Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chlamydia trachomatis is an obligate intracellular bacterium that infects mucosal surfaces of humans, including oropharyngeal, anogenital, and conjunctival surfaces. C. trachomatis can be classified through molecular typing into strains causing ocular infections (trachoma), nonulcerative oropharyngeal and/or anogenital infections (chlamydia), and a distinct ulcerative syndrome called lymphogranuloma venereum (LGV). C. trachomatis infections are highly prevalent worldwide, especially in adolescents and young adults, and can cause substantial reproductive tract morbidity. The majority of persons with chlamydia are asymptomatic, with normal genital examination findings; diagnosis of chlamydia usually relies on testing for the bacterium. Despite highly sensitive tests and highly effective therapy, the number of reported chlamydial infections continues to rise. As a chlamydial vaccine is not yet available, better prevention and control efforts are needed. This chapter reviews the epidemiology, clinical features, diagnosis, treatment, and prevention of chlamydia and LGV.
A 19-year-old female presents to the emergency department (ED) complaining of lower abdominal pain, nausea, and fever. Her abdominal pain began one day prior and rapidly progressed, causing her to present to the ED. She describes the pain as severe, constant abdominal cramping, 8/10 in severity, and located in her lower abdomen. One week prior, she had experienced a vaginal discharge and some mild dysuria. She has no known medical problems. Her only medication is an oral contraceptive medication; she has no known drug allergies. She denies tobacco, alcohol, and drug use. She is sexually active with a single male partner and does not use barrier protection; she has had six lifetime sexual partners. She has never had a sexually transmitted infection (STI) before. She was last sexually active 2 days ago and reports mild pain during sexual intercourse as well as postcoital bleeding. A review of systems is positive for nausea and two episodes of vomiting within the last 12 hours.
Her vital signs include an oral temperature of 101.5°F, pulse of 110 beats/min, respirations 12 breaths/min, and an oxygen saturation of 100% on room air. She has no evidence of conjunctivitis and her oropharynx is not erythematous. On physical examination, she is noted to have mild tenderness on palpation of her lower abdomen. A pelvic examination by speculum reveals a white vaginal discharge and a normal-appearing cervical os without cervical discharge. Bimanual examination reveals cervical motion tenderness and fundal tenderness but no adnexal tenderness. A complete blood count reveals a mild leukocytosis; a pregnancy test is negative. A cervical swab is obtained and sent for a nucleic acid amplification test (NAAT) for gonorrhea and chlamydia.
The patient is diagnosed with pelvic inflammatory disease (PID) and admitted to the hospital, given the concern that she will not tolerate oral outpatient antibiotics. Intravenous antibiotics are administered and, 24 hours later, she is markedly improved, with resolution of her nausea and improvement in her abdominal pain. Her gonorrhea test is negative, but her chlamydia test is positive. Given her clinical improvement, her intravenous antibiotics are stopped and oral antibiotics are started. She is discharged home with instructions to avoid sexual activity until she has completed her antibiotics, her symptoms have resolved, and her partner has been treated. Seventy-two hours later, she reports tolerating her antibiotics well, near complete resolution of her symptoms, and confirms that her partner has been treated. Three months later, she undergoes repeat chlamydia testing, which is negative.
Chlamydia is highly prevalent worldwide in both industrialized and developing countries. The World Health Organization (WHO) estimates that approximately 127 million new cases of chlamydia occur worldwide each year. Chlamydia is a reportable infection in the United States and remains the most commonly reported bacterial STI. Despite declining prevalence in some geographic areas with successful screening and treatment programs, the total number of chlamydia cases reported in the United States continues to increase each year and now exceeds 1.7 million annually. The majority of chlamydia cases in the United States remain undiagnosed, and it is estimated that the true prevalence may be up to 3 million new chlamydial infections each year. Nationally representative surveys of chlamydia prevalence in adolescents and young adults have revealed a prevalence of up to 5% in females and up to 4% in males. The actual prevalence of chlamydia may be much higher, often greater than 10%, in some venues such as clinics aimed at STI care and screening and in correctional facilities. In the United States, the region with the highest burden of chlamydia is the Southeast.
Females may experience significant health consequences from chlamydial complications. One important complication in women is PID, in which the infection spreads to the upper genital tract and can involve the uterus (endometritis), fallopian tubes (salpingitis), ovaries, and/or peritoneum. In the United States, it is estimated that there are over 1 million new cases of PID each year and that at least 10% of women with PID will become infertile (with repeated PID episodes resulting in higher infertility rates). In the United States, the estimated annual cost attributable to chlamydia and its complications is several billion dollars.
There are no reliable estimates of the worldwide burden of LGV because the diagnosis is often missed or is made on clinical findings rather than the results of diagnostic testing. In general, LGV was previously thought to be rare and sporadic in industrialized countries. However, following an LGV outbreak in the Netherlands in 2013, LGV infections have become more frequent in Europe, North America, and Australia, primarily among men who have sex with men (MSM) and mostly occurring at the anogenital site. In developing countries, LGV may be endemic and is a cause of genital ulcers. Hundreds to thousands of LGV cases occur annually in parts of East and West Africa, Southeast Asia, India, South America, and the Caribbean.
Among many risk factors associated with chlamydia, young age is the most prominent. Surveillance studies from the US Centers for Disease Control and Prevention (CDC) have demonstrated that the highest rates are in persons younger than 25 years of age. The chlamydia rate declines significantly in persons 30 years of age and older but still may be substantial in those who have other relevant risk factors, including unprotected sexual intercourse with a new partner or multiple sexual partners and engaging in transactional sex (trading sex for money or drugs). A prior chlamydial infection is a major risk factor, primarily because of high rates of reinfection from untreated partners. Use of human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) (taking HIV medications to prevent HIV acquisition) is associated with an increased risk for multiple STIs, including chlamydia; most chlamydia infections in this setting are likely due to non-LGV serovars, but some anorectal LGV infections also likely occur. Although chlamydia is common in all racial and ethnic groups in the United States, African Americans have the highest rate; Hispanics and American Indian/Alaska Natives are also disproportionately affected compared with Caucasians.
In addition to risk factors related primarily to higher-risk sexual behaviors or socioeconomic status (which may be a proxy for limited access to healthcare and availability of educational or prevention measures), biologic factors can also increase chlamydia risk. Cervical ectopy (expansion of intracervical columnar epithelial cells across the surface of the cervix) likely increases the risk of chlamydia acquisition by providing a greater surface area of susceptible cells for chlamydial infection. Cervical ectopy is more common in younger females, especially those on hormonal contraceptive therapy. Bacterial vaginosis (BV) has also been associated with a twofold increased risk of having chlamydia. The bacterial species present in BV may alter vaginal levels of specific metabolites (increased indole, decreased D-lactic acid) that favor C. trachomatis acquisition and survival. Finally, host immune responses and host genetic determinants (e.g., human leukocyte antigen [HLA] types and immune gene polymorphisms) likely play a role in susceptibility to or protection against chlamydia. Limited data suggest that interferon gamma–producing CD4 + T cells protect against chlamydia incidence and reinfection. HLA-DQB1∗06 is a risk for chlamydia reinfection and complications, but more research is needed on the role of immunogenetic factors in chlamydia and its complications.
Studies of LGV outbreaks in industrialized countries have revealed that most LGV cases were anorectal infections in MSM who practiced unprotected receptive and/or insertive anal intercourse. Other identified LGV risk factors included having multiple sexual partners, engaging in anonymous or group sex, having sex under the influence of drugs (“chemsex”), and being infected with HIV. Although risk factors for endemic genital LGV in developing countries are less well understood, they likely resemble traditional chlamydia risk factors except that, unlike with chlamydia, LGV can also be transmitted by skin-to-skin genital contact.
As discussed in more detail in the following paragraphs, chlamydia causes a diverse spectrum of clinical syndromes ( Table 58.1 ). The incubation period for chlamydia is estimated to be between 7 and 21 days.
| Women | Men | Neonates a |
|---|---|---|
|
In the female genital tract, C. trachomatis primarily infects the endocervix, but it may also infect the urethra and Bartholin glands. The majority (>75%) of women with endocervical chlamydia are asymptomatic; even when symptoms are present, they are frequently nonspecific and overlap with those caused by other vaginal infections (e.g., trichomoniasis or BV) or endocervical infections (e.g., gonorrhea). Symptoms may include new or increased vaginal discharge, intermenstrual bleeding, or pain during intercourse (dyspareunia). Even in the absence of symptoms, 10% or more of women with chlamydia will have signs of infection on pelvic examination. Endocervical discharge (purulent, cloudy, or bloody, Fig. 58.1 ), easily induced endocervical bleeding (“friability”) on insertion of an endocervical swab, and edematous ectopy are the examination findings most suggestive of endocervical chlamydia, yet they are nonspecific. The presence of one or more of these findings supports the clinical diagnosis of cervicitis. Abnormal vaginal discharge originating from the endocervix may also be present. Although cervical ectopy may predispose to chlamydia, the presence of cervical ectopy without edema or congestion is not indicative of chlamydia; ectopy is very common in female adolescents and young adults.

The majority of women with endocervical chlamydia will have concomitant urethral infection, and a small proportion of these urethral infections may cause painful urination and/or increased urinary frequency. In young sexually active women, dysuria due to urethral chlamydia is often misdiagnosed as a urinary tract infection (UTI) and treated with antibiotics that are not effective against chlamydia (e.g., trimethoprim-sulfamethoxazole). Consideration should be given to screening sexually active young women with urinary symptoms for chlamydia. Concomitant infection of the Bartholin glands may rarely occur and manifests as ductal erythema and swelling, typically unilateral and often with purulent ductal exudate.
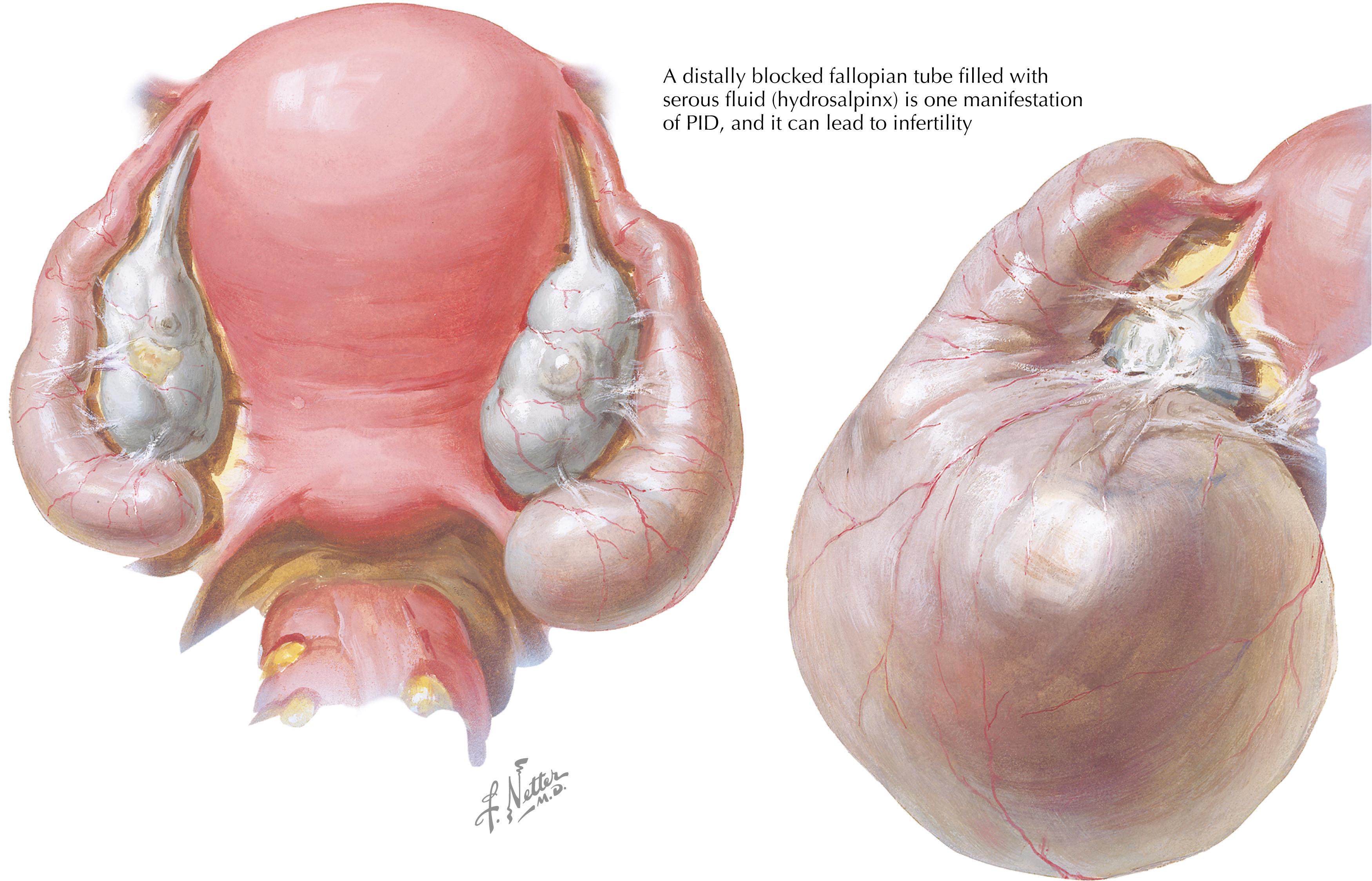
Symptoms of PID include fever, nausea, and pelvic or lower abdominal pain (especially with intercourse). Pelvic examination findings of PID may include cervical motion tenderness and/or tenderness of the uterus, fallopian tubes, ovaries, and/or lower abdomen. A distally blocked fallopian tube can fill with serous fluid and become substantially dilated, a condition called hydrosalpinx ( Fig. 58.2 ). The majority of subjects with PID will have either no symptoms or mild pelvic complaints. Short-term complications of PID include tubal and/or ovarian abscesses; long-term complications include chronic pelvic pain, ectopic pregnancy, and infertility. Chlamydia is a leading preventable cause of ectopic pregnancy and infertility worldwide. Overall, the most common clinical presentation of genital chlamydia in females is the absence of symptoms with normal pelvic examination findings (i.e., no cervical signs).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here