Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infection after penetrating keratoplasty can be a serious complication and can threaten the integrity of the graft.
Microbial keratitis in an eye that has undergone penetrating keratoplasty must be recognized early and treated aggressively, as it can progress to endophthalmitis.
Suture abscesses require careful and aggressive treatment as they can lead to wound dehiscence and endophthalmitis.
Endophthalmitis after penetrating keratoplasty is uncommon but often results in very poor outcomes.
Recurrence rates of host infections can be modified with medical therapy and surgical technique, and herpetic keratitis in corneal grafts can be reduced with prophylactic antivirals.
The Eye Bank Association of America has stringent guidelines designed to prevent the transmission of unusual infections such as rabies and human immunodeficiency virus.
Although the number of cases of penetrating keratoplasty (PK) performed annually in the United States has been surpassed by the number of endothelial keratoplasty cases, approximately 20,000 penetrating grafts are still performed there annually, and it remains a common corneal procedure worldwide. Therefore complications from this surgery are still frequently encountered. Infection after endothelial keratoplasty and keratoprosthesis implantation represents a different clinical scenario; these circumstances are discussed in other chapters.
The onset of an infection following PK can be a serious complication and can threaten the integrity of the graft. Infectious organisms may be introduced at the time of surgery or during the postoperative period. Patients undergoing PK are often predisposed to infection because of an altered immune defense system due to preexisting disease or as a result of frequent topical corticosteroids administered during the postoperative period. Suture-related infections also occur. Although infection is a severe complication, it can often be treated effectively with early diagnosis and prompt appropriate intervention.
Microbial keratitis after corneal transplantation presents a serious and challenging problem for the ophthalmologist and is often devastating for the patient. Reports of the incidence in the literature vary from 1.76% to 12.1% of eyes undergoing corneal transplantation. Most cases of microbial keratitis after keratoplasty present within the first postoperative year. The median time for the development of corneal infection ranged from 5.5 months to 3 years in different series. , , , ,
The causes of infection in the immediate postoperative period can be classified into three general categories: contaminated donor button, intraoperative contamination, or recurrence of host infection. Improved eye banking techniques have succeeded in decreasing the risk of donor transmission of infectious disease by means of careful review of the donor history and serologic testing. Similarly, strict adherence to a sterile operative technique by both the surgical and nursing staff decreases the likelihood of a perioperative infection.
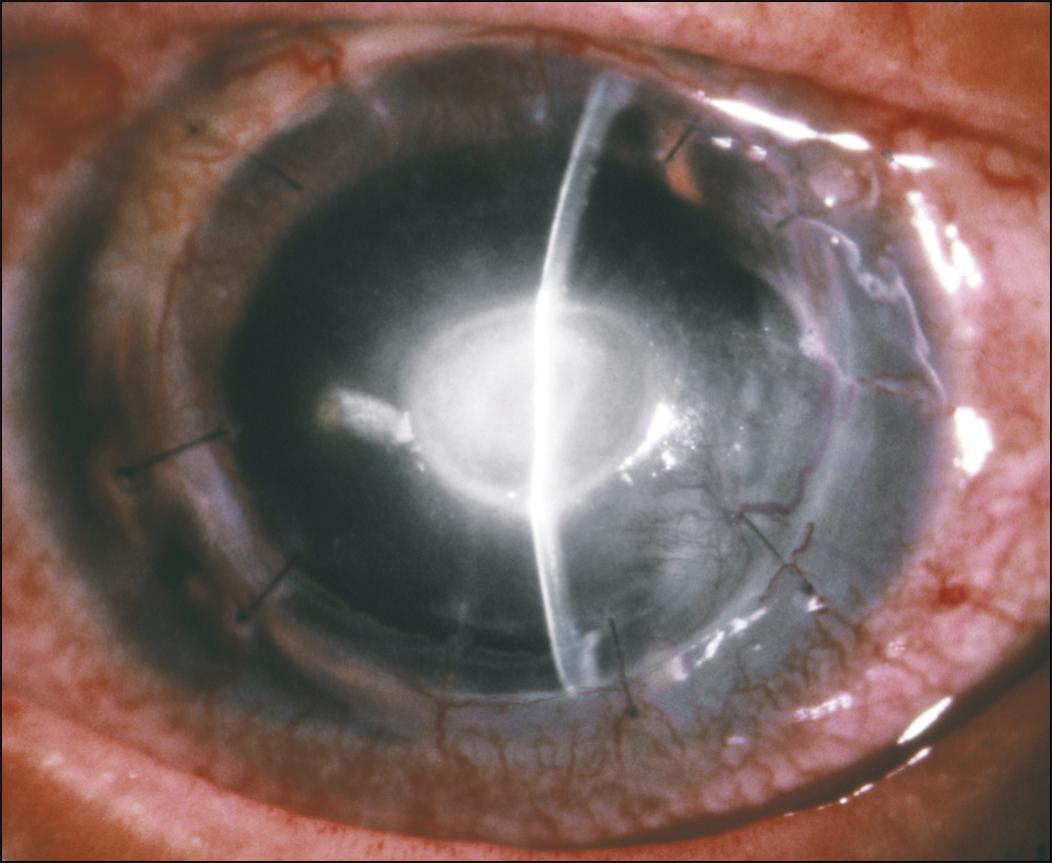
In the late postoperative period, microbial keratitis is usually due to an acquired infection ( Fig. 116.1 ). Predisposing risk factors for microbial keratitis after PK include suture-related problems, epithelial defects, bandage contact lens wear, a history of herpetic keratitis, topical corticosteroid use, ocular surface disorders, and eyelid and ocular adnexal abnormalities. ,
Broken or loose suture material or the recent removal of sutures have been implicated as the cause of microbial keratitis in a significant percentage of cases, ranging from 14% to 50%. , , , , , This topic is covered in a separate section further on.
Epithelial defects have been implicated as the cause of microbial keratitis after PK in 11.4%–64% of cases in reported series. , , In the case of persistent epithelial defects, many surgeons will use bandage contact lenses to promote healing of the defects. Unfortunately the use of bandage contact lenses itself has led to microbial keratitis after corneal transplantation in up to 45% of cases. The role of bandage contact lenses in the pathogenesis of corneal ulceration after keratoplasty remains controversial. It is unclear whether the bandage contact lens or the chronic epithelial defect being treated with a bandage contact lens predisposes these eyes to infection. Paglen and associates reported 41 PK patients who wore therapeutic bandage contact lenses postoperatively with no incidence of microbial keratitis. However, all lenses were removed by 4.4 months, and no persistent epithelial defects were reported. A more recent review of postkeratoplasty infectious keratitis by Davila and Mian reports a rate of 20%–28% of infections related to contact lens use.
Eyes that have undergone PK for herpetic keratitis have a higher risk of developing infectious keratitis than eyes that have been grafted for other indications. In one series, almost one-third of bacterial infections occurred in grafts performed for herpetic keratitis. In another series, 23% of microbial keratitis cases involved grafts performed for herpetic keratitis, although only 3% of all grafts had been performed for this indication.
It is difficult to implicate the long-term use of topical corticosteroids in the pathogenesis of postkeratoplasty ulceration since the overwhelming majority of patients receive topical corticosteroids on an extended basis without complications. It should be remembered that many eyes undergoing keratoplasty are compromised by the primary disorder; moreover, multiple mechanisms that alter host immune defenses are at work.
The status of the ocular surface and eyelids has been implicated in the pathogenesis of corneal ulceration. Attempts should be made both pre- and postoperatively to assess and treat ocular surface disease such as dry eyes, and eyelid abnormalities such as trichiasis and blepharitis.
No statistically significant correlations of postoperative microbial keratitis with systemic diseases have been identified. In one series of postoperative microbial keratitis, the prevalence of diabetes mellitus was 12%, systemic immunosuppression 9%, atopy 3%, and alcoholism 2%.
Many types of pathogens have been implicated in causing microbial keratitis after corneal transplantation. In Bates’s series of approximately 1700 keratoplasties, the predominating pathogens implicated in microbial keratitis were Streptococcus pneumoniae (27%) and Staphylococcus aureus (20%), followed by gram-negative organisms (20%) and fungal organisms (13%). However, in many cases, organisms not normally considered pathogenic may become opportunistic under certain conditions in a compromised eye. In contrast, in a series of over 1000 keratoplasties, Lamensdorf and colleagues reported that the most common pathogens were Candida albicans (33%), Staphylococcus epidermidis (22%), and S. aureus (15%). Overall, fungal infections ( Fig. 116.2 ) have been documented in 6%–36% of cases of microbial keratitis after keratoplasty. , , , , , In a review of adverse reactions reported to the Eye Bank Association of America (EBAA) from 2007 to 2014, the rate of fungal infections (keratitis and endophthalmitis) in all forms of keratoplasty was 0.023% (83 of 354,930). In the same report, the EBAA noted an increasing trend in the incidence of fungal infection after corneal transplantation. In contrast to the previous series, Tavakkoli and Sugar reported that of 885 penetrating keratoplasties, Pseudomonas aeruginosa (22.3%) was the predominant organisms causing postkeratoplasty microbial keratitis.
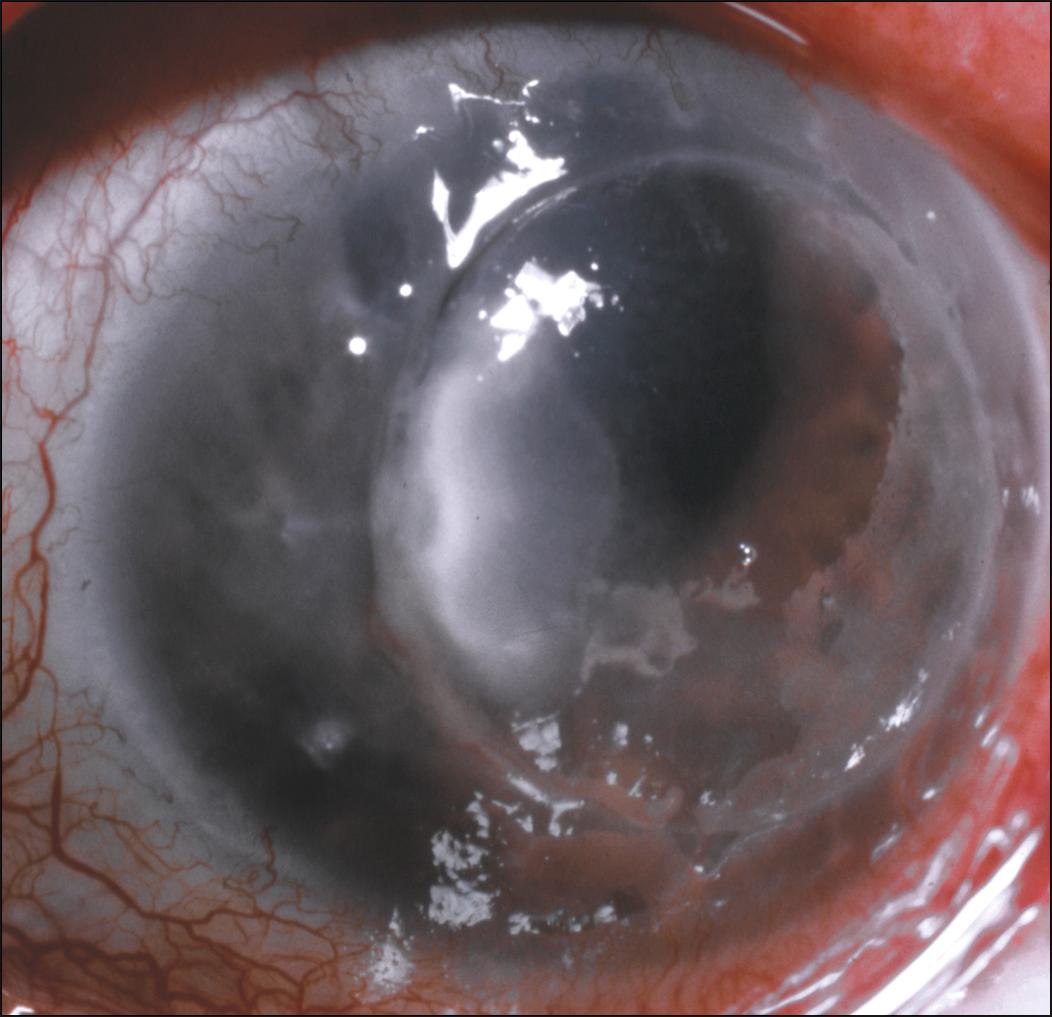
Scrapings or biopsy of the corneal infiltrates yielded positive cultures in 71.4% and 100% of cases, respectively. , , , , In the series by Tuberville and Wood, none of the patients receiving prophylactic antibiotics were culture positive for a resistant organism. However, in later series, an alarming 50%–89% of organisms demonstrated resistance to the prophylactic antibiotic used. , , This difference in resistance to prophylactic antibiotics may represent the recent emergence of resistant bacterial strains.
The ulcer should be characterized at the slit lamp and then documented with a carefully detailed drawing in the medical record. Measurements of the size of the epithelial defect, the infiltrate, and any hypopyon should also be made. This drawing will serve as a reference to monitor improvement or progression of the disease process. The wound must be carefully inspected for structural integrity. If the equipment is available, slit lamp photography should also be performed.
In order to identify the responsible organism, scrapings for cultures and smears should be obtained promptly. A sterile Kimura spatula or #15 scalpel blade is used to scrape the corneal surface and to obtain infected material for direct inoculation onto culture plates including blood, chocolate, and Sabouraud agar as well as into thioglycolate broth. If a contact lens is in place, it should be removed and placed on a separate culture plate or in a tube of thioglycolate broth.
If, at the time of keratoplasty, cultures of the donor rim were obtained, the culture results may help to guide the therapeutic regimen in the immediate postoperative period. However, the selection of an antibiotic should not be based exclusively on this information.
An intensive regimen of broad-spectrum antibiotic eye drops should be implemented as soon as scrapings for cultures and smears have been obtained. Recent clinical practice for the treatment of corneal ulcers in non-PK patients has shifted in some centers toward treatment with fourth-generation fluoroquinolones such as moxifloxacin or gatifloxacin hourly, based on in vitro susceptibility data , and ease of access to the drugs for the patient. Further studies to directly compare fourth-generation fluoroquinolones with fortified antibiotics have not been published to date, and this warrants further investigation. The practice of monotherapy with fourth-generation fluoroquinolones could be extrapolated to approach treatment in patients with bacterial keratitis postkeratoplasty. However, it is important to treat this high-risk population aggressively, and a low threshold for initiating fortified antibiotics should be maintained. If fortified antibiotics are chosen, empiric combination therapy with fortified cefazolin 33–50 mg/mL (or vancomycin 25–50 mg/mL) and fortified tobramycin 9.1–14 mg/mL eye drops should be given every 30 minutes around the clock in the first 24 hours. Subconjunctival antibiotic injection should be considered when compliance may be a problem or the administration of eye drops may be delayed.
If the patient is to be managed as an outpatient, the patient’s home situation and the availability of responsible family members or friends who can administer the eye drops should be investigated. If there is any doubt regarding compliance, the patient should be hospitalized with an explanation to the nursing staff stressing the importance of adhering to the eye drop schedule. Patients should be instructed to wash their hands before putting in their drops and not to touch the eye or eyelids with the dropper tip.
Topical corticosteroids should be stopped in the presence of an acute graft infection. Only when the organism has been identified and the infection brought under control should the clinician consider restarting corticosteroid therapy. Preferably, the epithelium should be intact before corticosteroids are reintroduced. Use of topical corticosteroids in the treatment of corneal ulcers in general has traditionally been a highly debated topic, , but recent evidence suggests that corticosteroid use is safe in most cases of bacterial keratitis. Although it is unclear if these results can be extrapolated to PK eyes, consideration could be given to restarting earlier or even continuing topical steroids at low doses in certain cases where there is a high risk for rejection and the organism has been identified. If the patient is taking other eyedrops (e.g., glaucoma medications), these bottles should be discarded because they may be contaminated. New prescriptions can be written so that the patient can obtain sterile medication.
If trichiasis is present, the offending lashes should be epilated. Elimination of ocular surface problems may play an important role in the patient’s recovery from a corneal ulcer. Punctal occlusion or frequent preservative-free tear replacement therapy can be used to manage coexisting dry eye. A temporary or permanent tarsorrhaphy can be performed to manage exposure keratitis or severe dry eye.
The patient should be examined daily to monitor the clinical response for progression or improvement of microbial keratitis. Signs of worsening include an increasing epithelial defect, enlarging infiltrate, stromal thinning or perforation, and increasing hypopyon. Signs of improvement include a smaller or healed epithelial defect, consolidation of the infiltrate, decreasing white cell reaction in the stroma, lack of stromal thinning or necrosis, and decreasing anterior chamber reaction. Careful inspection of the wound interface should be performed at every visit to assess its integrity and to determine if wound dehiscence has developed.
Once culture results have become available, antibiotic therapy can be tailored to the individual organism or organisms. Tapering of topical antibiotics can be started after the ulcer has demonstrated improvement or when signs of antibiotic toxicity are evident. Signs of toxicity include an enlarging epithelial defect, worsening conjunctival injection, and chemosis in the presence of an improved infiltrate. Antibiotics should be tapered gradually.
If cultures are negative and the patient continues to worsen despite frequent fortified antibiotic therapy, a diagnostic corneal biopsy should be considered. Adjunctive imaging techniques such as confocal microscopy can also be utilized.
The prognosis for graft survival after infectious keratitis is relatively poor. The overall prognosis depends on the causative organism, location of the infiltrate, severity of the infection, underlying disease, and host defenses, including tear and eyelid function.
Outcome data of several published reports of microbial keratitis after keratoplasty reveal that only 23%–50% of original grafts remained clear despite intensive treatment. , , , , Approximately 10.3%–26% of corneal grafts with microbial keratitis later perforated. , , , , , Endophthalmitis developed in 2.7%–13% of cases of infectious corneal ulceration after keratoplasty. , , , , , Emergent regrafting was required in 19%–35% of cases for dehiscence or perforation. , , Overall, repeat PK was performed in 30.8%–70% of eyes. , , , , , , Enucleation or evisceration was performed in 9% of eyes. ,
Visual outcome after microbial keratitis in the setting of a previous PK varies by report. Tuberville and Wood reported a series of corneal ulcers in grafts showing that visual acuity was reduced in 46% of eyes. However, patients with a history of herpetic keratitis demonstrated an 88% incidence of decreased vision. Loss of light perception has been reported to occur in 10%–15% of eyes. , In contrast, more recent studies have reported a best corrected visual acuity of 20/200 or better in 56%–76% of patients, including those who were regrafted. ,
Poor visual outcome may also be associated with extremes of age. Wagoner and colleagues reported no patients with visual outcomes better than 20/200 among patients less than 12 years of age (10 eyes) or older than 60 years of age (45 eyes) compared with 17 (36.2%) of eyes of patients between those ages. In addition to a poor prognosis, the pediatric population may also have a higher risk for microbial keratitis. In a separate study, Wagoner and associates found that culture-positive bacterial keratitis developed in 17.3% of their series of 202 primary penetrating keratoplasties in children 12 years of age or younger compared with 1.76%–12.1% reported in the general population. Gram-positive organisms accounted for 77.6% of isolates, among which S. pneumoniae was the most common organism. Despite aggressive therapy, graft clarity was maintained in only 11.4%, no eyes achieved a visual acuity of 20/40 or better, and 65.7% were hand motions vision or worse.
A suture abscess is defined as an infiltrate in either the donor or recipient cornea that is in direct contact with suture material or adjacent to it ( Figs. 116.3 and 116.4 ). Suture abscesses have been reported to occur in 1%–3.3% of penetrating keratoplasties after an average of 21.5–41.5 months from the time of surgery. Organisms most commonly responsible for suture abscess formation are S. epidermidis , S. pneumoniae , and S. aureus , although cases of gram-negative infection are well documented. , Risk factors for suture abscess formation include a loose or broken suture ( Fig. 116.5 ), recent suture removal, topical corticosteroid use, a persistent epithelial defect, use of soft contact lenses, keratoconjunctivitis sicca, or previous herpes simplex virus (HSV) keratitis.
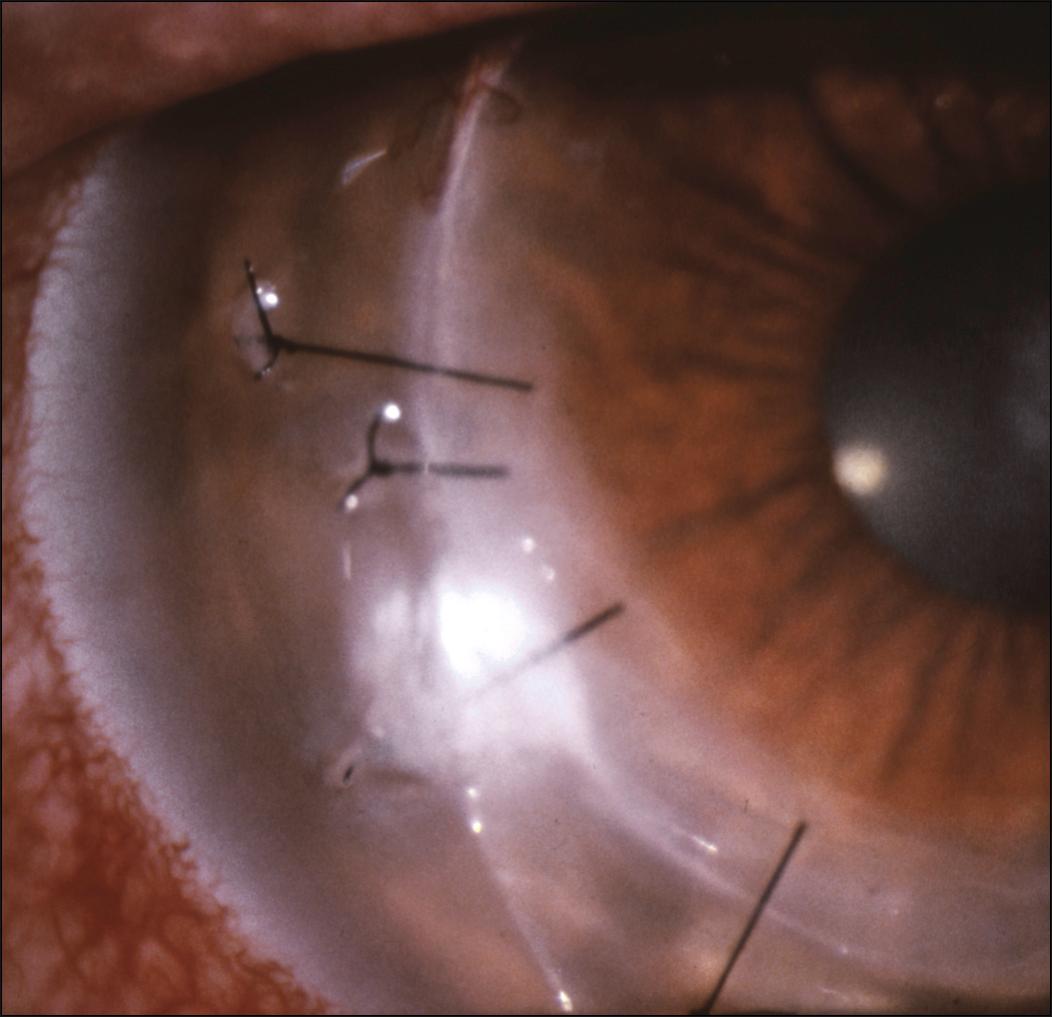
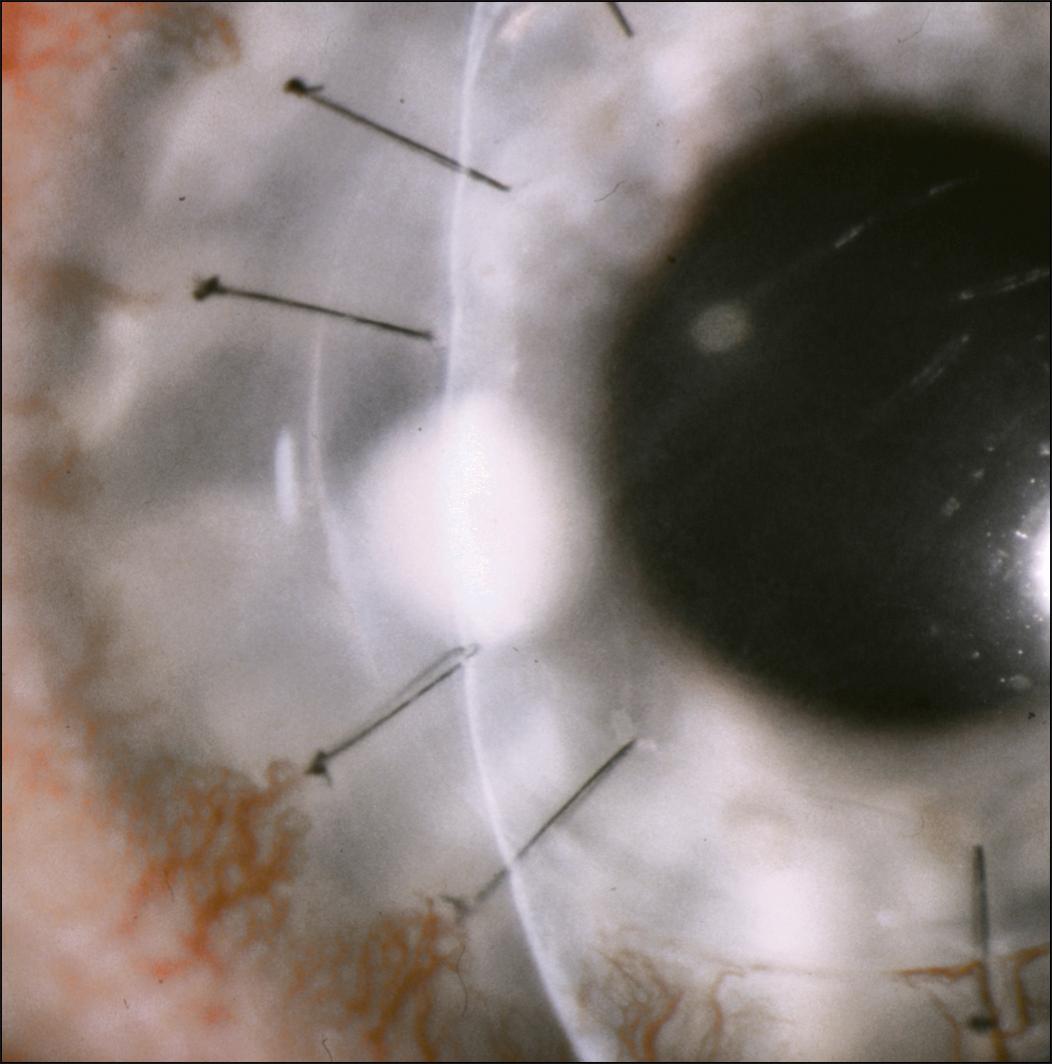
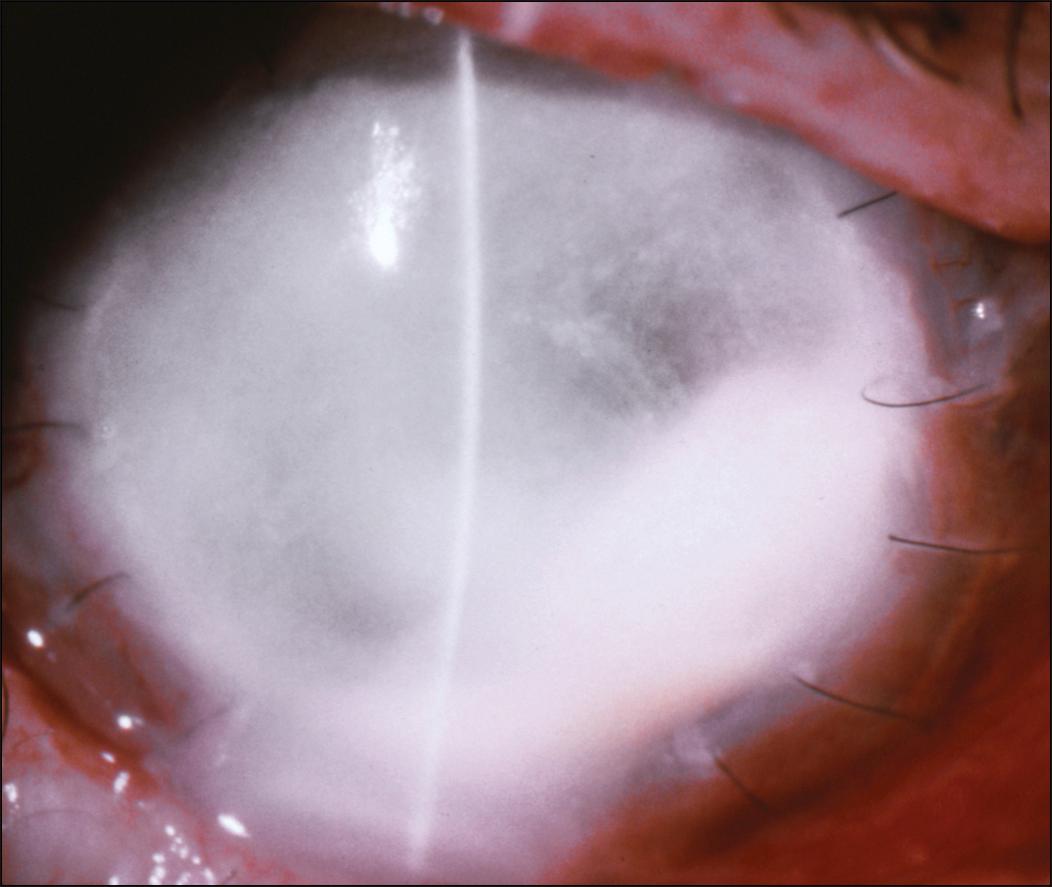
An infectious suture abscess must be differentiated from sterile suture infiltrates. A suture abscess is typically a single lesion associated with an eroded suture and an overlying epithelial defect, which may occur on the host and/or recipient side of the graft. A sterile suture infiltrate occurs with an exaggerated inflammatory response, usually within the first few weeks after surgery. In this situation, there are usually multiple lesions typically on the host cornea and the overlying epithelium is intact.
Careful inspection of each suture should be made at every postoperative visit. Loose sutures should be removed promptly, since they may serve as a nidus for infection. Sutures can become loose due to cheese-wiring through corneal tissue, degradation of suture material over time, and wound shrinkage. Suture removal must be performed carefully, including the removal of mucous debris and avoiding the tracking of exposed sutures through the corneal stroma.
Prompt treatment of a suture abscess is warranted because it can lead to wound dehiscence ( Fig. 116.6 ), scarring, graft failure, perforation, and endophthalmitis. , Management should include careful removal of the offending suture, followed by corneal scrapings for smears, cultures, and sensitivities. The suture itself can also be placed directly onto a culture plate or into a tube of thioglycolate broth. Broad-spectrum fortified antibiotic eye drops with an intensive dosing regimen should then be given. In the past, it was taught that only after the infection had been identified and controlled should topical steroids be restarted in order to prevent or treat graft rejection and scarring. However, given the newer evidence regarding the use of corticosteroids for bacterial keratitis, it may be appropriate to restart earlier or even continue topical steroids at low doses in certain cases of suture abscesses (as in other cases of microbial keratitis in the setting of PK) in which there is a high risk for rejection and the organism has been identified.
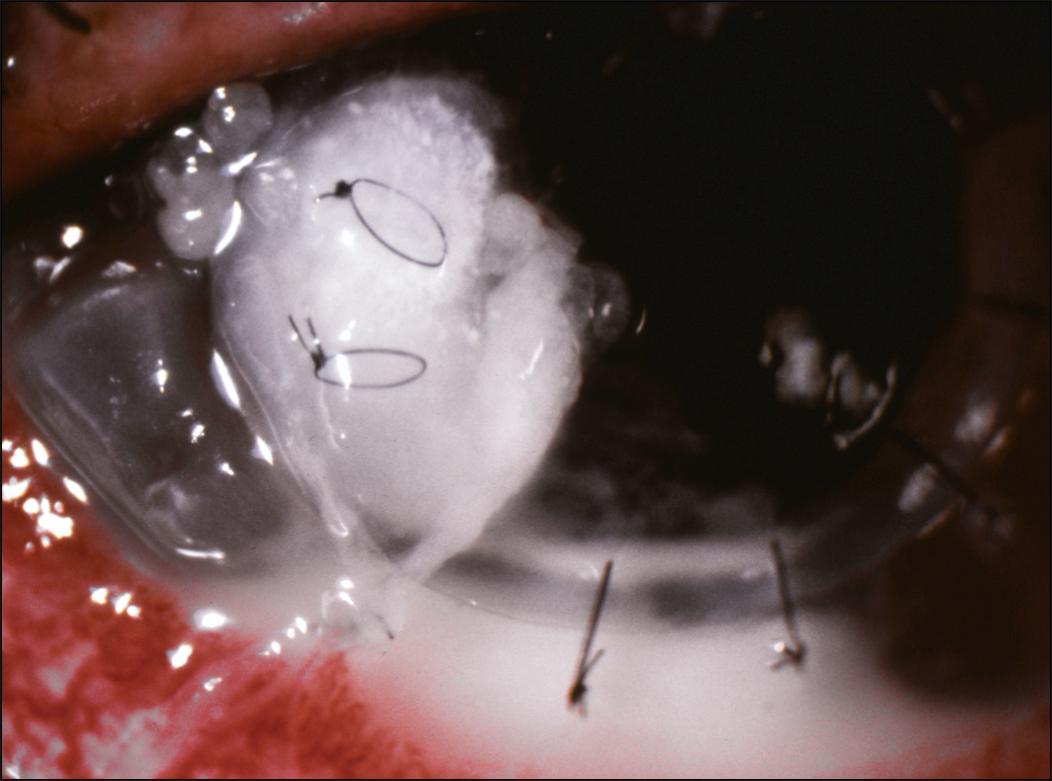
Surgical intervention is required when the graft-host interface becomes unstable or wound dehiscence occurs. Additional sutures may be placed to secure the wound. However, the area involved may be necrotic or too friable for sutures to be held in place securely. Long interrupted suture bites or sutures placed in a figure-eight or horizontal mattress configuration through healthy tissue should be attempted. Attention must also be given to the wound area 180 degrees away from the dehiscence, since this area is placed under greater tension during the resuturing process. Small-diameter tectonic keratoplasty involving the graft-host junction has also been described to be an effective treatment modality for PK wound dehiscence secondary to suture abscess. , High or irregular astigmatism can result from these techniques. If the suture abscess has caused extensive destruction of the cornea, a large PK (recipient bed >9.5 mm) can be performed. Postoperatively, however, an increased risk for graft rejection and increased intraocular pressures must often be addressed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here