Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The manifestations of immune deficiencies that affect multiple cell types range from profound to mild; these conditions can present with severe infection, recurrent infections, unusual infections, or autoimmunity. The most profound disorder is severe combined immunodeficiency. Other combined immunodeficiencies include defects of innate immunity and defects leading to immune dysregulation; the latter category is typically associated with profound autoimmunity. Combined immunodeficiencies are characterized by a predisposition to viral infections, and the innate immunodeficiencies are susceptible to a range of bacteria.
SCID
newborn screening
lymphoid development
Severe combined immunodeficiency ( SCID ) is caused by diverse genetic mutations that lead to absence of T- and B-cell function. Patients with this group of disorders have the most severe immunodeficiency.
SCID is caused by mutations in genes crucial for lymphoid cell development ( Table 152.1 and Fig. 152.1 ). All patients with SCID have very small thymuses that contain no thymocytes and lack corticomedullary distinction or Hassall corpuscles. The thymic epithelium appears histologically normal. Both the follicular and the paracortical areas of the spleen are depleted of lymphocytes. Lymph nodes, tonsils, adenoids, and Peyer patches are absent or extremely underdeveloped.
| DISEASE | INHERITANCE | PRESUMED PATHOGENESIS | ADDITIONAL FEATURES | TREATMENT |
|---|---|---|---|---|
| Reticular dysgenesis | AR | Impaired mitochondrial energy metabolism and leukocyte differentiation | Severe neutropenia, deafness. Mutations in adenylate kinase 2 | GCSF, HSCT |
| Adenosine deaminase deficiency | AR | Accumulation of toxic purine nucleosides | Neurologic, hepatic, renal, lung, and skeletal and bone marrow abnormalities | HSCT, PEG-ADA, gene therapy |
| IL-2Rγ deficiency | X-linked | Abnormal signaling through by IL-2 receptor and other receptors containing γc (IL-4, -7, -9, -15, -21) | None | HSCT |
| Jak3 deficiency | AR | Abnormal signaling downstream of γc | None | HSCT |
| RAG1 and RAG2 deficiency | AR | Defective V(D)J recombination | None | HSCT |
| Artemis deficiency | AR | Defective V(D)J recombination, radiation sensitivity | DCLERE1C gene defects | HSCT |
| DNA-PK deficiency | AR | Defective V(D)J recombination | None | HSCT |
| DNA ligase IV deficiency | AR | Defective V(D)J recombination, radiation sensitivity | Growth delay, microcephaly, bone marrow abnormalities, lymphoid malignancies | HSCT |
| Cernunnos-XLF | AR | Defective V(D)J recombination, radiation sensitivity | Growth delay, microcephaly, birdlike facies, bone defects | HSCT |
| CD3δ deficiency | AR | Arrest of thymocytes differentiation at CD4 − CD8 − stage | Thymus size may be normal | HSCT |
| CD3ε deficiency | AR | Arrest of thymocytes differentiation at CD4 − CD8 − stage | γ/δ T cells absent | HSCT |
| CD3ζ deficiency | AR | Abnormal signaling | None | HSCT |
| IL-7Rα deficiency | AR | Abnormal IL-7R signaling | Thymus absent | HSCT |
| CD45 deficiency | AR | None | HSCT | |
| Coronin-1A deficiency | AR | Abnormal T-cell egress from thymus and lymph nodes | Normal thymus size. Attention deficit disorder. | HSCT |
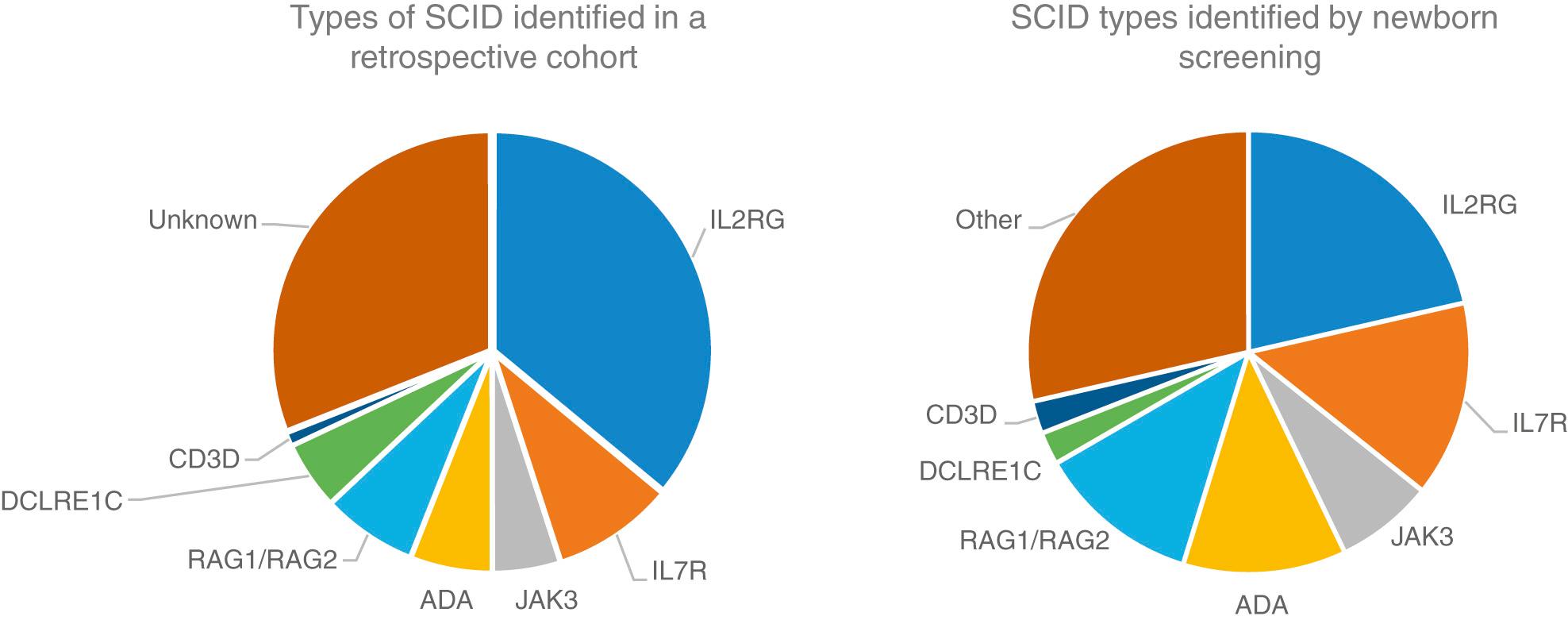
SCID is included in the newborn screening program in many states. Thus, infants are identified prior to symptoms, which has dramatically improved the survival of infants with SCID. A few genetic types of SCID are not detected by newborn screening, and there are a few states where newborn screening for SCID is not yet performed.
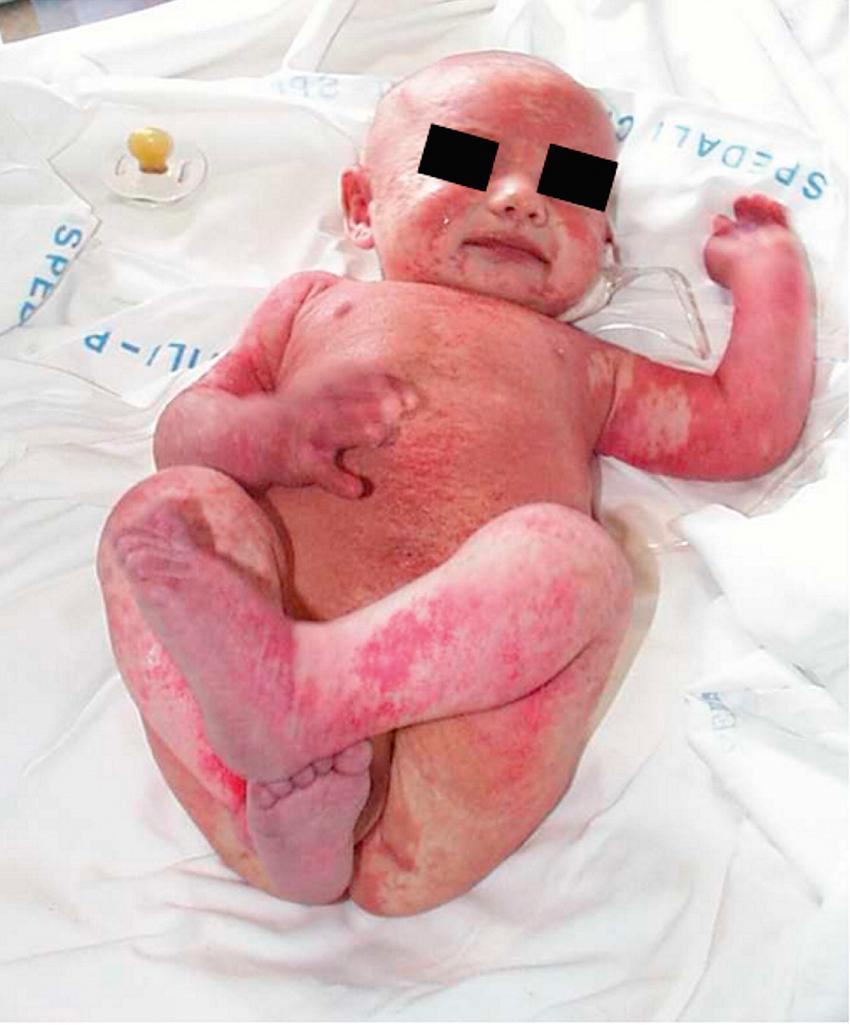
When infants with SCID are not detected through newborn screening, they most often present with infection . Diarrhea, pneumonia, otitis media, sepsis, and cutaneous infections are common presentations. Infections with a variety of opportunistic organisms, either through direct exposure or immunization, can lead to death. Potential threats include Candida albicans, Pneumocystis jiroveci , parainfluenza 3 virus, adenovirus, respiratory syncytial virus (RSV), rotavirus vaccine, cytomegalovirus (CMV), Epstein-Barr virus (EBV), varicella-zoster virus, measles virus, MMRV (measles, mumps, rubella, varicella) vaccine, or bacille Calmette-Guérin (BCG) vaccine. Affected infants also lack the ability to reject foreign tissue and are therefore at risk for severe or fatal graft-versus-host disease (GVHD) from T lymphocytes in nonirradiated blood products or maternal immunocompetent T cells that crossed the placenta while the infant was in utero. This devastating presentation is characterized by expansion of the allogeneic cells, rash, hepatosplenomegaly and diarrhea. A 3rd presentation is often called Omenn syndrome , in which a few cells generated in the infant expand and cause a clinical picture similar to GVHD ( Fig. 152.2 ). The difference in this case is that the cells are the infant's own cells.
A key feature of SCID is that almost all patients will have a low lymphocyte count. A combination of opportunistic infections and a persistently low lymphocyte count is an indication to test for SCID. The diagnostic strategy both for symptomatic infants and those detected by newborn screening is to perform flow cytometry to quantitate the T, B, and natural killer (NK) cells in the infant. The CD45RA and CD45RO markers can be helpful to distinguish maternal engraftment and Omenn syndrome. T-cell function is also often assessed by measuring proliferative responses to stimulation.
All genetic types of SCID are associated with profound immunodeficiency. A small number have other associated features or atypical features that are important to recognize. Adenosine deaminase (ADA) deficiency can be associated with pulmonary alveolar proteinosis and chondroosseous dysplasia. Adenylate kinase 2 (AK2) deficiency causes a picture referred to as reticular dysgenesis where neutrophils, myeloid cells, and lymphocytes are all low. This condition is also often associated with deafness.
SCID is a true pediatric immunologic emergency. Unless immunologic reconstitution is achieved through hematopoietic stem cell transplantation ( HSCT ) or gene therapy, death usually occurs during the 1st yr of life and almost invariably before 2 yr of age. HSCT in an infant prior to infection is associated with a 95% survival rate. ADA-deficient SCID and X-linked SCID have been treated successfully with gene therapy. Early trials of gene therapy were associated with a risk of malignancy, but this has not been seen in trials with new vectors. ADA-deficient SCID can also be treated with repeated injections of polyethylene glycol–modified bovine ADA ( PEG-ADA ), although the immune reconstitution achieved is not as effective as with stem cell or gene therapy.
The 4 most common types of SCID are the X-linked form, caused by mutations in CD132 ; autosomal recessive RAG1 and RAG2 deficiencies; and ADA deficiency. Additional forms are listed in Table 152.1 . For X-linked SCID and ADA deficiency, gene therapy exists, but genetic counseling is the most compelling reason for genetic sequencing to identify the gene defect. Several specific gene defects are associated with increased sensitivity to radiation and chemotherapy, and their early identification can lead to a better transplant experience.
Sequencing is often done by requesting a SCID gene panel. There are certain laboratory features that predict specific gene defects. When both T cells and B cells are low, often a gene encoding a protein involved in V(D)J recombination is the cause. Similarly, certain cytokine receptor defects are associated with specific lymphocyte phenotypes.
Hypomorphic mutations in genes most often associated with SCID can lead to varied phenotypes. This condition is often referred to as leaky SCID, referring to the mutation being “leaky” for some lymphocyte development. Leaky phenotypes range from the dramatic Omenn syndrome phenotype to later-onset immunodeficiency, granulomas, and autoimmunity.
cartilage-hair hypoplasia
Wiskott-Aldrich syndrome
lymphopenia
Combined immunodeficiency ( CID ) is distinguished from SCID by the presence of low but not absent T-cell function. CID is a syndrome of diverse genetic causes. Patients with CID have recurrent or chronic pulmonary infections, failure to thrive, oral or cutaneous candidiasis, chronic diarrhea, recurrent skin infections, gram-negative bacterial sepsis, urinary tract infections, and severe varicella in infancy. Although they usually survive longer than infants with SCID, they fail to thrive and often die before adulthood. Neutropenia and eosinophilia are common. Serum immunoglobulins may be normal or elevated for all classes, but selective IgA deficiency, marked elevation of IgE, and elevated IgD levels occur in some cases. Although antibody-forming capacity is impaired in most patients, it is not absent.
Studies of cellular immune function show lymphopenia, deficiencies of T cells, and extremely low but not absent lymphocyte proliferative responses to mitogens, antigens, and allogeneic cells in vitro. Peripheral lymphoid tissues demonstrate paracortical lymphocyte depletion. The thymus is usually small, with a paucity of thymocytes and usually no Hassall corpuscles.
Cartilage-hair hypoplasia (CHH) is an unusual form of short-limbed dwarfism with frequent and severe infections. It occurs with a high frequency among the Amish and Finnish people.
CHH is an autosomal recessive condition. Numerous mutations that cosegregate with the CHH phenotype have been identified in the untranslated RNase MRP ( RMRP ) gene. The RMRP endoribonuclease consists of an RNA molecule bound to several proteins and has at least 2 functions: cleavage of RNA in mitochondrial DNA synthesis and nucleolar cleaving of pre-RNA. Mutations in RMRP cause CHH by disrupting a function of RMRP RNA that affects multiple organ systems. In vitro studies show decreased numbers of T cells and defective T-cell proliferation because of an intrinsic defect related to the G1 phase, resulting in a longer cell cycle for individual cells. NK cells are increased in number and function.
Clinical features include short, pudgy hands; redundant skin; hyperextensible joints of hands and feet but an inability to extend the elbows completely; and fine, sparse, light hair and eyebrows. Infections range from mild to severe. Associated conditions include deficient erythrogenesis, Hirschsprung disease, and an increased risk of malignancies. The bones radiographically show scalloping and sclerotic or cystic changes in the metaphyses and flaring of the costochondral junctions of the ribs. Some patients have been treated with HSCT.
Wiskott-Aldrich syndrome is an X-linked recessive disorder characterized by atopic dermatitis, thrombocytopenic purpura with normal-appearing megakaryocytes but small defective platelets, and susceptibility to infection.
The Wiskott-Aldrich syndrome protein (WASP) binds CDC42H2 and Rac, members of the Rho family of guanosine triphosphatases. WASP controls the assembly of actin filaments required for cell migration and cell-cell interactions.
Patients often have prolonged bleeding from the circumcision site or bloody diarrhea during infancy. The thrombocytopenia is not initially caused by antiplatelet antibodies. Atopic dermatitis and recurrent infections usually develop during the 1st yr of life. Streptococcus pneumoniae and other bacteria having polysaccharide capsules cause otitis media, pneumonia, meningitis, and sepsis. Later, infections with agents such as P. jiroveci and the herpesviruses become more frequent. Infections, bleeding, and EBV-associated malignancies are major causes of death.
Patients with this defect uniformly have an impaired humoral immune response to polysaccharide antigens, as evidenced by absent or greatly diminished isohemagglutinins, and poor or absent antibody responses after immunization with polysaccharide vaccines. The predominant immunoglobulin pattern is a low serum level of IgM, elevated IgA and IgE, and a normal or slightly low IgG concentration. Because of their profound antibody deficiencies, these patients should be given immunoglobulin replacement regardless of their serum levels of the different immunoglobulin isotypes. Percentages of T cells are moderately reduced, and lymphocyte responses to mitogens are variably depressed.
Good supportive care includes appropriate nutrition, immunoglobulin replacement, use of killed vaccines, and aggressive management of eczema and associated cutaneous infections. HSCT is the treatment of choice when a high-quality matched donor is available and is usually curative. Gene-corrected autologous HSCT has resulted in sustained benefits in 6 patients.
Ataxia-telangiectasia is a complex syndrome with immunologic, neurologic, endocrinologic, hepatic, and cutaneous abnormalities.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here