Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Image-guided tumor ablation is a minimally invasive strategy to treat a range of focal tumors by inducing irreversible cellular injury through the application of thermal and more recently nonthermal energy or chemical injection. This approach is being used to treat a range of focal tumors, most commonly primary and secondary malignancies of the liver, lung, kidney, adrenal glands, and bone. Specific advantages of percutaneous tumor ablation therapies include the ability to treat patients with limited disease who are not surgical candidates owing to medical comorbidities, lower associated morbidity and mortality compared to conventional surgery or systemic chemotherapy, relative cost-effectiveness, and equivalent or near-equivalent long-term outcomes in well-selected patient populations.
Here, concepts on image-guided tumor ablation will be presented, including the principles and goals of tumor ablation and a brief review of different ablation modalities. Key steps involved in tumor ablation, including initial patient evaluation, intraprocedural imaging guidance and adjunctive techniques, and postprocedure imaging and care will be reviewed. Finally, current applications in common parenchymal organs such as the liver and kidney will be reviewed.
Minimally invasive focal tumor ablation encompasses several specific objectives. First, the main goal of tumor ablation (regardless of organ or primary tumor type) is inducing focal cytotoxic injury throughout the entire target tumor by placing needle-like applicators at the center of the tumor to generate high temperatures (>60°C) or an equivalent injury.
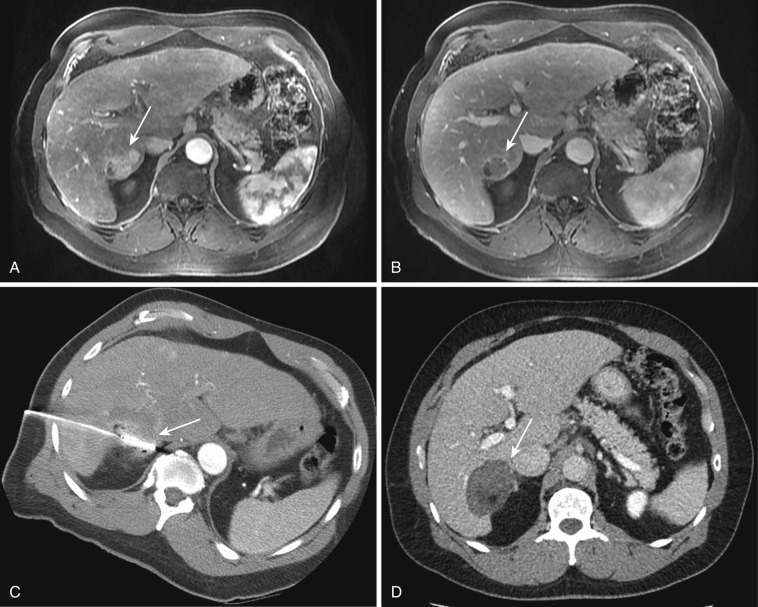
The second objective is to treat an adequate ablative margin of tissue surrounding the target tumor. An appropriate ablative margin is defined as adequately treating a rim of apparently normal tissue around the tumor, which often contains microscopic invasion of malignant cells at the tumor periphery ( Fig. 69-1 ). In most cases, this rim should be 5 to 10 mm thick, extending circumferentially beyond the edge of visible tumor, although less (e.g., for renal tumors) or more (e.g., for colorectal liver metastases) of a margin may be needed on a tumor-specific basis. This means that for 3- to 5-cm tumors, a single ablation treatment may not be sufficient to entirely encompass the target volume. In these cases, multiple overlapping ablations or simultaneous use of multiple applicators may be required to successfully treat the entire tumor and achieve an ablative margin. Additionally, if the target tumor (e.g., hepatic colorectal metastasis) has decreased in size from chemotherapy before ablation is being performed, the ablation zone should be sufficiently large to include the area originally involved by the tumor.
Finally, although complete treatment of the target tumor is of primary importance, specificity and accuracy are also highly preferred, with a secondary goal of incurring as little injury as possible to surrounding nontarget normal tissue. This ability to minimize damage to normal organ parenchyma is one of the significant advantages of minimally invasive percutaneous thermal ablation and can be critical in patients who have focal tumors in the setting of limited functional organ reserve. Examples of clinical situations where this is relevant include focal hepatic tumors in patients with underlying cirrhosis and limited hepatic reserve, patients with Von Hippel-Lindau syndrome who have limited renal function and require treatment of multiple renal tumors, and patients with primary lung tumors with extensive underlying emphysema and limited lung function. Many of these patients are not surgical candidates owing to limited native organ functional reserve placing them at a higher risk for postoperative complications or organ failure. Careful planning is required to ensure that each of these three objectives is met.
Several types of ablation modalities with differing mechanisms of cellular injury are used in current clinical practice. These can be divided into technologies that induce a thermal injury through high-temperature heating (most commonly using radiofrequency [RF] and microwave energy and to a lesser degree ultrasound [US] or laser), extreme cooling (i.e., cryoablation), exposure to cytotoxic chemicals (e.g., ethanol or acetic acid instillation), or nonthermal energy with irreversible electroporation. All of these modalities are based upon a similar premise—delivery occurs through percutaneous placement of needles directly into the tumor using imaging guidance. Finally, operator technique likely has as much influence on performance as device technology; for example, multiple applicators can be used to increase heating rather than switching to a different energy source.
A detailed discussion of different types of ablation modalities available for use falls beyond the scope of this chapter. However, it is important to be familiar with modalities that are commonly used in clinical practice, including RF- and microwave-based thermal ablation, cryoablation, and ethanol instillation. For each modality, mechanisms of cellular injury, advantages and disadvantages, and common clinical uses are presented in Table 69-1 . Of these available technologies, RF ablation (RFA) and microwave ablation are more ubiquitous in current clinical practice. Both types of ablation elevate tissue temperatures enough to create zones of irreversible cellular damage. RF energy is easy to generate but suffers in areas of high blood flow or high tissue impedance and requires use of electrode switching or multiple applicator use to achieve large ablation zones in a time-efficient manner. Microwave heating is fast and efficient and thus appears better equipped to overcome heat sinks and treat large tumor volumes. In current clinical practice, RF-based platforms are most commonly used and have the largest set of relevant long-term clinical data (i.e., studies were performed with systems still used in clinical practice). However, there are now a number of commercially available microwave-based systems with increasing data becoming available, demonstrating at least equivalency to RFA and potentially superiority for larger tumors. This is particularly relevant in situations (i.e., hepatic colorectal metastases) where the need to achieve a 10-mm ablative margin necessitates larger ablation zones (e.g., 5-cm ablations for 3-cm tumors). Cryoablation and ethanol instillation also offer specific clinical advantages. Cryoablation procedures are largely painless, and the ablation zone is readily visible on noncontrast computed tomography (CT), allowing careful monitoring of the ablation zone during treatment in situations where greater accuracy is required. Ethanol instillation is one of the most cost-effective treatments available (requiring only a 21-gauge needle and dehydrated alcohol), though this is offset by poorer long-term efficacy compared to thermal ablation. Finally, there are several less commonly used techniques with platforms that are not as well studied and with less overall clinical experience. These include thermal ablation using laser energy or US (either through focused energy platforms or with percutaneous applicators), which are cytotoxic using thermal injury similar to RFA or microwave ablation. Irreversible electroporation is an emerging technology that employs administration of electrical pulses to disrupt cellular membranes (and is therefore often considered less of a thermal modality), though additional clinical and experimental studies using this modality are ongoing.
| Modality | Mechanism | Advantages | Disadvantages | Commonly Used Sites Within the Abdomen |
|---|---|---|---|---|
| RFA | High-temperature thermal injury (60-100°C) Friction induced by current oscillation around electrode Tissue injury dependent on diffusion of heat through tissues |
Established long-term clinical experience Device platforms with significant clinical experience Multiple electrodes can be used. |
Susceptible to heat sink effects from blood flow cooling Requires two poles (electrode and grounding site) |
Liver, kidney, adrenal, bone |
| Microwave ablation | High-temperature thermal injury (60-150°C) Friction from water molecule agitation within tissues Active heat generation occurs farther/deeper in tissues; less dependence on thermal transmission |
Less susceptible to heat sink effects Larger ablation zones compared to similarly sized RFA platforms Multiple antennae can be used. |
Greater individual variation between available platforms (less long-term clinical experience) Higher temperatures; potentially greater risk of nontarget thermal injury |
Liver, kidney, adrenal, bone (similar to RFA but with overall fewer long-term studies) |
| Cryoablation | Alternating freeze/thaw cycles induce injury with intracellular ice formation and vascular thrombosis | Ablation zone easily visible on noncontrast CT; permits close intraprocedural monitoring Multiple probes can be used. |
Has been associated with systemic inflammation/side effects during hepatic ablation | Kidney Liver Bone |
| Ethanol instillation | Protein denaturation and cellular homeostasis changes | Extensive clinical experience in some situations Very cost-effective Low-profile needles, minimally invasive delivery |
Limited to use for HCC Long-term studies demonstrate higher recurrence compared to RFA Limited uniformity of injury owing to differences in intratumoral diffusion |
Liver (HCC) |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here