Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
At birth, the fetal-neonatal immune system gets activated. It is critical for survival to recognize and eliminate all the potentially dangerous pathogens in the infant’s external and its own enteric environment.
The innate arm is particularly necessary because the adaptive immunity is still developing.
In immunodeficiency states, the aberrant development of the immune system puts the infant at risk of infections, and all the chaotic attempts at host defense often cause dysregulated and excessive inflammation.
The neutrophils are the first line of defense against invading pathogens. There are known defects in adhesion and transepithelial migration, cellular life span, chemotaxis, and bacterial killing.
Most defects in the monocyte-macrophage system are parts of multisystem disorders and are recognized in the neonatal period more often due to manifestations in other organs.
Most defects in adaptive immunity in term infants manifest later in infancy once the maternally derived immunoglobulins fade away.
Severe combined immunodeficiency (SCID) presents early in life with severe infections such as respiratory infections, diarrhea, and sepsis; it is a genetically heterogeneous disorder that is comprised of many genetic defects. The most frequently seen subtype is inherited as an X-linked trait and is T− B+ NK−. Fifty to seventy percent of infants have lymphopenia, particularly in T cells. Early recognition of SCID syndromes can help in survival. Testing for these conditions is part of the neonatal screening.
At birth, the fetus suddenly moves out of a relatively sterile intrauterine sanctuary into an open environment that is full of microflora. In utero, the fetal immune system is hypoactive and tolerant, which may actually be beneficial in evading recognition as an allograft by the maternal immune system. However, after birth, the same fetal-neonatal immune responses become critical for survival, to recognize and eliminate all the potentially dangerous pathogens in the external and the enteric environment. The innate arm is particularly necessary because the adaptive immunity is still developing. There is also a need for a functional dichotomy in the innate immunity that will help harmonize with the friendlier commensals but at the same time “contain/eliminate” the confrontational pathogens. In immunodeficiency states, the aberrant development of the immune system puts the infant at risk of infections, and with all the chaotic attempts at host defense, it often causes dysregulated and excessive inflammation.
In this review, we present an overview of various immunodeficiency syndromes that present during early infancy. We have included evidence from our own studies and from an extensive literature search in the databases PubMed, Embase, and Scopus. To avoid bias in the identification of studies, keywords were short-listed a priori from anecdotal experience and PubMed’s Medical Subject Heading thesaurus.
Neutrophils are the first line of defense against invading pathogens. Once activated by infection and/or associated inflammation, this amphibious “naval” force of leukocytes patrolling in the bloodstream gets rapidly deployed into the affected tissues. The activated neutrophils emarginate out of the circulation, attach to the vascular endothelium, migrate across the capillary walls, move rapidly with focused chemotaxis, and then eliminate the pathogens through phagocytic ingestion or extracellular killing. The major limitations in the role of neutrophils in host defense can be due to decreased neutrophil number and/or function.
In neonates, neutropenia is a frequent occurrence. It is defined as an absolute neutrophil count (ANC) < 1000/μL or an ANC less than the 5th percentile for age. , There are existing norms for gestational and postnatal age-dependent neutrophil counts. Neutropenia is frequently seen in extremely premature infants, with some studies having noted it in as many as half of all very low birth weight infants in the first week after birth.
In terms of kinetics, neutropenia can be secondary to decreased neutrophil production, increased destruction, or a combination of these mechanisms. Based on etiology, duration, and severity, neonatal neutropenia can be broadly classified into several groups:
Transient neonatal neutropenia of the neonate: Small-for-gestational-age infants, particularly those born to mothers with preeclampsia or pregnancy-induced hypertension, are frequently neutropenic in the first 3 to 5 days after birth. These infants may have some inhibitors of normal granulocyte colony stimulating factor (G-CSF) production and consequent suppression of neutrophil production. Neutropenia may also be seen for 2 to 3 days after birth in donor twins in twin-twin transfusion syndromes.
Congenital neutropenia syndromes: Neutropenia may be severe and protracted with ANCs as low as <200 μL/mL. These conditions are rare and are noted only when these infants have recurrent infections and are noted to be severely neutropenic. Table 57.1 summarizes important congenital neutropenia syndromes. Kostmann disease is an important example that is caused by mutations in HAX1 , with consequent arrest of neutrophil maturation at the promyelocytic stage. Cyclic neutropenia is a rare autosomal dominant condition with mutations in the ELANE gene. These patients have regular drops in ANCs to levels <250 μL/mL at 3-weekly intervals. Bone marrow studies in these patients demonstrate an arrest in neutrophil maturation that precedes or coincides with severe neutropenia.
| Neutropenia Syndrome | Gene | Inheritance | Hematologic Features | Nonhematologic Features | Treatment |
|---|---|---|---|---|---|
| Severe congenital neutropenia | ELANE | AR | Neutropenia | — | rG-CSF |
| Kostmann disease | HAX1 | AR | Neutropenia | — | rG-CSF |
| Severe congenital neutropenia—AD forms | GFI1 PRDM5 PFAAP5 |
AD | Neutropenia | — | rG-CSF |
| Cyclic neutropenia | ELANE | AD | Neutropenia | — | rG-CSF |
| Barth syndrome | TAZ1 | X-linked | Neutropenia | Cardiomyopathy, growth retardation, muscle weakness | rG-CSF |
| Shwachman-Bodian-Diamond syndrome | SDBS | AR | Neutropenia, anemia, thrombocytopenia | Pancreatic insufficiency, skeletal anomalies, short stature, failure to thrive, delay in development | rG-CSF, HSCT |
| Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome | CXCR4 | AD | Neutropenia, lymphopenia, thrombocytopenia, myelokathexis | Cardiac anomalies | rG-CSF , CXCR4 antagonist |
| Hermansky-Pudlak syndrome type 2 | AP3B1 | AR | Neutropenia, platelet dysfunction | Albinism | |
| Glycogen storage type IB | G6PT1 | AR | Neutropenia, neutrophil dysfunction | Hypoglycemia, lactic acidemia, hyperlipidemia | rG-CSF |
| Cartilage hair hypoplasia | RMPR | AR | Neutropenia, lymphopenia, macrocytic anemia | Short stature, aganglionic megacolon, fine sparse hair | rG-CSF |
| Chediak-Higashi syndrome | LYST | AR | Neutropenia, giant neutrophil granules, bleeding diathesis | Albinism, peripheral neuropathy, hemophagocytic syndrome | HSCT |
| Cohen syndrome | VPS13B | AR | Neutropenia | Facial dysmorphism, retinochoroidal dystrophy, delay in development | |
| Schimke immuno-osseous dysplasia | SMARCAL1 | AR | Neutropenia, lymphopenia | Spondyloepiphyseal dysplasia, postnatal growth failure, proteinuria | G-CSF , HSCT |
a Genetic causes, clinical features, and current treatment modalities for disorders with congenital neutropenia are highlighted. AD, Autosomal dominant; AR, autosomal recessive; HSCT, hematopoietic stem cell transplant; rG-CSF, recombinant granulocyte colony stimulating factor.
Immune-mediated neonatal neutropenias: These conditions should be considered in infants with persistent neutropenia. Neonatal alloimmune neutropenia (NAIN) occurs when the mother develops antibodies against fetal red blood cells carrying a paternally derived human neutrophil antigen, and these antibodies are then transmitted across the placenta back into the fetus to cause neutropenia. NAIN can be severe and protracted. Neonatal autoimmune neutropenia results when mothers have antineutrophil autoantibodies that cross the placental and bind fetal neutrophils. This form of neutropenia is usually milder than NAIN. In addition to allo- and autoimmune neutropenia, some patients have been reported with the so-called autoimmune neutropenia of infancy. This relatively transient form of immune neutropenia is caused by antineutrophil autoantibodies produced by the neonate’s own immune system.
Sepsis: Neonates, particularly those born preterm, can become neutropenic during sepsis due to exhaustion of neutrophil reserves. Neonates have a limited neutrophil storage pool in the bone marrow. In the proliferative pool, the progenitors have a higher steady-stage rate of proliferation at baseline, which reduces the ability of these cells to substantially increase production when needed, resulting in neutropenia.
Idiopathic neutropenia of prematurity: This is seen in some growing premature infants, possibly because a large fraction of the marrow progenitors is committed to erythroid differentiation to compensate for anemia of prematurity. This is a benign form of neutropenia that typically presents after the early neonatal period (week 4–10). Neutropenia is usually transient and recovers spontaneously in these patients.
A complete blood count with differential leukocyte counts is important in the initial evaluation of patients with neutropenia. Concomitant presence of anemia and thrombocytopenia would indicate marrow failure syndromes. On the other hand, an increased number of immature neutrophils and a high immature/total ratio indicates increased neutrophil destruction and marrow that is actively proliferating to restore neutrophil numbers. In comparison, a normal immature/total ratio may indicate decreased neutrophil production. If autoimmune or alloimmune neutropenia is being considered, maternal anti-NAIN antibody titers and typing for NAIN antigens in the mother, father, and infant is indicated. In infants with prolonged neutropenia (2 weeks to months), a bone marrow biopsy should also be considered.
Therapy with recombinant granulocyte colony stimulating factor (rG-CSF) and recombinant granulocyte-macrophage colony stimulating factor (rGM-CSF), which are myeloid growth factors, can be used in neutropenic infants. Unlike G-CSF, which primarily affects neutrophil populations, GM-CSF stimulates proliferation of both neutrophil and macrophage precursors. Both G-CSF and GM-CSF can increase neutrophil counts, but G-CSF is more effective. G-CSF (rG-CSF) can also raise neutrophil counts in many patients with congenital neutropenia, such as those with Kostmann syndrome and cyclic neutropenia, and it can reduce the frequency of infections. G-CSF is also an effective therapy in immune-mediated neutropenia.
Recombinant G-CSF treatment has been evaluated in neonatal sepsis. Smaller studies have shown some success, but no convincing effects were seen in a Cochrane review by Carr et al. Intravenous immunoglobin can mobilize neutrophils from the storage pool into circulation and has been tried. Similarly, the efficacy of granulocyte transfusions in sepsis is uncertain.
Leucocyte adhesion deficiency (LAD) is a rare autosomal recessive (AR) disorder impairing adhesion, migration, and phagocytic ability of neutrophils ( Fig. 57.1 ). Many infants present with delayed separation of the umbilical cord and sometimes omphalitis. Some infants go on to have recurrent bacterial infections, recurrent skin and periodontal infections, and deep tissue abscesses. In a patient with typical clinical features, neutrophilia raises the clinical suspicion, which can be confirmed by flow cytometry of peripheral blood leukocytes to measure CD18 expression on neutrophils.
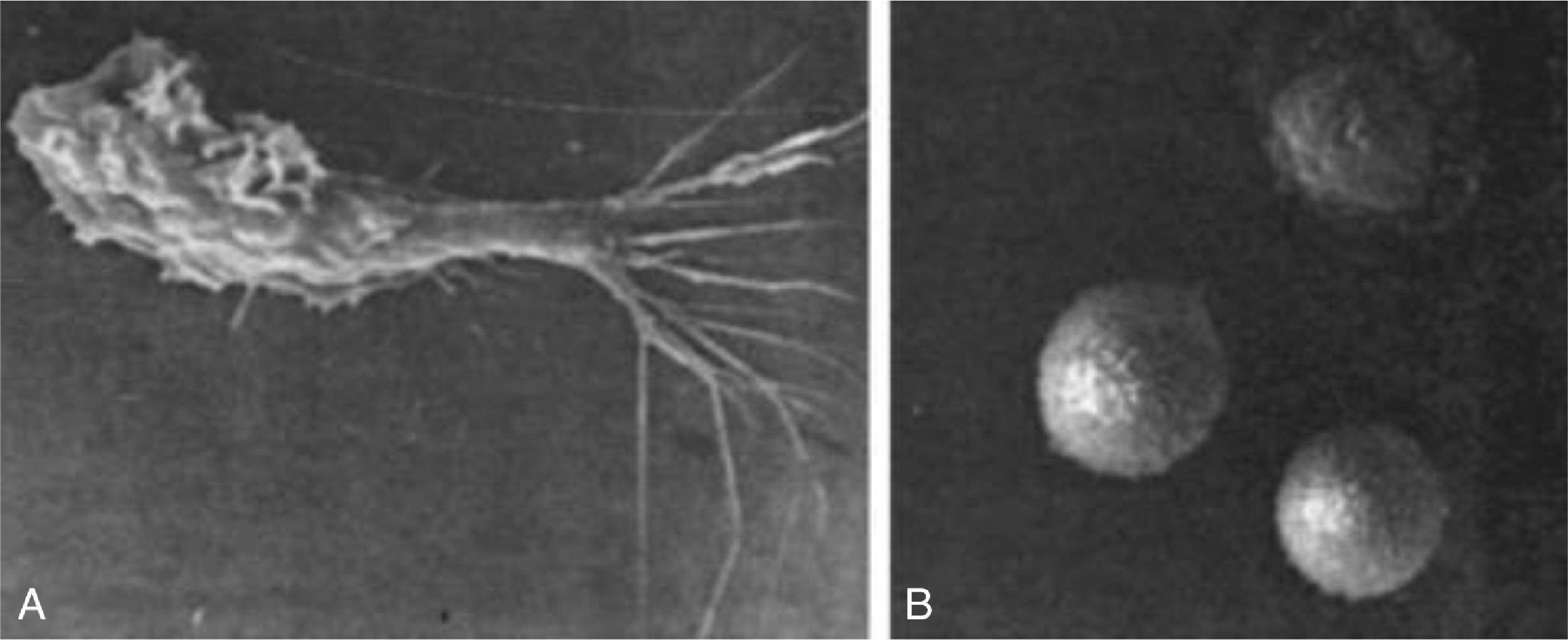
There are three types of LAD. LAD-1 results from mutation in the integrin β 2 (ITBG2) gene, which is located on chromosome 21q22.3 and encodes the CD18 subunit of β 2 integrins. More than 100 mutations have been reported in the ITGB2 gene, including many that are missense, involving the splice site, small deletions, large deletions, and nonsense mutations. The degree of clinical severity and prognosis depends on CD18 expression, and hence, LAD1 can be classified into mild (CD18 expression >30%), moderate (2%–30%), and severe (<2%). The clinical suspicion can be confirmed by flow cytometry for lack of expression of CD18 on neutrophils or by genetic testing.
Hematopoietic stem cell transplantation (HSCT) is the treatment of choice, although it is used mostly in patients with severe LAD1. Recently, adipose tissue-derived mesenchymal stem cells have shown some promise in improving wound healing in murine LAD1 via adaptive release of transforming growth factor beta (TGF-β1). Similarly, gene therapy studies using different vectors have shown promising short- as well as long-term results in canine LAD1. These findings have increased the enthusiasm for future research in humans. ,
LAD-2 is an extremely rare AR disorder caused by mutations in the Solute Carrier Family 35 Member C1 (SLC35C1) gene, which encodes the guanosine diphosphate–fucose transporter 1. The clinical hallmark of LAD2 is recurrent bacterial infections (less severe than LAD1) and developmental abnormalities including psychomotor and mental impairment, dysmorphic facies (coarse facial features, broad nasal tip, hypertelorism, and microtia), and short stature (not seen in LAD1). , The SLC35C1 gene is located on chromosome 11p11.2, and mutations result in decreased expression of fucosylated glycans on the cell surface, including the sialyl Lewis X ligand that binds selectins. Therefore LAD2 is also known as congenital disorder of glycosylation type IIc. Flow cytometry can confirm the diagnosis by showing a lack of sialyl Lewis X (CD15s) expression on the leukocyte cell surface and the absence of H antigen on the red blood cell surface (Bombay blood type). Oral supplementation with high-dose fucose has benefitted some patients, but the effect is not consistent.
LAD3 is also a very rare AR disorder resulting from mutations in the FERMT3 (FERM Domain Containing Kindlin 3; F for 4.1 protein, E for ezrin, R for radixin, and M for moesin) gene located on chromosome 11q13.1. FERMT3 mutations lower the expression of kindlin-3, causing impaired integrin activation and impaired leukocyte and platelet adhesion. LAD3 shares the clinical features with LAD1 with an additional platelet aggregation defect; the diagnosis is suspected when a patient presents with typical clinical manifestations but flow cytometry shows normal expression of β 2 -integrins. Therefore confirmation of diagnosis requires next-generation sequencing looking at mutation in the FERMT3 gene. The high tendency for bleeding (even in the intracranial area) and risk of infections qualifies these patients for early HSCT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here