EV often have impaired cell-mediated immunity, which is believed to be important with regard to the manner in which they respond to infections by this subset of cutaneous HPVs.
About one third of EV patients develop skin cancers in association with their lesions. Most of the malignant tumors remain local, but regional and distant metastases may occur. The risk of malignant progression is limited to the pityriasis-like lesions, which are the lesions that contain the beta-HPV types. HPV5 and HPV8 appear to be the most oncogenic, because most of the skin cancers contain one of these two types. The EV carcinomas usually arise in sun-exposed areas, suggesting that ultraviolet radiation may play a co-carcinogenic role with the specific HPVs in the etiology of these cancers. Mutations in the p53 gene are common in EV-associated cancer, in contrast to the mucosal cancers associated with HPV. Studies have also established that the viral genomes are transcriptionally active within these carcinomas.
Nonmelanomas skin cancers (NMSCs) are subdivided into basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs). They generally arise on exposed areas, and UV exposure is a predominant risk factor. Immunosuppressed individuals are at high risk for developing warts as well as premalignant lesions and NMSC, especially SCC, in sun-exposed areas. The consistent finding of certain beta genus HPV types in SCC associated with EV and other immunosuppressed individuals makes HPV infection an attractive etiologic agent for at least some NMSCs in individuals who do not have EV. Beta HPVs encode potential viral oncoproteins that could interfere with UV-induced apoptosis, which might allow keratinocytes with UV-induced mutations to survive and progress to carcinomas. Although beta HPV DNA can be frequently detected in SCC using sensitive PCR-based detection methods, it is also frequently detected in normal skin. The genome copy number is usually much less than one copy per tumor cell. A recent study employing an unbiased analysis involving high-throughput sequencing of randomly primed mRNAs detected virtually no HPV transcripts in SCC specimens. Overall, the association between HPV infection and NMSC is therefore considered weak at present, because expression of predominant HPV types has not been as clearly identified in NMSC as it is in EV-associated skin cancers or in mucosal cancers associated with the high-risk alpha genus HPV types. It is formally possible that the beta HPV types have a role in the initiation of NMSC but that they are not required for cancer maintenance.
A major advance in the prevention of human cancer has been the development of an effective preventive vaccine for the major genital tract HPVs. The vaccine is a subunit vaccine consisting of the major capsid protein (L1) that can self-assemble into virus-like particles (VLPs), which are empty capsids that closely resemble authentic virions morphologically and immunologically. The L1 VLPs are highly immunogenic, inducing high titers of neutralizing antibodies that are conformationally dependent and type specific. Two commercial prophylactic HPV vaccines have been developed and approved by the FDA. GlaxoSmithKline’s Cervarix is a bivalent vaccine composed of L1 VLPs of HPV16 and 18, whereas Merck’s Gardasil is a quadrivalent vaccine composed of L1 VLPs of HPV6, 11, 16, and 18. Both vaccines are generally safe, able to induce high titers of capsid-reactive antibodies, and highly effective at preventing acquisition of cervical infection and low- and high-grade CIN caused by the types targeted by the vaccine. Both vaccines also induce a modest degree of protection against cervical infection caused by specific nonvaccine types closely related to HPV16 or 18. For instance, both vaccines induced partial protection against persistent infection by HPV31. Cervarix, but not Gardasil, induced significant protection against HPV45, and neither vaccine protected significantly against HPV35 or 58. One can anticipate that second-generation VLP vaccines may be able to protect against an even higher proportion of HPV infection by incorporating VLPs from a larger number of HPV types. Although 70% of cervical cancers are caused by HPV16 or HPV18, 30% are caused by the other high-risk HPV types.
Although Cervarix and Gardasil have now been licensed in more than 100 countries, they have been introduced into the national vaccination programs of only about 30 countries, mostly the most developed ones. National programs have been centered on vaccination of preadolescent or adolescent girls, ages 9 to 15 years, because more than 90% of HPV-associated cancer worldwide occurs in women. However, recent evidence indicates that Gardasil protects young men from genital warts and anal cancer precursors, providing a rationale for considering male vaccination programs. Furthermore, the increase in HPV-positive head and neck oropharyngeal cancer also suggests a rationale for male vaccination programs.
There are several important unresolved issues for the current VLP vaccines. For instance, the VLP vaccine is expensive and is not heat stable, two characteristics that might impede its use in developing countries where the cervical cancer disease burden is greatest. Because of the type specificity, the current vaccines are unlikely to protect against a substantial proportion of other high-risk HPV-type infections, so it will be important for vaccinated women to continue to undergo cervical cancer screening. Additional approaches to improve the vaccine seem warranted. Merck has indicated that a nonavalent (nine HPV targets) VLP vaccine is currently in clinical trials. In addition, the use of L2 represents a potential alternative approach to developing a prophylactic vaccine against a broader spectrum of HPV types. Although they are not as immunogenic as the L1 neutralization epitopes, at least some of the L2 neutralization epitopes induce cross-neutralizing antibodies against papillomaviruses from different types. In addition, modifications of the L1 capsid protein allow the self-assembly of capsomeres that are highly immunoprotective, can be produced in bacteria, and are more stable.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here