Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human immune system has evolved to protect an individual from infectious microbes. It does this by using a complex interactive network of cells, proteins, and organs. Rapidly expanding research of inborn errors of immunity has taught us that despite the apparent redundancy of the system, quantitative and qualitative defects of individual components or pathways, or both, result in abnormal function and susceptibility to particular infections. Taken together, the prevalence of these inborn errors of immunity is now thought to be close to ∼1 per 1,000–1 per 5,000 births.
This chapter provides a general overview of the development of innate and adaptive immune responses, addresses some immunologic developmental characteristics unique to the fetus and newborn, and addresses susceptibility to pathogens in general terms.
The innate immune system represents the first line of host defense against infection and includes the following: (1) antimicrobial products and physical barriers such as skin and mucosal surfaces; (2) receptors for pathogens, including pattern-recognition receptors (PRRs), Toll-like receptors (TLRs), C-type lectin receptors, nucleotide-binding oligomerization domain (NOD) proteins, leucine-rich repeat-containing receptors (NLRs), and retinoic acid-inducible gene I protein (RIG-I) helicase receptors; (3) phagocytic cells such as neutrophils and macrophages; (4) dendritic cells (DCs); (5) complement proteins; and (6) natural killer (NK) cells.
In mammals, epithelial cells are capable of secreting antimicrobial peptides such as defensins and cathelicidins. Defensins contribute to host defense by disrupting the cytoplasmic membranes of microbes and inducing DC and lymphocyte chemotaxis. There are two defensin types in humans, α-defensins, which are produced by neutrophils, monocytes, and Paneth cells of the gut, and β-defensins that are produced by epithelial cells. Both have been implicated in antiviral activity against herpes simplex virus 1 (HSV-1), HSV-2, cytomegalovirus, varicella-zoster virus, and human papillomavirus (HPV). Cathelicidins exhibit both antimicrobial and immunomodulatory activity in the setting of injury and infection, and they are expressed by epithelial and peripheral blood cells.
The skin’s tight junctions between epithelial cells, thickness, and dry environment offer a shield against microbes. Loss of skin integrity, as occurs with wounds, burns, and inflammation, allows pathogens to breach this barrier. Antimicrobial peptides decrease the susceptibility to infection when skin barriers are compromised. Psoriasis and atopic dermatitis (AD), which are inflammatory skin conditions associated with skin disruption, demonstrate the role of these molecules in modulating infection risk. Although infection rarely is associated with psoriasis, patients with AD commonly are colonized and/or infected with Staphylococcus aureus, with some susceptibility thought to be secondary to filaggrin deficiency.
At the level of the mucosal-epithelial interface, the expression of interleukin (IL)-17 by Th17 cells and the synthesis of defensins by keratinocytes prevent invasion by Candida spp. Experimental models of mucocutaneous candidiasis have shed light on the pathogenesis of Candida infections in patients with hyperimmunoglobulinemia E (hyper-IgE), the autoimmune polyendocrinopathy syndrome, in patients with ectodermal dysplasia (APECED), and in patients with defects in the caspase recruitment domain-9 (CARD-9) protein. ,
The more common entry pathway for pathogens is through the mucosal barrier. Mucosal-epithelial cells secrete mucus that contains antimicrobial peptides. Mucus coats pathogens, thus allowing antimicrobial peptides to exert their action, and provides a vehicle for particles and pathogens to be cleared by the action of cilia. Within the respiratory tract, cilia move the mucus toward the upper airways, where it is either expelled by cough mechanism or swallowed. Ineffective clearance, as seen in patients with immotile cilia syndrome, cystic fibrosis, or after lung transplantation favors pathogen colonization. Cathelicidin LL-37 promotes bacterial phagocytosis by macrophages and has also been proposed as a signal molecule to promote an inflammasome-mediated death and trigger a protective inflammatory response to uncontrolled Pseudomonas aeruginosa infections, thereby contributing to pathogen clearance. In this context, evolving therapeutic approaches using novel antimicrobial peptides in the setting of decreased mucosal clearance and antibiotic resistance show promise.
Surfactant-associated proteins, specifically surfactant protein A (SP-A) and surfactant protein D (SP-D), contribute to innate immune responses in the lung. Produced by type II pneumocytes and nonciliated respiratory epithelial cells, SPs belong to the family of proteins called collectins . SP-A and SP-D can interact with microorganisms, modulate local inflammatory responses, modulate neutrophil responses in vitro, and participate in the clearance of pollens and other complex organic antigens. SP-A is known to bind to Mycoplasma pneumoniae , helps modulate inflammation, and induces eosinophil peroxidase release, which particularly affects patients with asthma leading to exacerbations. In patients with severe, treatment-resistant asthma, SP-D levels in bronchoalveolar lavage fluids decline, whereas serum concentrations increase. Exciting prospects for the role of recombinant SP-D have been explored, including its ability to downregulate inflammation caused by influenza A, which otherwise would lead to cell death and lung damage. Lysozyme, lactoferrin, histatins, and phospholipase A2 in tears and saliva also are potent antibacterial enzymes. While digestive enzymes, bile salts, fatty acids, and lysolipids protect the gastrointestinal tract, Paneth cells secrete α-defensins and influence the virulence of orally ingested bacteria. For example, children and adults with infections caused by Shigella or virulent Salmonella strains have decreased synthesis of HBD1 and cathelicidin LL-37 by colonic enterocytes. HBD2 expression also is reduced in enterocytes of patients with Crohn disease, and gastric mucosa–derived β-defensins are decreased in Helicobacter pylori infections. Endogenous antimicrobial peptides also appear to play a role in the regulation of host commensal bacteria and in the composition of gut microbiota.
There are a variety of signaling tools within the innate immune system that facilitate the communication and amplification of responses, specifically activating and releasing IL-1β, when presented with a pathogen. These include, but are not limited to, pathogen receptors , TLRs , and inflammasomes . Pathogen-associated molecular patterns (PAMPs) represent pathogen-specific carbohydrates and lipoproteins or nucleic acids expressed as part of their life cycle (i.e., bacterial DNA as unmethylated repeats of dinucleotide CpG, double-stranded [ds], or single-stranded [ss] RNA). Host proteins capable of recognizing such specific microbial patterns are called PRRs. An example is mannose-binding lectin (MBL), a circulating soluble protein that binds mannose or fucose, residues on microbes and facilitates phagocytosis. Macrophages carry a C-type lectin, called macrophage mannose receptor (MMR), which binds carbohydrate moieties found on the surface of bacteria and also recognizes viruses such as the human immunodeficiency virus (HIV) and fungi. ,
TLRs are a type of PRR capable of linking the innate and adaptive immune systems (see Chapter 10 ). These proteins are present on many cells including airway and gut epithelial cells, antigen-presenting cells (B lymphocytes, macrophages, DCs, monocytes), hematopoietic stem cells (HSCs), mast cells, regulatory T lymphocytes (Tregs), NK cells, and endothelial cells. TLRs have been identified in humans: TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are found on cell surfaces, whereas TLR3, TLR7, TLR8, and TLR9 are localized within the endosomes. TLR2 is involved in responses to gram-positive bacteria (peptidoglycans and lipoproteins) and yeast. TLR4 mediates the interaction of gram-negative bacteria by transducing signals derived from lipopolysaccharides (LPS). A mouse model for TLR4 mutations renders the animal resistant to endotoxin but highly susceptible to gram-negative infection. All TLRs are capable of interacting with different ligands (see Table 10.1 in Chapter 10 ). Respiratory syncytial virus (RSV) F protein, LPS, and Pseudomonas exoenzyme S have been shown to interact with TLR4, whereas flagellin is recognized by TLR5. TLR2 recognizes envelope proteins of herpes simplex virus (HSV), whereas TLR9 identifies CpG motifs within the viral genome.
TLR recognition of microbial products triggers the activation of downstream signaling pathways where myeloid differentiation factor 88 (MyD88) and Toll–IL-1 receptor domain-containing adaptor-inducing interferon β (IFNβ) (TRIF) lead to the activation of NF-κB and subsequent transcription of proinflammatory cytokines: tumor necrosis factor (TNF), IL-1, and IL-6. MyD88 recruits the IL-1R–associated kinase (IRAK) family of proteins: IRAK1 and IRAK4. Humans and mice with deficiencies in IRAK4 or MyD88 demonstrate severe impairment of IL-1 and TLR downstream signaling and are susceptible to recurrent and severe bacterial infections. TRIF signaling results in activation of IFN regulatory factor-3 (IRF-3) and induction of type 1 IFN genes such as IFNα and IFNβ, thus enhancing viral clearance.
TLR polymorphisms have been linked to a variety of diseases. Polymorphisms in TLR1 and TLR10 may increase the risk of asthma in adolescents who had bronchiolitis as infants. TLR4 has been implicated in its role in exaggerated bacterial signaling leading to necrotizing enterocolitis in premature infants. TLR-9 has been linked to hepatic injury following severe renal ischemia reperfusion, and a deletion of the signaling domain of TLR5 has been found to increase susceptibility to Legionnaire disease. Toll signaling pathways have been implicated in the pathogenesis of sepsis and shock, and defects in TLR3 have identified inherited susceptibility to HSV encephalitis. The stage of human development in which Toll receptors appear is not clearly established. However, evidence indicates that TLR2 is developmentally expressed during fetal life from the early pseudoglandular to the canalicular stages of human lung development.
A group of PRRs, called nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), are intracellular sensors that detect bacterial infection and cellular damage. NLRs are distinguished based on the types of protein domains they contain. An important NLR subfamily is the nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing protein (NLRP) family. Specifically, NLRP3 has been studied in the formation of a multiprotein complex known as the inflammasome. Inactivated NLRP3 resides in the cytoplasm until it is activated by reduced intracellular potassium, the generation of reactive oxygen species, or the disruption of lysosomes. Upon activation, NLRP3 molecules aggregate and interact with another protein, ASC, which then binds to inactive protease pro-caspase 1, forming what is known as the inflammasome and releasing an active caspase 1. With the help of TLR-mediated priming, inflammasome activation ultimately leads to the production of inflammatory cytokines, specifically IL1β and IL18. NLRP3’s role in the innate immune system’s response to infectious diseases has been studied in patients who have died from SARS-CoV-2, with high levels of IL-1β detected in autopsy tissues. Additionally, it has been proven that ion channel activity of the SARS-CoV 3a protein is essential for triggering the NLRP3 inflammasome. NLRP3 inflammation may also play a role in disease tolerance, as proven by its dampening observed in bats that could explain their lack of response when exposed to high-dose Ebola and MERS-CoV viruses. However, when dysregulated, inflammasomes have proven to have a role in a variety of conditions including pediatric febrile seizures, periodic fever syndromes, and inflammatory bowel disease.
More specifically, mutations in NLRP3 have now been established as a spectrum of a monogenetic diseases known as cryopyrin-associated periodic syndromes (CAPS). These include the phenotypes of chronic infantile neurologic cutaneous articular (CINCA) syndrome, neonatal onset multi-system inflammatory disease (NOMID), and overlap with Muckle–Wells syndrome (MWS). CAPS patients will present with cutaneous, musculoskeletal, ocular, and central nervous system involvement. Urticarial rashes are typically the first symptoms noted, with fever, myalgia, arthralgia, headache, fatigue, conjunctivitis, and keratitis symptoms following. Complications can include sensorineural hearing loss, sterile meningitis, developmental delay, increased intracranial pressure, and amyloidosis.
Interferons are types of antiviral cytokines, which are divided into three types. Type I interferons include IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω. IFN-γ is the sole type II interferon. Type III interferons include IFN-λ1 (IL-29; non-functional pseudogene in mice), IFN-λ2 (IL-28A), IFN-λ3 (IL-28B), and the most recently described human IFN-λ4. Type I interferons have several major functions. They induce resistance to viral replication of uninfected cells, induce major histocompatibility complex (MHC) class I expression that enhances resistance to NK cells, while also activating NK cells to selectively kill virally infected cells. Additionally, interferon stimulates the production of chemotaxins , including CXCL9, CXCL10, and CXCL 11 that recruit lymphocytes to infections. Type I interferons can be induced by a variety of signaling cascades including those initiated through TLRs, specifically TLR 3 and TLR 7, RIG–1–like receptors (RLRs), and stimulator of interferon genes (STING).
The inability to produce type I or type III interferons during the neonatal period has been found to predispose these children to an increased risk of lower respiratory tract infections and subsequent development of persistent wheezing. In addition, interferon alpha 2 receptor (IFNA2R) deficiency causes fatal susceptibility to live virus vaccines. The last decade has seen the recognition of a group of inherited disorders known as the interferonopathies recognized as the results of constitutive upregulation of type I interferon activity, the prototype of which is the Aicardi–Goutières syndrome.
The major phagocytic cells are neutrophils and macrophages. In humans, myeloid precursors are found in the yolk sac by day 19 of development, 2 days before the onset of blood circulation. Hematopoiesis then shifts to the fetal liver and finally to the bone marrow. In the bone marrow, phagocyte development is under the control of multiple growth factors, including IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and macrophage colony-stimulating factor (M-CSF). The marrow pool of neutrophils in adults is 20 times the number of neutrophils in circulation. The mechanisms responsible for the release of neutrophils from the marrow are complex. In familial neutropenias associated with maturational arrest, genes such as HAX-1 , CSF3R , ELANE , and WAS have been identified as essential for neutrophil development. The release or retention of neutrophils within the marrow compartment is regulated by the interaction of signaling proteins on the cell surface, called chemokines, which interact with their ligands on bone marrow stromal cells. An example is the CXCR4/CXCL12 pair that permits retention of neutrophils in the marrow. Mutations in CXCR4 lead to a rare condition called WHIM syndrome (warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis). Myelokathexis indicates that neutrophils are retained in the marrow compartment, unable to be released, causing peripheral neutropenia. Normally, once in the circulation, neutrophils circulate for a few hours and then move into the tissues where they are active for 2–6 days.
With birth at term gestation, the neonatal peripheral neutrophil count is higher than that of adults, but a reserved capacity to respond with an outpouring of phagocytic cells, which often are immature, appears to be minimal during infection. Newborn infants with septicemia often have severe neutropenia and depletion of phagocytic storage pools, a finding associated with high mortality. The cause of this rapid depletion remains unknown. It is possible that decreased numbers of neutrophils at tissue sites of infection contribute to the susceptibility of neonates to pneumonia and skin infection and to the development of multiple sites of infection after bacteremia or fungemia. The lack of adequate numbers of cells at the site of infection can cause or result in functional deficiencies.
Monocytes also move from the circulation to tissue spaces, where they develop into macrophages, live for 2–3 months, and assume specialized characteristics generally determined by their location (e.g., lung, liver, or spleen). Circulating monocytes also have chemotactic and phagocytic activities and have receptors for immunoglobulin G (IgG) Fc domains (FcR), complement, and TLRs 2–9 along with CD14, an important molecule that mediates TLR4 activation by LPS.
The function of phagocytes, which are particularly important in defense against bacteria and fungi, requires not only an adequate number of cells, but these cells need to be able to sense and migrate toward the site of infection ( chemotaxis ) and to ingest and kill microorganisms ( phagocytosis ). These processes are mediated by the expression of adhesion molecules, opsonins (complement and antibodies), and chemoattractants. IL-8 is the most important chemokine that attracts neutrophils. At the site of infection, neutrophils engulf microbes and particulate matter by the mechanism of phagocytosis and kill the pathogen. Additionally, neutrophils secrete chemokines, release neutrophil extracellular traps (NETS), and die by apoptosis once their mission is accomplished. Macrophages subsequently clear any cellular debris. Monogenic defects at different stages of this process define unique syndromes ( Fig. 9.1 ).
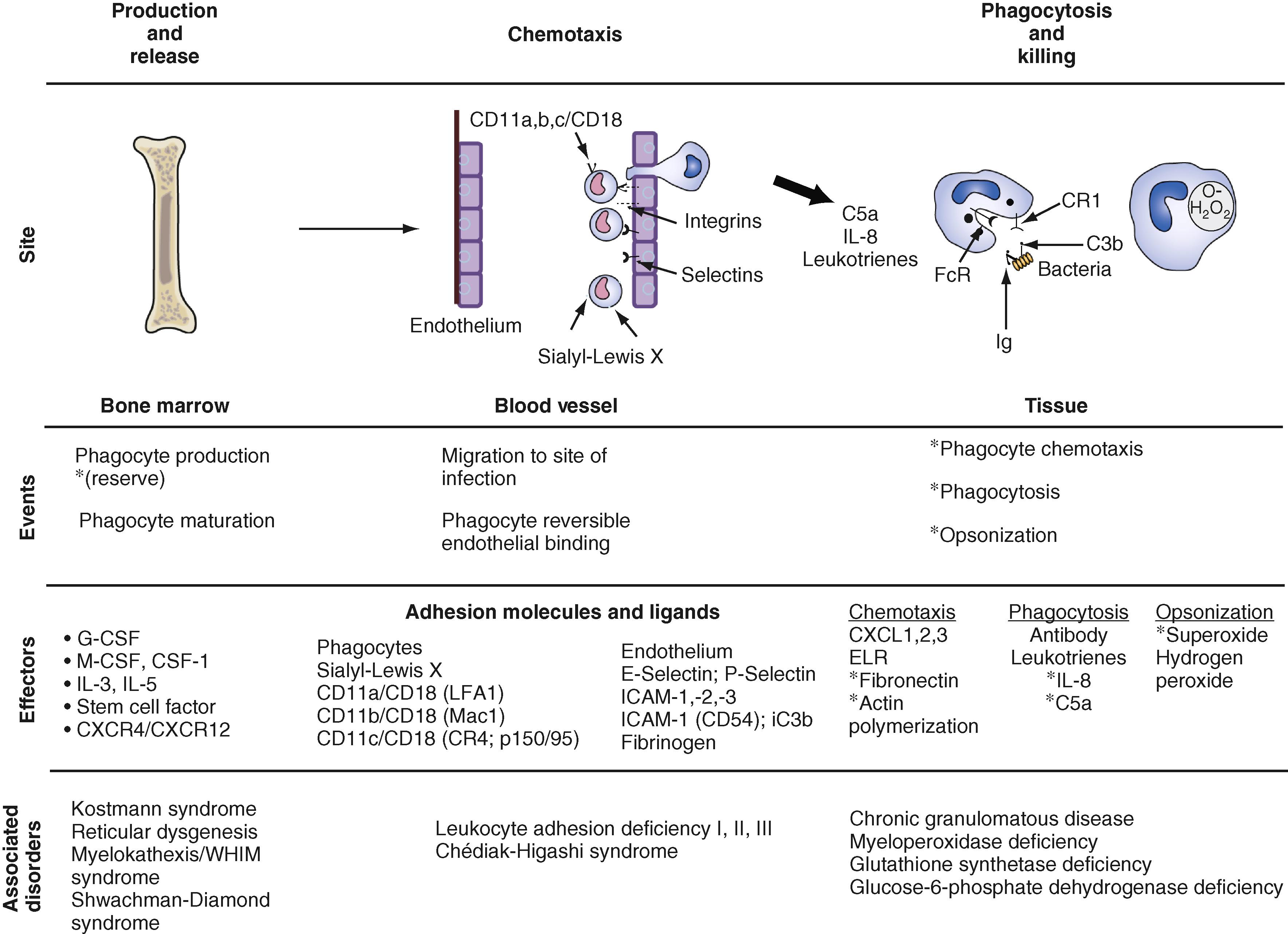
Chemotaxis is the process by which phagocytes reach the site of infection. As a result of a local inflammatory response, endothelial cells within local vessels express adhesion molecules called selectins (CD62E, CD62P). These molecules reversibly bind to ligands on neutrophils (sialyl-Lewis X and PSGL1) and consequently make the neutrophil slow down and “roll” along the endothelium. Subsequently, another group of adhesion molecules, called integrins , is upregulated on the surface of neutrophils. Integrins are composed of one of three different α chains: CD11a, CD11b (CR3), or CD11c (CR4); they are noncovalently linked to a β chain, CD18, thus forming a CD11a/CD18 or LFX1, CD11b/CD18, or MAC-1, and CD11c/CD18 or p150,95 integrin complex. Integrin molecules “stop” the neutrophil, which then undergoes skeletal changes and migrates through the vascular lumen into the extravascular space by adhering to intracellular adhesion molecules (ICAMs). The sialic constituent of the group B Streptococcus (GBS) capsular polysaccharide mimics the human Lewis X antigen, thus making this a poor immunogen, and perhaps renders the neonate especially susceptible to this organism. Defects in the expression of adhesion molecules have been described in humans: leukocyte adhesion defect (LAD) types 1, 2, and 3. The lack of integrin expression causes LAD-1, lack of sialyl-Lewis X expression causes LAD-2, and defects of integrin activation cause LAD-3. Patients have persistent leukocytosis, delayed separation of the umbilical cord, skin ulcers, periodontitis, and delayed wound healing. L-selectin (CD62L) levels on fetal and immature infant neutrophils are comparable to those of adults. However, L-selectin expression is downregulated in the term neonate and is further diminished during acute bacterial infection in vivo.
Phagocytosis is an active process by which a previously bound pathogen is engulfed by a phagocyte in a membrane-bound vesicle called a phagosome. Both macrophages and neutrophils contain lysosomes , which are membrane-bound acidic organelles containing proteolytic enzymes capable of producing toxic products: nitric oxide (NO), superoxide anion (O 2 − ), and hydrogen peroxide (H 2 O 2 ). The fusion of the lysosome and phagosome membranes is necessary to kill the organism. Within azurophilic granules, a bactericidal/permeability-increasing protein (BPI) binds to bacterial LPS and kills gram-negative bacteria. BPI dysfunction has been implicated in both neonatal sepsis and chronic Pseudomonas infection in patients with cystic fibrosis. ,
The production of O 2 − and H 2 O 2 depends on the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzyme complex (see Chapter 104 ). Defects in the different components of this enzyme complex result in the immunodeficiency known as chronic granulomatous disease. Affected patients are susceptible to infections with catalase-producing organisms, Aspergillus and Nocardia species. Because phagocytes are unable to kill the microbes, the host tries to contain the infection by calling in more macrophages and lymphocytes, with resulting granuloma formation. Once bacteria are killed, neutrophils die, whereas macrophages can generate new lysosomes. Patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency and glutathione synthetase deficiency also have neutrophil dysfunction and can have clinical features similar to those of chronic granulomatous disease.
The capability of the newborn’s phagocytes for non-opsonic adherence to organisms and phagocytosis is nearly equal to that of adult cells. However, deficiencies in chemotaxis and O 2 − production have been described. The bacterial killing of umbilical cord blood phagocytes is effective against Escherichia coli and Streptococcus pyogenes and is similar to the bacterial killing of phagocytes in adults, but it appears abrogated against GBS. Abnormalities in chemoattractants (IL-8, complement fragment C5a, fibronectin) and defective expression of complement receptors, such as C5a receptor, caused by the C5a-mediated exocytosis of myeloperoxidase also have been described. , Defects in membrane fluidity and cytoskeletal changes that affect microfilamentous and cytoskeleton responses can contribute to neutrophil motility defects as well.
DCs are the prototypic antigen-presenting cell. In humans, CD34 + HSCs capable of generating DCs are detected in the fetal liver at 20 weeks of gestation, after which they are found mainly in the bone marrow. Originating in the bone marrow, their lineage-specific markers are still being defined, but the final steps in the differentiation process are controlled by transcription factors, eventually dividing into lymphoid organs and tissues under the control of FLT3LG (Fms Related Receptor Tyrosine Kinase 3 Ligand). DCs are capable of regulating both innate and adaptive immune responses. With unique morphologic characteristics when activated, several pathogen receptors have been identified. Examples include TLRs, scavenger receptors that mediate bacterial internalization, such as MAC-1 (CD11b/CD18) and the CR3 complex. DCs induce antiviral cytotoxic T-lymphocyte responses producing IL-12 and IL-15, 2 cytokines critical for cytotoxic CD8 + T-lymphocyte differentiation. Certain DC subsets activate CD4 + T lymphocytes through MHC II activation, while others are involved in a large production of type I interferon and the initiation of antiviral innate and adaptive immune responses. Furthermore, activated DCs can promote B-lymphocyte proliferation into plasma cells by releasing soluble factors or by direct cell-cell interaction. In the skin, DCs are localized to the basal and suprabasal layers of the epidermis; in the murine gut, DCs are found in the Peyer patches; and in the human lungs, they can be found within the airway epithelium, alveolar septa, visceral pleura, and vascular walls.
While surveying the tissue environment, DCs can be recognized by an “immature” phenotype (CD11b bright , CD11c mo d, CD86 low , ClassII low , CD4 − ). On uptake of antigen or microbial products by different mechanisms of phagocytosis, DCs migrate through the afferent lymphatics to the regional lymph node, where they arrive as mature, nonphagocytic DCs (CD40 high ). These DCs can produce inflammatory cytokines and chemokines. Bacterial uptake of Mycobacterium tuberculosis , bacille Calmette-Guérin (BCG), Saccharomyces cerevisiae , Corynebacterium parvum , S. aureus , Leishmania spp., Borrelia burgdorferi, and Staphylococcus aureus has been demonstrated in vitro.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here