Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1981, the acquired immunodeficiency syndrome (AIDS) was first recognized in humans as an outbreak of Pneumocystis carinii pneumonia among gay men in the United States, , having spread from Africa to Haiti to the United States, and then globally. , The disease is caused by the human immunodeficiency virus type 1 (HIV-1), a lentivirus that is transmitted through varied routes of transmission including cervicovaginal, penile, rectal, oral, percutaneous, intravenous, and from pregnant women to children in utero or at the time of parturition, such that a variety of cell types are candidates for early infection including CD4 T-cells, tissue macrophages, Langerhan’s cells, and dendritic cells. AIDS is characterized by progressive depletion of CD4 + helper T-cells, and by chronic activation of the immune system. This results in an immunodeficiency syndrome that leads to opportunistic infections, including Mycobacterium tuberculosis , Pneumocystis jiroveci pneumonia, Mycobacterium avium-intracellulare infections, toxoplasmosis, candidiasis, cryptosporidiosis, and a variety of viral infections (cytomegalovirus, hepatitis C, herpes simplex) as well as cancers, such as Kaposi sarcoma (caused by human herpes virus-8) and non-Hodgkin B cell lymphomas.
Since 1981, an estimated 76 million people have been infected with HIV-1, and approximately 33 million people have died. Globally some progress is being made in control of HIV-1. In 2004, 1.7 million people died from AIDS-related illnesses, while this number was 690,000 in 2019, a reduction of 60%. Since 2010, new HIV-1 infections have declined 23% from 2.1 million to 1.7 million and over this same period of time, new infections have declined in children by 52% to ∼150,000. Recently infected subjects are potentially more infectious than individuals in the chronic phase of the disease. It has been suggested that up to 50% of new HIV-1 infections are acquired from newly infected individuals. The importance of the acute infection period for subsequent transmission events is underscored by high plasma viral loads during the early phase of disease and interferon-resistance of transmitted/founder viruses that initiate infection. , Treatment as prevention involves treatment of infected individuals to prevent spread of HIV-1 since infections decrease with reduced viral load during sexual contact. Thus, the current treatment guidelines suggest all HIV-1 infected individuals be treated with antiretroviral drugs regardless of the stage of infection, to prevent CD4 cell loss, mitigate clinical symptoms of immunodeficiency and to prevent HIV-1 transmission.
Much of the progress in prevention of HIV-1 infections and death can be attributed to progressive wide-spread use of antiretroviral treatment (treatment as prevention and prevention of mother-to-child transmission) and multiple additional prevention strategies such as pre-exposure prophylaxis, condoms, microbicides and male circumcision. In spite of this progress, the global AIDS pandemic continues, and each week approximately 5500 women between 15 and 24 years of age still become infected with HIV-1. Thus, a successful HIV-1 vaccine will be key to ending the HIV-1 pandemic.
HIV-1 encompasses four distinct lineages, groups M, N, O, and P, all of which cluster in phylogenetic trees with chimpanzee or gorilla simian immunodeficiency viruses (SIVs). All HIV-1 groups can cause the clinical syndrome of AIDS with CD4 + T-cell depletion, but differ in their distribution in human populations. Group M, or “major” group of HIV-1, caused the global pandemic that was first recognized in 1981. Group O was discovered in 1990 and represents ∼1% of global infections and is localized to Cameroon, Gabon, and surrounding countries. Group N was discovered in 1998 and is limited to scattered cases in Cameroon, while Group P was isolated in 2009 and has been limited to two individuals in Cameroon. Groups N and M are of chimpanzee SIV origin, group P of gorilla SIV origin and group O of chimpanzee or gorilla SIV origin. Thus, the AIDS epidemic represents four separate ape-to-man transmission events that likely occurred as a result of blood or membrane exposure during bushmeat hunting in west central Africa. Molecular analyses of HIV-1 sequences have estimated the onset of Group M and O epidemics to the early 1900s , while the Group N and Group P transmissions to humans occurred later.
A second less pathogenic lentivirus, HIV-2, that originated from the SIV of sooty mangabey monkeys, can also cause AIDS, but does so less frequently than HIV-1, and is also less transmissible, likely due to lower viral loads. Similar to HIV-1, HIV-2 encompasses eight groups, A–H, with each group representing a monkey to human transmission event. HIV-2 infection is restricted to West Africa where bushmeat hunting of sooty mangabey monkeys is common.
HIV-1 and HIV-2 belong in the same group of lentiviruses that infect a wide range of nonhuman primate species in Africa, where multiple cross-species transmissions in the wild have been documented. , , SIVs have been present in African primates for more than 30,000 years, suggesting that humans probably had many chances of sporadic encounter with these viruses for millennia. Humans are still naturally exposed today to infection by diverse SIVs, as illustrated by the high frequency and diversity of lentiviruses that can be encountered in primate bushmeat in Southeastern Cameroon. , Thus, while the etiologic agent of AIDS can be a number of forms of simian retroviruses that can infect humans, HIV-1 vaccine efforts are targeted to HIV-1 Group M as it is the continuing cause of the global AIDS pandemic.
HIV-1 belongs to the genus lentivirus in the family Retroviridae. Lentiviruses are enveloped RNA viruses that typically produce slow, progressive infections after long periods of latency and persist in the host despite the host’s active immune response . Their replication depends on the presence of reverse transcriptase (RT), which transforms the RNA genome into a proviral DNA copy that integrates into the host cell chromosomes. Other lentiviruses are simian, feline, and bovine immunodeficiency viruses, the ovine Visna virus, the caprine arthritis-encephalitis virus, and the equine infectious anemia virus.
The HIV-1 virion encapsulates two copies of a single-stranded positive-sense RNA genome that is 9.5 kb in length. The HIV-1 genome encodes the typical retrovirus structural proteins Gag , further cleaved into p17 gag (matrix), p24 gag (capsid), and p7 gag and p9 gag (nucleocapsid) proteins; Pol, itself cleaved into p12 pol (protease), p68 pol (RT), and p31 pol (integrase, IN); and envelope (Env), a 160-kDa glycoprotein eventually cleaved into a gp120 env external subunit and a gp41 env transmembrane subunit that together form trimeric spikes on the surface of the virion. In addition, the genome encodes a variety of nonstructural proteins, such as the regulatory proteins Tat and Rev and the accessory proteins Nef, Vif, Vpr, and Vpu ( Fig. 31.1 ). Within the HIV-1 virion, the two RNA genomes are bound to individual molecules of RT. Upon entry into a permissive cell, virus replication is initiated by RT, which reverse transcribes the RNA genome into proviral DNA. The proviral DNA in complex with host and viral proteins form the preintegration complex that traffics to the nucleus. The proviral DNA and IN enter the host cell nucleus through interactions with nuclear pores and various nucleoporins. Inside the nucleus, IN integrates the proviral DNA into host cell chromatin using a three-step process that includes trimming the 3ʹ ends of the HIV-1 provirus, viral and host strand transfer, and DNA repair to fill the 5ʹ gaps between viral and host DNA. The HIV-1 provirus preferentially integrates into active transcription sites. The integrated provirus is eventually transcribed into a set of unspliced, singularly spliced, or multiply spliced messenger RNAs (mRNAs) that encode the various viral proteins or full-length viral RNA. The full-length viral mRNAs are transported out of the nucleus to the cytoplasm by interactions between the viral protein Rev and the Rev response element (RRE) in the genome. The full-length viral genome is encapsidated into progeny virions via the Psi packaging element in the genome. ,
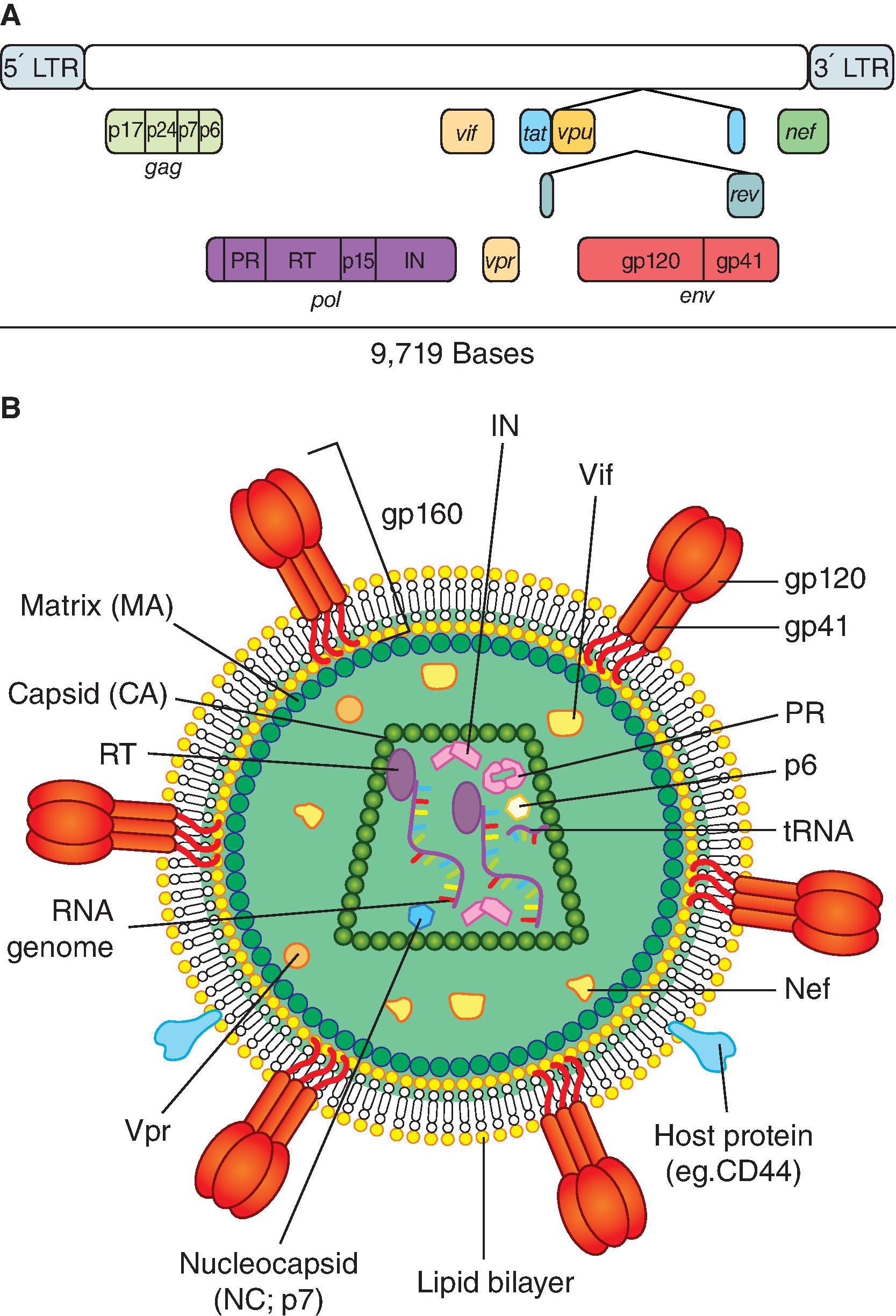
Virions emerge from the infected cell by budding as immature, noninfectious virus particles in which the Gag polyprotein is still intact and arranged in a radial fashion. Gag is eventually cleaved by the viral protease, resulting in the formation of a mature, infectious virus core. Assembly and egress of new virus particles most often occur at sites of transient contact between an infected cell and a naïve target cell, resulting in the formation of “virologic synapses” in which the membranes of the two cells form protrusions that come together like interlocking fingers. Virologic synapses allow the virus to directly spread to the uninfected target cell and shield progeny virions from neutralizing antibodies. ,
The HIV-1 virion consists of an internal cone-shaped capsid built from 1200 molecules of capsid protein (p24 gag ) and surrounded by the viral lipid envelope, wrapped on a scaffold of matrix protein (p17 gag ), and spiked with trimers of the highly glycosylated protein gp160 (gp120 + gp41). There is evidence that the lipid bilayer enclosing the virus also contains numerous molecules of type II human leukocyte antigen (HLA)-DR and β 2 -microglobulin borrowed from the host cell in which the virus last replicated, together with cell membrane proteins, including CD43, CD44, CD55, CD59, CD63, or CD71; leukocyte function–associated antigen (LFA)-1; and intercellular adhesion molecule (ICAM)-1.
The virus capsid contains two copies of the RNA genome associated with the nucleocapsid protein (p7 gag ), together with the transfer RNA molecules that serve as primers for their reverse transcription. The capsid also contains internal viral proteins p1, p2, and p6, copies of viral proteins Vpr, Nef, and Vif, which play a role in the early events of HIV replication, as well as host proteins such as cyclophilin A, Tsg101, calmodulin, actin, ezrin, and moesin. For example, cyclophilin A binds tripartite motif (TRIM)-5α, an important mediator of innate antiretroviral immunity which is the basis for the resistance of Old World monkeys to HIV-1 infection and an important factor in the susceptibility of macaque monkeys to SIV infection.
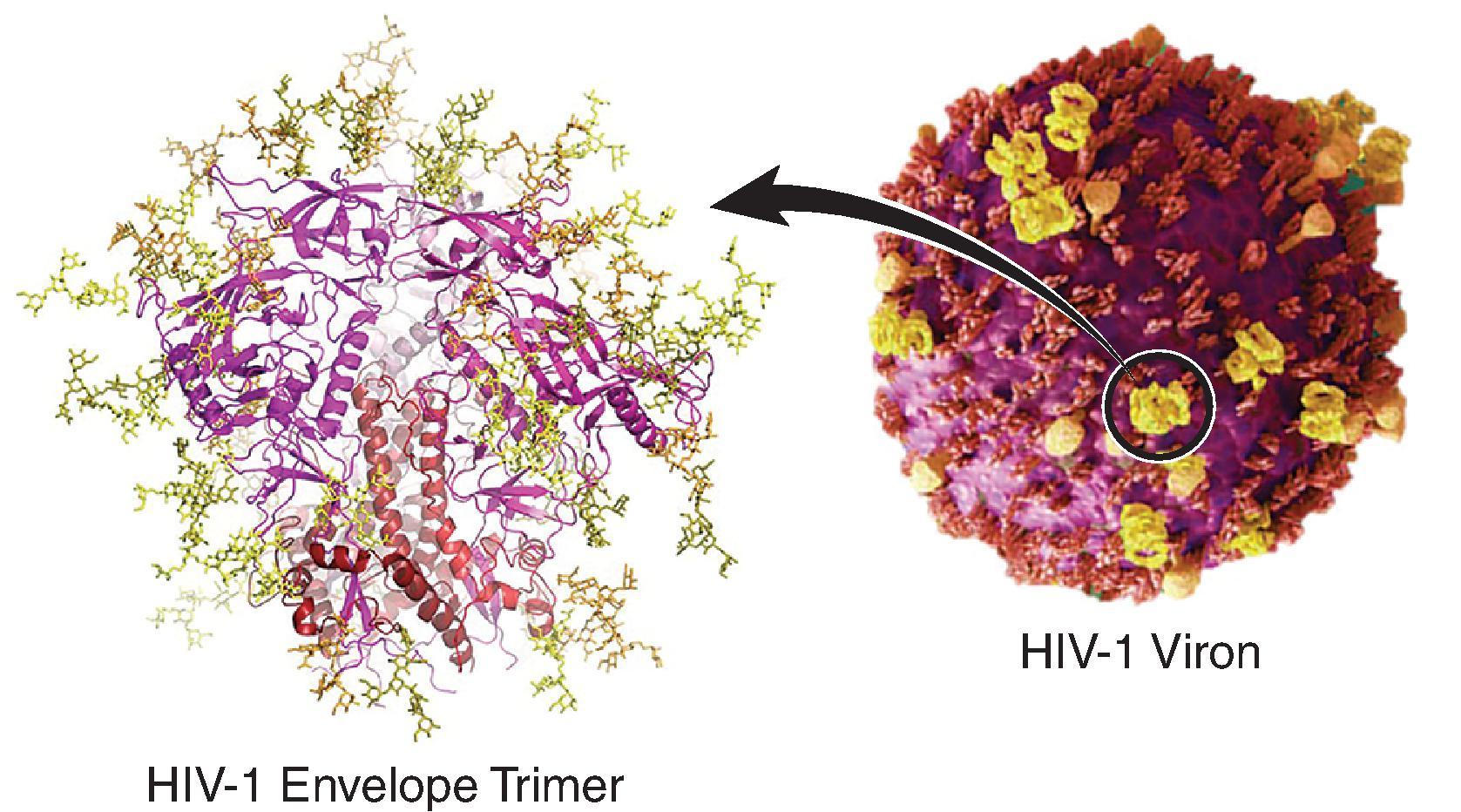
The env gene codes for the HIV-1 envelope (Env) gp160 glycoprotein that forms trimers of gp120-gp41 heterodimers on the virion surface and serves as the target for HIV-1 broadly neutralizing antibodies. Synthesis of gp160 as a single polypeptide, followed by cleavage into the noncovalently associated gp120 (the external chain) and gp41 (the transmembrane chain) subunits in the Golgi, trimerization, glycosylation, and Env transport to the cell surface have been studied in detail. , , Target cell tropism and attachment of the virus are determined by the gp120 subunit, which binds sequentially to the CD4 receptor then to a coreceptor. The coreceptor is typically CCR5 or CXCR4, but can also be other members of the G-coupled protein receptors, such as BOB for some rare strains of HIV-1. , There is evidence that the α 4 β 7 integrin, the gut mucosal homing receptor, also serves as a coreceptor , and provides a mechanism for HIV-1 to target α 4 β 7+ activated T-cells in gut-associated lymphoid tissue (GALT). The coreceptors were historically used to classify viruses as T-cell tropic or macrophage tropic, but these classifications are rarely used currently. Gp120 contains 20–30 glycosylation sites where a mixture of high-mannose and complex glycan-containing carbohydrates are attached to envelope, and comprise up to 50% of the mass of Env.
Relatively few trimers are present on HIV-1 ranging from 7 to 14 trimers per virion ( Fig. 31.2 ). , Env gp41 anchors viral envelope spikes in the viral envelope and maintains their trimeric organization. , Gp41 has the classical features of a type 1 fusion protein including an N-terminal fusion peptide that inserts into the target cell plasma membrane, and two long alpha helices per gp41 called the heptad repeat regions 1 and 2. Each pair of gp41 alpha helices undergoes conformational changes that insert the fusion peptide into the target cell membrane during the membrane fusion process. Another set of conformational changes in the alpha helices results in fusion of target cell and viral membranes.
The gag gene is transcribed into either a gag or a gag - pol mRNA, whose translation yields Pr55 gag and p160 gag-pol , respectively. Pr55 gag is eventually cleaved to generate the p17 gag matrix protein, the p24 gag capsid protein, and the p7 gag nucleocapsid protein. The p7 gag nucleocapsid protein, which remains in tight association with the RNA genome, contains two zinc finger motifs that bind a zinc ion in a tetrahedral conformation necessary for packaging of viral RNA and infectivity. ,
The pol gene encodes key viral enzymes, RT, which is associated with an RNase H activity; integrase, which allows the integration of the provirus DNA into the host T-cell genome; and the aspartyl proteinase, a homodimer of two subunits, which each contributes the amino acid triplet DTG to form the active site of the enzyme. The viral protease is required for virus maturation.
The tat gene encodes the trans -activator of transcription (Tat) protein that prevents premature termination of provirus transcription through binding to a 59-residue stem-loop at the 5′ end of the nascent viral mRNA molecules called the Tat-responsive element , increasing production of viral mRNA several hundred-fold. The process involves the Tat-cdk9/cyclin T1 phosphorylation of RNA polymerase II C-terminal domain, which enhances enzyme processivity. , Tat also plays a role in AIDS pathogenesis: free Tat molecules are released by HIV-1–infected cells , and activate, at a distance, uninfected CD4 + T-cells through interaction with cell membrane lipid rafts and internalization followed by transport to the cell nucleus. Anti-Tat antibodies are associated with long-term nonprogression.
The rev gene encodes a protein that binds to a specific sequence element, the Rev-responsive element, which is present on the unspliced or single-spliced viral mRNAs that encode Gag, Pol, and Env. This allows their transportation from the nucleus to the cytoplasm and permits the expression of structural proteins Gag, Pol, and Env. Rev thus controls the switch from an early phase of the virus replication cycle, when only regulatory proteins Tat and Rev and accessory proteins Nef , Vif , Vpu, and Vpx are synthesized from multispliced mRNAs, to a late phase, when structural proteins are made and virions assembled.
The nef gene encodes a major virulence factor, the myristoylated Nef protein, which contributes to the maintenance of high viral loads and promotes activation of resting T-cells by selectively binding the p21-associated kinase 2. Extracellular Nef also contributes to the depletion of CD4 + T-cells by interacting with the CXCR4 receptor and inducing Fas ligand–mediated apoptosis of uninfected bystander CD4 + T-cells. , Intracellular Nef downmodulates cell-surface expression of both CD4 and class I MHC (HLA) antigens, thus providing infected cells with a mechanism for evading the host cellular immune response. Transgenic mice that express the SIV nef gene in their CD4 + T-cells develop an AIDS-like disease with CD4 + T-cell depletion, activation, and apoptosis. , As a corollary, SIV with a deletion in the nef gene (SIV Δnef) is attenuated and can serve as a live attenuated virus vaccine in monkeys, but is not of use clinically due to reversion of the attenuated virus to pathogenicity. The Nef protein also increases viral infectivity by inhibiting the incorporation into progeny virions of two host-cell antiviral proteins, SERINC3 and SERINC5. ,
Vpr binds cyclophilin A and induces cell-cycle arrest in G 2 and apoptosis , ; it also enhances HIV replication in macrophages and nondividing cells by allowing entry of the viral preintegration complex into the nucleus of the cell. Vpu forms ion channels in the cell membrane, which promotes the release of progeny virions from infected cells. Vpu also antagonizes the action of tetherin (CD317/BST2), which opposes the release of fully formed virions at the surface of infected cells, , a role played by Nef in NHP lentiviruses , as well as in HIV-1 group O virus strains. , Vpu also binds CD4 molecules and targets them to the proteasome, leading to their degradation. HIV-2 and SIV lack a vpu gene but carry another accessory gene, vpx , which appears to be a duplication of vpr and counteracts the restriction factor SAMHD1 (sterile alpha motif and histidine-aspartate–domain-containing protein 1).
Vif prevents HIV inactivation by APOBEC3G (A3G), which can edit the HIV-1 early reverse transcripts through cytidine deamination, leading to degradation of the provirus DNA. Vif connects A3G to a polyubiquitination complex that leads to its proteasomal degradation. A3G expression is one of the key determinants of innate immunity that counter HIV-1 infection in humans and SIV infection in macaques. The Vif protein also associates with cytoskeleton intermediate filaments and facilitates transport of incoming virus to the cell nucleus.
HIV-1 exhibits a remarkable degree of genetic variability in the host that results from the error-prone nature of RT (approximately 3 × 10 −5 errors per replication cycle), together with high rates of virus production (up to 10 10 particles per day per infected person during the acute early phase of infection) and high frequency of recombination (seven to 30 crossovers per genome, per replication round). ,
HIV-1 strains in group M are found worldwide and show major diversity. They have been divided into nine major subtypes , or clades , designated by letters A, B, C, D, F, G, H, J, and K, and in subgroups such as A1, A2, F1, F2, etc. Recombinant viral strains have been detected, an indication that superinfection by a second HIV-1 strain is common. Recombinant virus strains which contain genetic material from more than one subtype and which are circulating widely in the population have been designated circulating recombinant forms ( CRFs ). , Currently there are three major recombinant forms, CRF01_AE, CRF02_AG, and CRF07_BC in the Los Alamos HIV Sequence Database ( www.lanl.gov ) ( Fig. 31.3 ). HIV-1 subtype C accounts for nearly half (48%) of all global infections and predominates in Southern Africa, Eastern Africa, and India. Subtypes A and D are more common in Central Africa, with subtype A appearing in Eastern Europe. East African HIV-1 infections are predominantly caused by subtype C and to a lesser extent by subtypes A and D. Clade B predominates in the United States, Europe, Central and South America, and Australia ( Fig. 31.3 ).
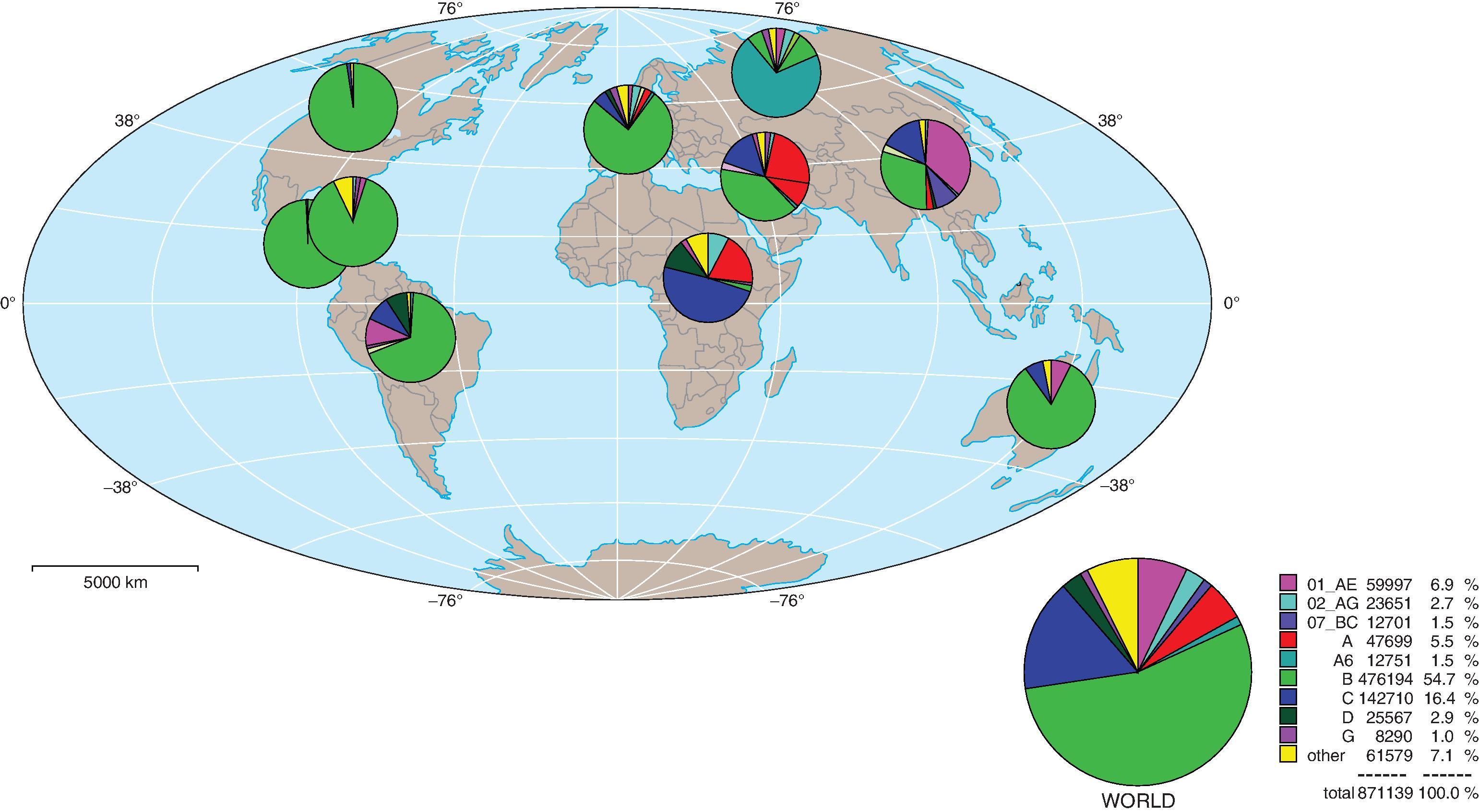
The greatest diversity of HIV-1 subtypes and recombinants is found in Africa where all clades and many CRFs are present. In West Africa, all clades are also detected with the dominant variants being CRF02_AG and subtype G. All major subtypes and many CRFs and unique recombinant forms are similarly found in Western and Central Europe. CRF03_AB is prevalent in Russia and Central Asia, whereas in East Asia, the epidemic is dominated by CRF07_BC, CRF08_BC, and CRF01_AE, and in Southeast Asia by CRF01_AE. HIV-1 diversity is continuing to expand in a “big bang” pattern with ever-increasing diversity due both to the generation of escape mutations from host-virus interactions and due to virus recombination.
The impact of HIV-1 variability on vaccine design cannot be overstated. There are two general vaccine strategies to overcome HIV-1 diversity. The first is to design sequential Env immunogens that engage the germline B cell receptors (BCRs) of bnAb B cell lineages and select intermediate antibodies to acquire broadly neutralizing antibody (bnAb) breadth. By targeting the induction of bnAbs, by definition, the vaccine is targeting the most conserved HIV-1 Env epitopes. The second strategy is to design T-cell immunogens that are comprised of synthetic HIV-1 genes that are produced by homologous recombination of T-cell epitopes in silico, such that genes are produced that cover the most conserved T-cell epitopes for CD4 and CD8 T-cell recognition. Such genes have been designed and have been termed ancestral, consensus, mosaic, and Epigraph. The goal of each of these designs is to overcome HIV-1 diversity by including conserved regions of genes and as well, including the most common T-cell epitopes. Optimally such genes are constructed with native joining sequences between each T-cell epitope so as to not induce immune responses to non-native epitopes. Mosaic HIV-1 genes have found to be superior to many wildtype immunogens for inducing cross-subtype T-cell and B-cell immune responses. Mosaic T-cell genes have now been incorporated into multiple clinical trials, including two Adenovirus 26 prime, Env protein boost, HIV-1 vaccine efficacy trials, HVTN 705 and HVTN 706, that are targeted at inducing protective non-neutralizing antibodies and CD8 T-cell responses to HIV-1 ( Table 31.1 ).
| Trial | Start | End | Vaccine | Location | Result | References |
|---|---|---|---|---|---|---|
| VAX004 (NCT00002441) | 1999 | Jan 2000 | Bivalent Clade B gp120 in Alum | United States, Europe | No efficacy | , , |
| VAX003 (NCT00006327) | Mar 1999 | Aug 2000 | Bivalent CRF_01AE/B gp120 in Alum | Thailand | No efficacy | |
| HVTN 502 (Step) (NCT00095576) | Nov 2004 | Sep 2009 | Adenovirus type 5 Clade B gag/pol/nef | United States | No efficacy; increased infection in vaccines | , |
| HVTN 503 (Phambili) (NCT00413725) |
Dec 2006 | Jul 2015 | Adenovirus type 5 Clade B gag/pol/nef | South Africa | No efficacy; increased infection in male vaccinees | , , |
| RV144 (NCT00223080) | Sep 2005 | Apr 2019 | ALVAC with gag/pro/Env; Bivalent CRF_01AE/B gp120 in Alum | Thailand | Estimated 31.2% vaccine efficacy at 42 months; 12-month efficacy, 60% | , , |
| HVTN 505 (NCT00865566) | May 2009 | Oct 2017 | DNAs with Clade B gag/pol/nef and DNAs with Clade A, B, C Envs Adenovirus type 5 with gag/pol and Clades A,B,C Envs | United States | No efficacy | , , |
| HVTN 703/ HPTN 081 (NCT02568215) |
May 2016 | Mar 2021 | Antibody-Mediated Protection (AMP) Trial of VRC01 neutralizing antibody infusion IV | Sub-Saharan Africa | No overall efficacy; protection from only highly sensitive HIV-1 strains | , , |
| HVTN 704/ HPTN085 (NCT02716675) | Apr 2016 | Dec 2020 | Antibody-Mediated Protection (AMP) Trial of VRC01 neutralizing antibody infusion IV | North America, South America, Switzerland | No overall efficacy; protection from only highly sensitive HIV-1 strains | , , |
| HVTN 702 Uhambo (NCT02968849) | Oct 2016 | . Sep 2021 | ALVAC-C with gag/pol/Env; Bivalent gp120s in MF59 | South Africa | No efficacy | , |
| HVTN 705 Imbokodo (NCT03060629) | Nov 2017 | Ongoing (est. Jul 2023) | Ad26, 4 valent T-cell mosaic genes, boost with clade C gp140 Env | Sub-Saharan Africa | No efficacy | |
| HVTN 706 Mosaico (NCT03964415) | Oct 2019 | Ongoing (est. Mar 2024) | Ad26, 4 valent T-cell mosaic genes, boost with clade C gp140 Env + B-cell mosaic gp140 Env | United States, Spain, Central/South America | Ongoing | N/A |
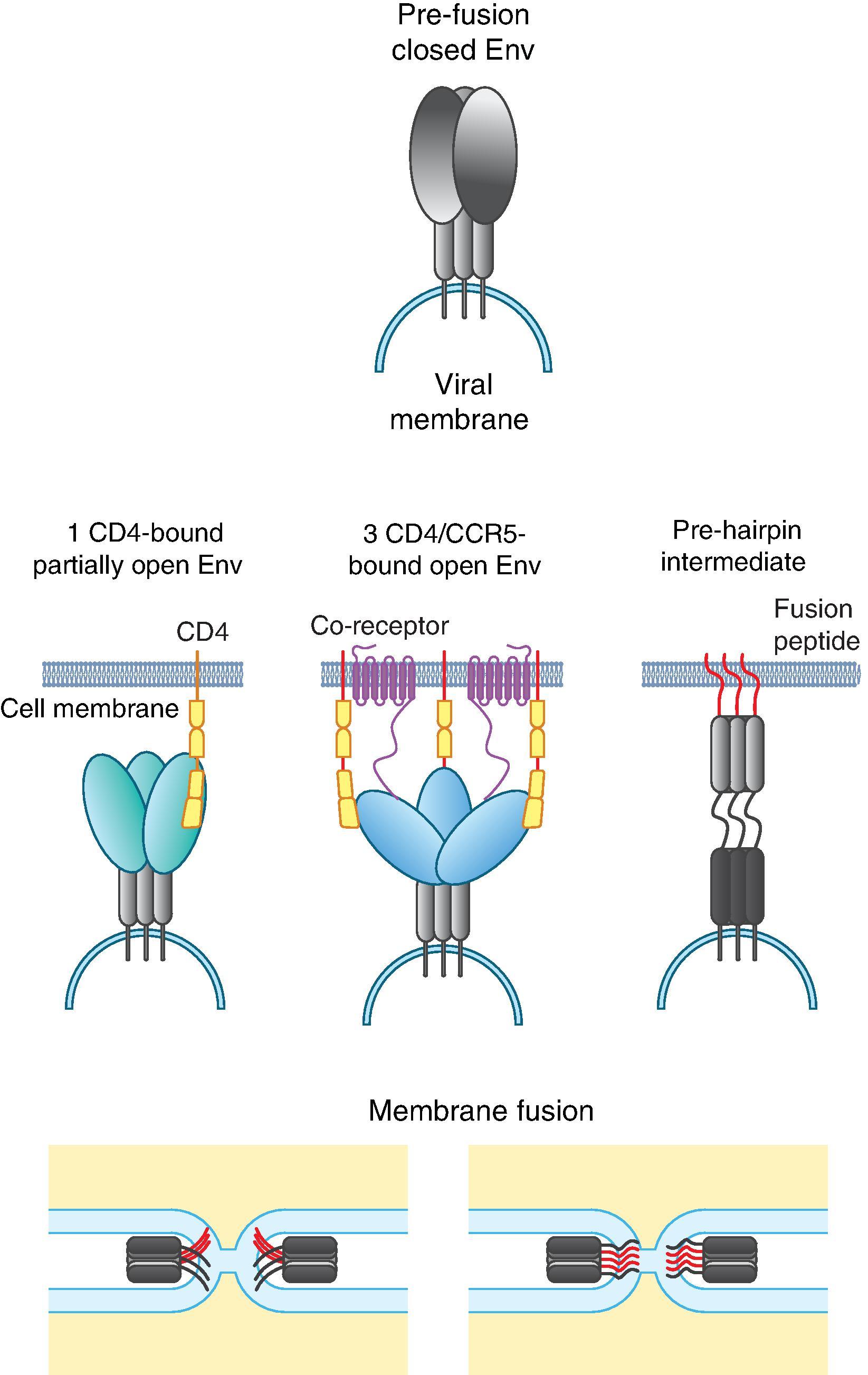
HIV-1 entry into host cells is a multistep process involving sequential interactions of the Env glycoprotein with primary receptor CD4 and coreceptor, CCR5 or CXCR4 ( Fig. 31.4 ). Most HIV-1 isolates obtained at the time of primary infection and during the asymptomatic period of the disease use the CCR5 coreceptor. HIV-1 strains using CXCR4 appear late in infection and have been associated with faster decline of CD4+ T-cells. Atomic level details of the interaction between Env and host receptors have been revealed by x-ray crystallography and cryo-EM structures determined using either monomeric gp120 or a soluble mimic of the Env trimer. These structures, as well as cryo-electron tomography (cryo-ET) studies in the context of membrane-bound Env , have provided information on receptor-binding induced Env conformational changes. Entry is initiated by the first contact of Env with a single CD4 molecule, which triggers conformational changes in Env leading to the binding of additional CD4 molecules and exposure of the coreceptor binding site. CD4-induced conformational changes include rigid body motions of the Env protomers, accompanied with re-organization of the gp120 V1/V2 and V3 loops. Coreceptor binding leads to further conformational changes in gp41 resulting in exposure of the highly hydrophobic, glycine-rich, N-terminal fusion peptide, which is inserted into the target cell membrane to initiate virus–cell fusion. The rod-like trimeric structure of gp41 comprises three parallel N-terminal α-helices (“heptad repeats”) assembled as a coil in the center with three antiparallel C-terminal α-helices packed on the outside. Electron tomography studies have revealed spoke-like structures that likely represent the prehairpin intermediate connecting the virus and host cells ( Fig. 31.4 ). ,
CD4 present on the surface of T-helper lymphocytes (CD4 + T-cells), monocyte-macrophages, lymph node follicular DCs, Langerhans cells in the skin, and microglia in the central nervous system is the primary receptor used by HIV-1, HIV-2, and SIV. , The virus can also infect CD4 − cells such as glial cells, natural killer (NK) cells, brain endothelial cells, gut epithelial cells, and probably epithelial cells in the genital tract by using the glycolipid galactosyl ceramide as a receptor.
Each step in the HIV-1 entry pathway presents an opportunity for intervention ( Fig. 31.4 ). Virus entry can be effectively prevented by targeting the CD4 binding site on gp120 using a composite molecule such as the eCD4-Ig, a broadly neutralizing monoclonal antibody such as VRC01 or 3BNC117 or small molecule inhibitors of CD4 binding such as the drug fomtemsavir. HIV-1 Env interaction with coreceptor is another critical step that can be targeted to inhibit entry. The FDA licensed drug maraviroc binds CCR5 and alters its conformation, disrupting its interaction with Env. Remarkable resistance to HIV-1 infection was observed in individuals with a 32-bp deletion in both alleles of the CCR5 gene, which encodes the CCR5 coreceptor (CCR5Δ32). The most important clinical significance of the CCR5Δ32 mutation is that the first patient cured of HIV-1 infection, Timothy Ray Brown, was cured by receiving two CCR5Δ32 bone marrow transplants for acute myelogenous leukemia. Other genetic polymorphisms in the chemokine receptors that can affect host resistance or susceptibility to HIV-1 infection have also been identified. , HIV-1 entry can also be inhibited by targeting steps that occur downstream of receptor interactions; Enfuvirtide, a 36 amino acid peptide, binds to the first heptad-repeat (HR1) in the gp41subunit and prevents the conformational changes required for the fusion of viral and cellular membranes. ,
Non-specific interactions between the HIV-1 Env and charged groups at the surface of the cell, such as heparin sulfate proteoglycans, facilitate adhesion of the virions to target cells. Major HIV-1 attachment factors are syndecans such as DC-SIGN, a mannose-binding lectin on the surface of dendritic cells (DCs) and macrophages. Immature DCs capture the virus by their surface DC-SIGN, internalize it by endocytosis into multivesicular endosomal bodies, and transmit it to T-cells by exocytosis into the extracellular medium in association with vesicular exosomes. Transmission from DCs to T-cells thus does not require a second round of infection and provides HIV an avenue for escaping immune responses. Penetration of HIV-1 through the mucosal barrier of the genital or the intestinal mucosa can involve uptake of the virus by intraepithelial Langerhans cells or DCs, as is observed in stratified squamous epithelia such as in the vagina and exocervix, the anus, the oral cavity, and the esophagus. It can also happen by translocation of the virus across columnar epithelia such as those of the endocervix, rectum, and intestinal tract by transcytosis ( Fig. 31.5 ).
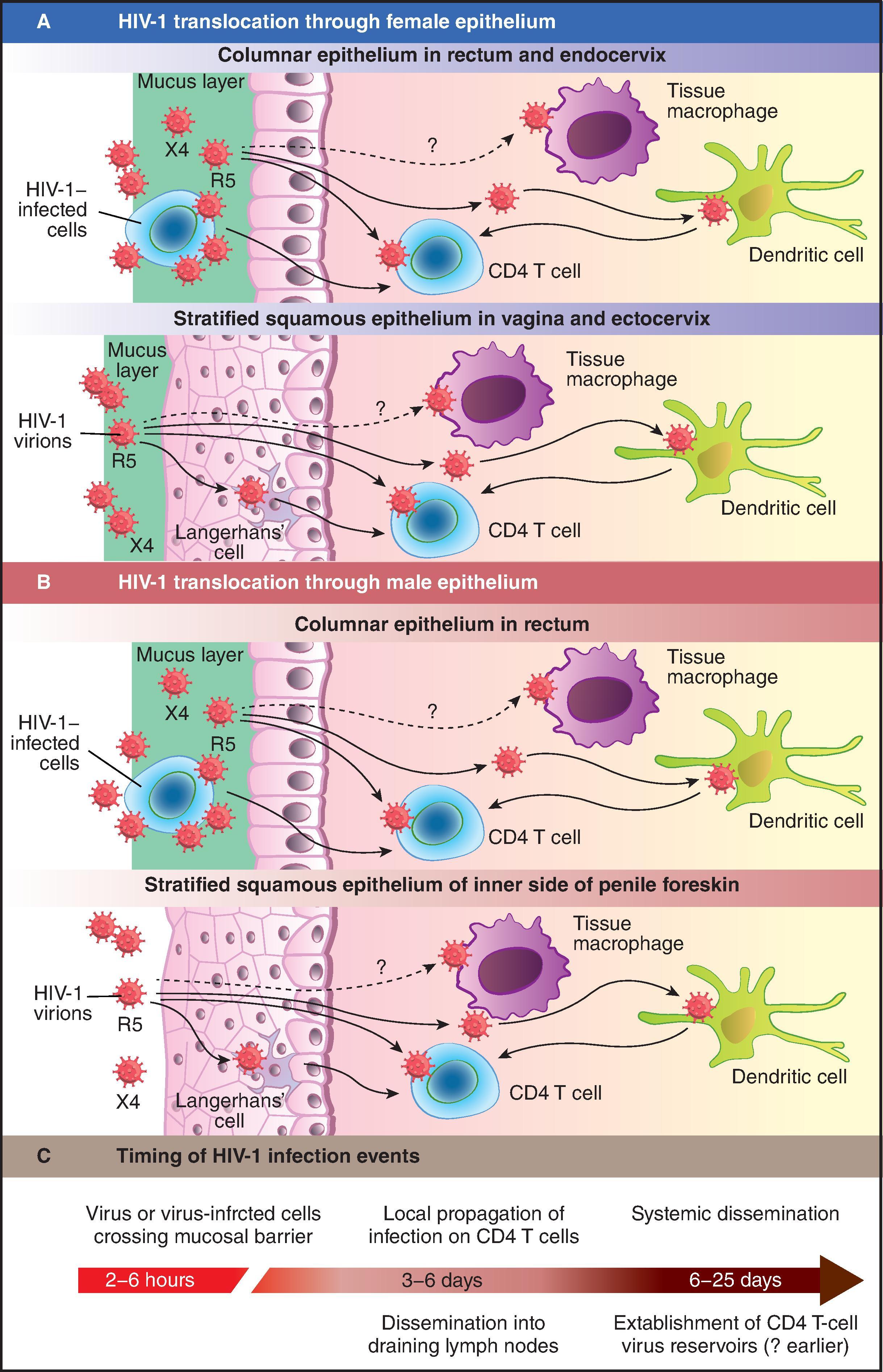
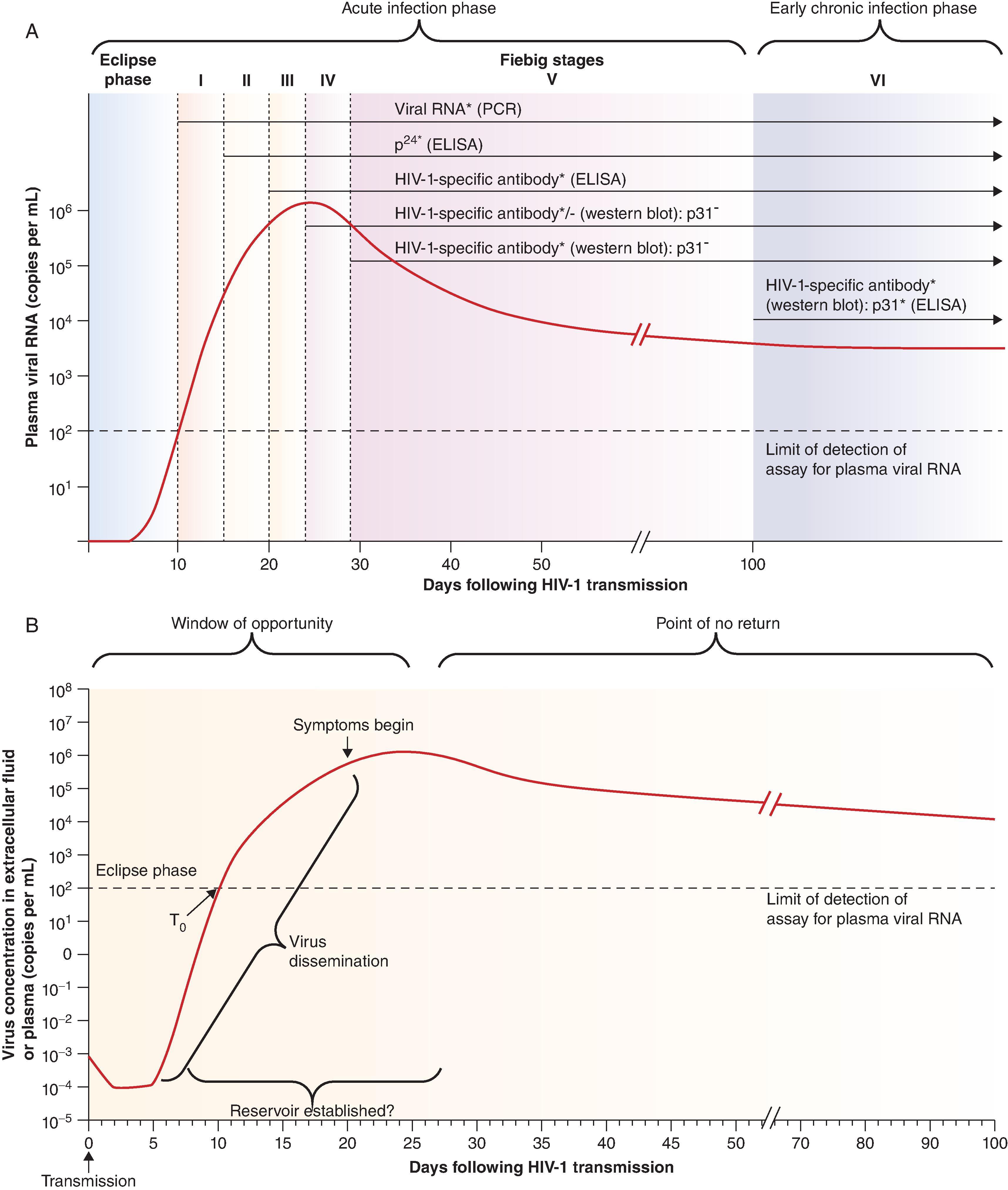
Acute HIV-1 Infection . For HIV-1 vaccine development, the critical time to determine what a successful vaccine needs to accomplish is the time around the transmission event during acute HIV-1 infection ( Fig. 31.6 ). , Analysis of blood or tissue samples from individuals early after infection with HIV-1 has revealed that the acute infection period can be divided into clinical stages that are defined by detection times of HIV-1 antigens and HIV-1-specific antibodies in diagnostic assays. The time between infection and the first detection of viral RNA in the plasma is the eclipse phase . Plasma virus levels then increase, peaking around 21–28 days after infection, followed by a steady decrease and eventual plateau ( Fig. 31.6A ). HIV-1–infected individuals can be categorized into Fiebig stages I–VI based on viral RNA measured by PCR, viral antigens measured by enzyme-linked immunosorbent assay (ELISA) and HIV-1-specific antibodies detected by ELISA or western blot. Progression from acute infection to the early chronic stage of infection occurs approximately 100 days following infection at the end of stage V ( Fig. 31.6A ). Fundamental events in acute HIV-1 infection (AHI) are initial viral replication locally in the mucosa and draining lymph nodes ( Fig. 31.6B ). This initial phase of infection occurs within the eclipse phase. The time when virus is first detected in the blood is referred to as T 0 , at which time significant depletion of gut and other mucosal CD4 + T-cells has occurred.
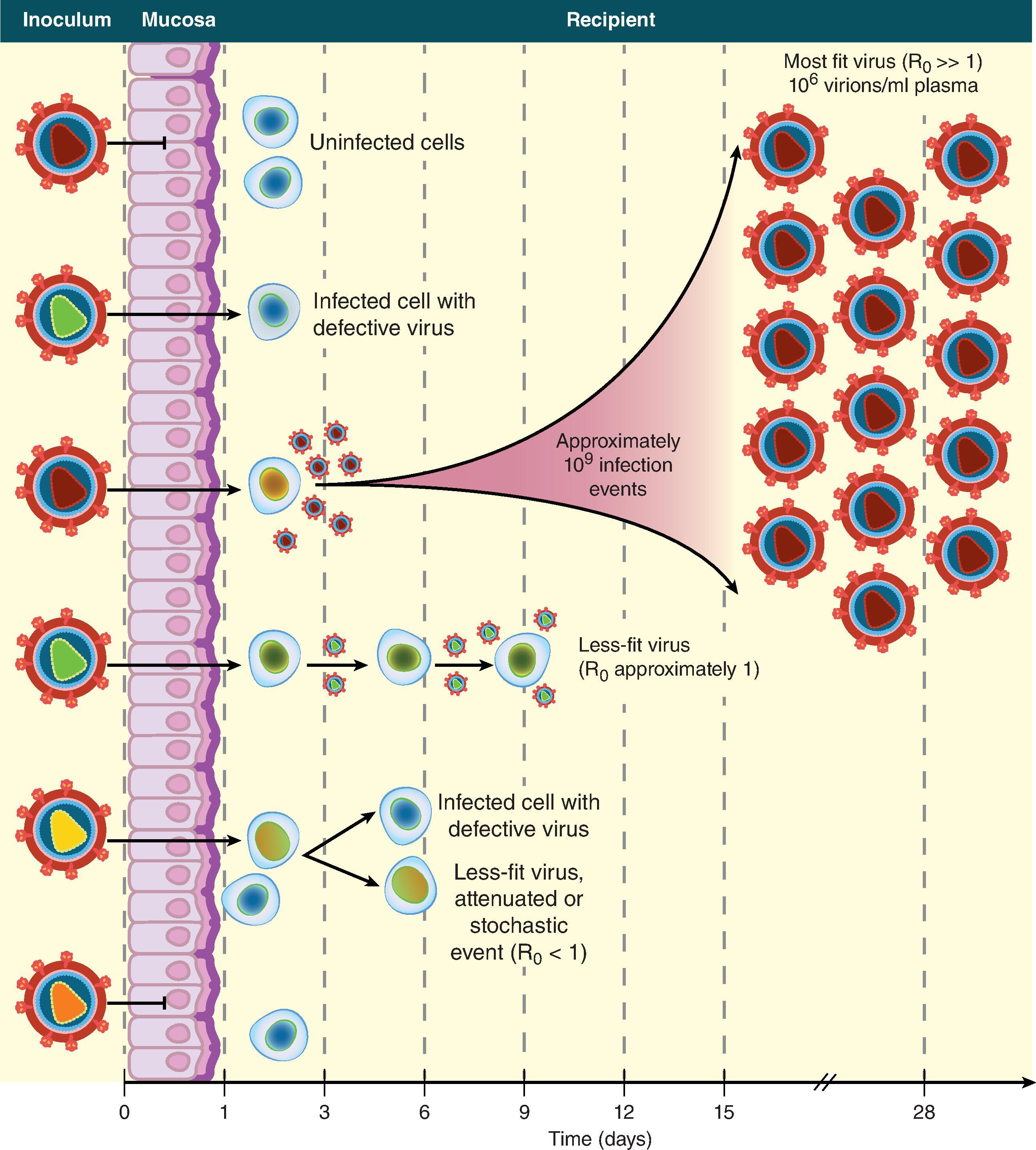
A key finding for HIV-1 vaccine development was the discovery that the transmitted/founder (TF) virus quasispecies that meditate transmission in the context of sexual transmission is limited to one or a few viruses in most cases ( Fig. 31.7 ). More viruses may also infect, but do not result in productive infection, presumably because they are either defective or less fit. R 0 represents the basic reproductive ratio, which is the number of secondary infections caused by one infected cell. If R 0 is below 1, infection is extinguished and does not occur. In AHI, R 0 is estimated to be ∼ 8. Once T/F viruses traverse the mucosal barrier, the first cells infected are submucosal CD4 T-cells and macrophages and virus binds to dendritic cells and are carried to draining lymph nodes ( Fig. 31.5 ). A characteristic of T/F viruses is their resistance to IFN-α, resulting in T/F virus resistance to an important innate antiviral cytokine response in the early phase of AHI.
The symptoms of AHI are rash, fever, headache, fatigue, sore throat, night sweats and loss of appetite, similar to the symptoms seen in acute mononucleosis caused by acute Epstein–Barr virus infection. Around peak viremia, AHI individuals may become symptomatic and reservoirs of latent virus are established in CD4 + T-cells. The “window of opportunity” between transmission and peak viremia, prior to massive CD4 + T-cell destruction and the establishment of viral reservoirs, is the time in which an HIV-1 vaccine must control viral replication, prevent extensive CD4 + T-cell depletion and prevent immune activation. , In AHI, treatment ∼10 days after HIV-1 transmission does not prevent establishment of the latent reservoir of CD4+ HIV-1–infected CD4+ T-cells. In rhesus macaques, studies have been performed to define the time of integration of SIV after transmission, and have shown that the viral reservoir of SIV is established within the first 1–3 days of SIV infection, and early antiretroviral therapy limits the size of the viral reservoir. Thus, a successful HIV-vaccine must induce durable protective responses that are present at the time of transmission to prevent virus infection and/or eliminate the first round of virus-infected CD4 T-cells and macrophages, prior to establishment of the reservoir of latently infected cells.
As viremia increases, so do the levels of cytokines and chemokines in plasma resulting in an intense cytokine storm ( Fig. 31.8 ). Levels of IL-15, type I interferons (IFNs) and CXC-chemokine ligand 10 (CXCL10) increase transiently. IL-18, TNF, IFNγ, IL-10, and IL-22 are sustained. The intense cytokine response during AHI promotes viral replication and mediates immunopathology.
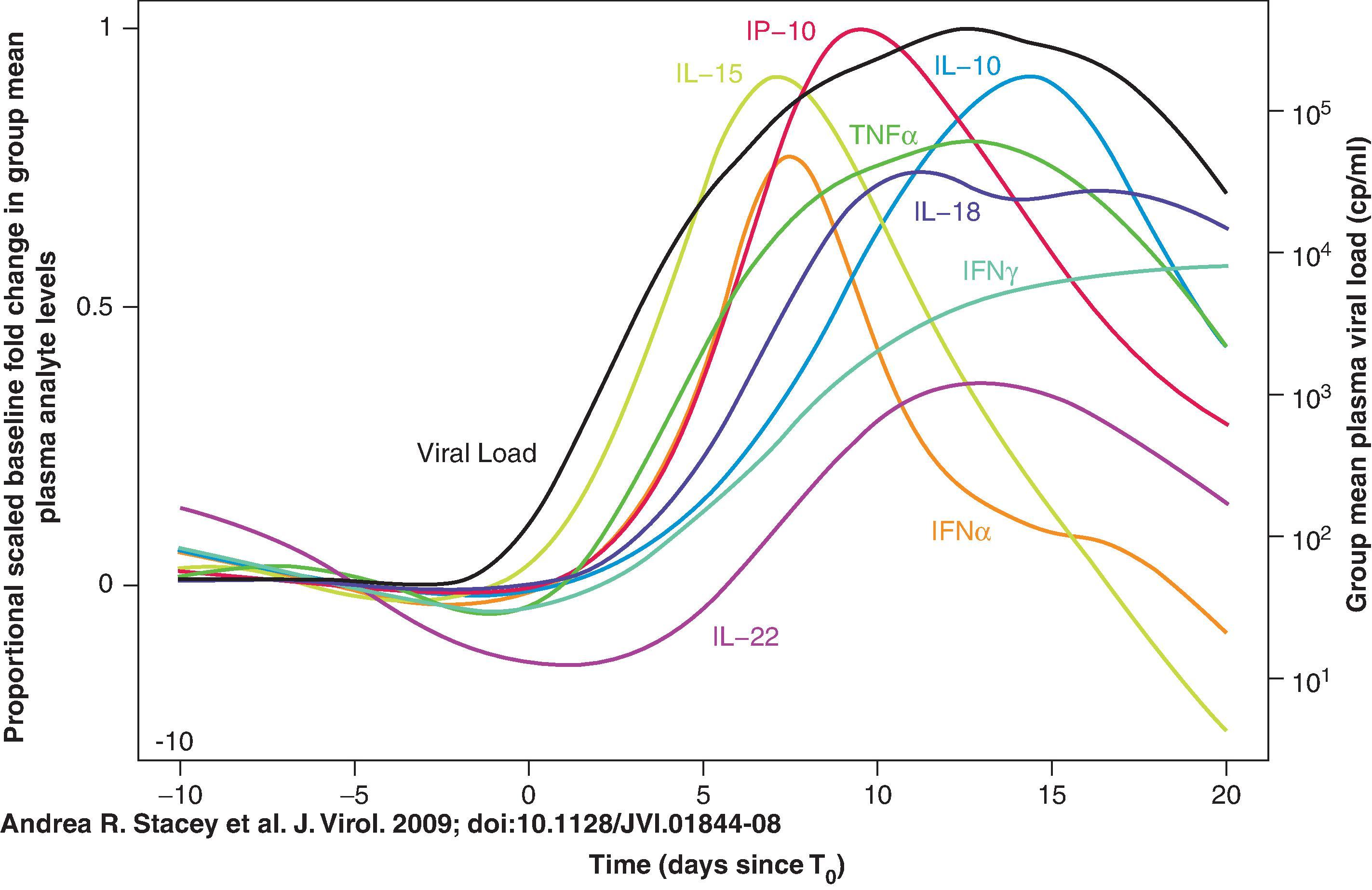
There are two initial cytokine waves in AHI: interleukin-15 (IL-15) and interferon-α (IFNα), followed by tumor necrosis factor (TNF), IL-18 and IL-10 ( Fig. 31.8 ). An increase in the level of soluble tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in the plasma is the first evidence of infection-induced apoptosis and/or immune activation and it occurs before the peak in viremia. Soon thereafter increases in the number of apoptotic microparticles are observed, followed by increased levels of soluble TNF receptor 2 (TNFR2) and soluble FAS ligand ( Fig. 31.9 ). A portion of the microparticles express CCR5, suggesting that they originate from cellular targets of HIV-1 infection. The appearance of these plasma components early during HIV-1 infection represents a pathological proinflammatory cascade of events that is associated with virus-induced CD4 + T-cell death, and are key events that must be prevented by a successful HIV-1 vaccine.
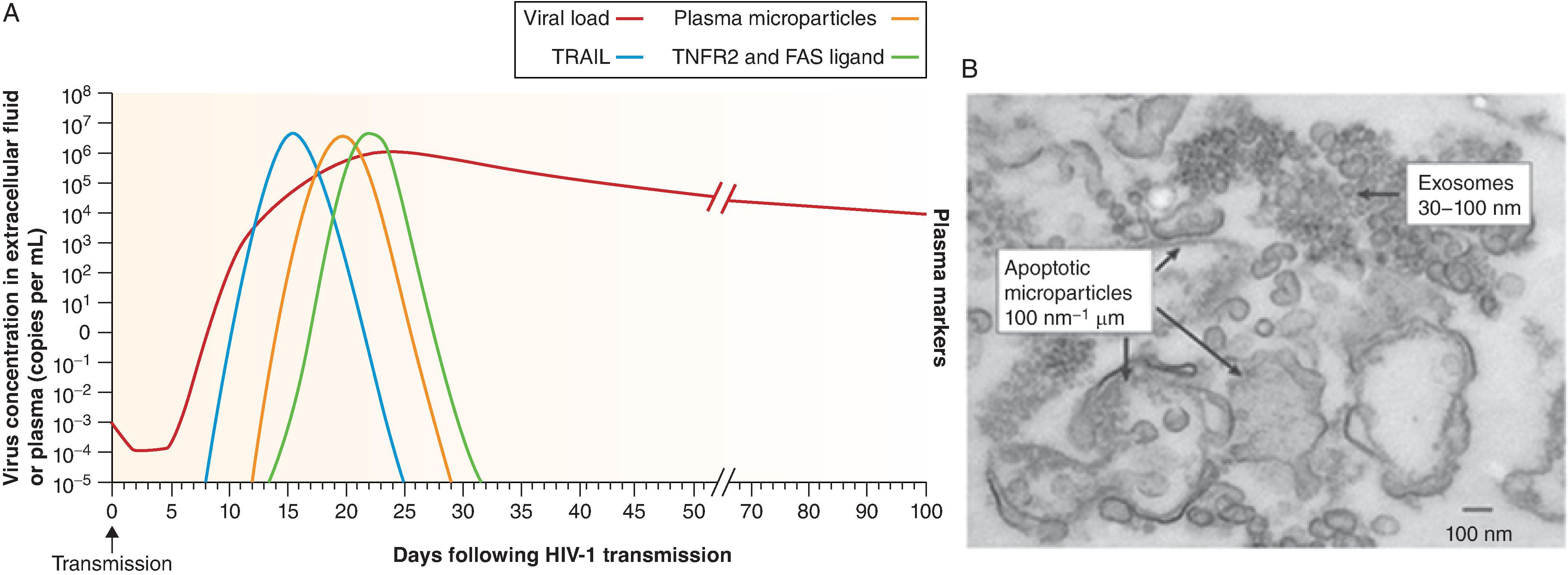
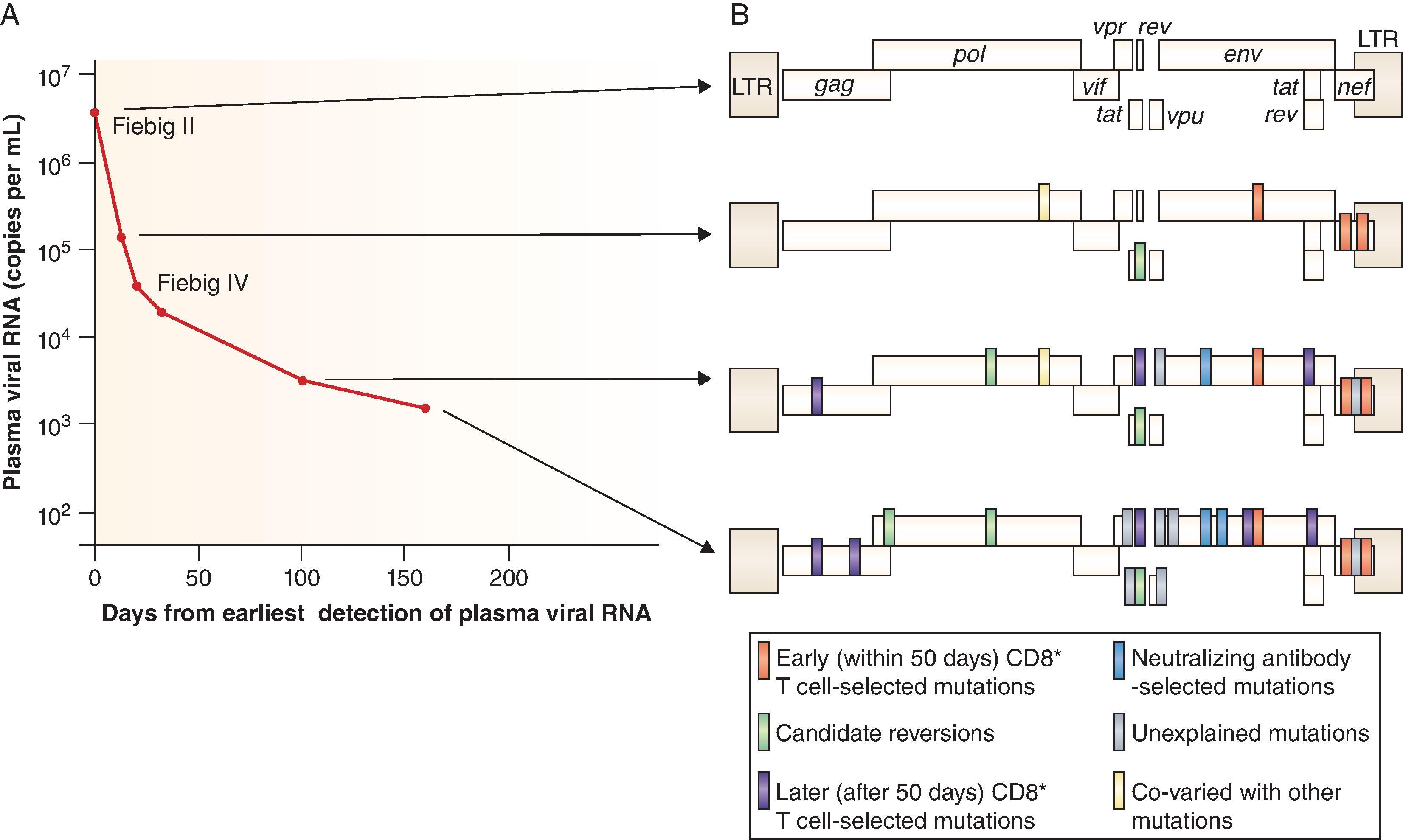
In addition to early integration of HIV-1 and formation of the latently infected reservoir of cells in AHI, other reasons for inability to control HIV-1 infection are ineffective initial innate, T and B cell responses to HIV-1. , , , T-cell responses occur early on in AHI and are initially successful, but the virus rapidly escapes and selects mutations at T-cell epitopes resulting in rapid replacement of blood T/F viruses ( Fig. 31.10 ). In contrast to T-cell responses, the initial B cell responses in AHI are non-neutralizing and have no effect on viremia until several weeks after transmission when autologous neutralizing antibodies arise, long after viral integration has occurred and the reservoir of latently infected CD4 cells has been established. , The earliest antibody responses after transmission occur in serum after ∼20 days and are non-neutralizing and directed to HIV-1 gp41 epitopes that cross-react with gut microbiome antigens. , , It has been postulated that these cross-reactive gp41 antibodies are pre-primed by gut microbiome and divert secondary antibody response following HIV-1 transmission. Interestingly non-neutralizing, cross reactive gp41 antibodies also occur early after HIV-1 Env vaccination in humans and in macaques. The next antibodies that occur 40–70 days after transmission are gp120 non-neutralizing antibodies to the CD4 binding site, the membrane proximal external region (MPER) and to CD4 inducible epitopes, followed by autologous neutralizing antibodies for the T/F and evolved variant viruses. Broadly neutralizing antibodies (bnAbs) at some titer typically only appear several years after transmission, and only appear in ∼50% of individuals, and only appear in high serum titers in 10–15% of HIV-1–infected people. Thus, a successful HIV-1 vaccine must reverse this order of antibody appearance that occurs in infection and induce a potent protective antibody response that is present at the time of transmission. The immunobiology of broadly neutralizing antibodies is discussed below in the section “ Vaccines to induce HIV-1 broadly neutralizing antibodies. ”
Recent data are available from the Antibody Mediated Protection (AMP) trial in which an HIV-1 bnAb against the CD4 binding site, called VRC01, was administered IV every 8 weeks for 10 doses to prevent HIV-1 acquisition (Table 34.1). While VRC01 did not prevent overall HIV-1 acquisition more effectively than placebo, VRC01 administration was 75% protective against VRC01 sensitive HIV-1 strains (inhibitory concentration at 80% neutralization, IC 80 <1 ug/mL). Of importance to HIV-1 vaccine development, the AMP trial demonstrated that a successful HIV-1 vaccine will need to elicit antibodies that can neutralize a wide breadth of HIV-1 strains and that are very potent with a consistently high titer of ∼1:250 inhibitory dilution at 80% neutralization (ID80) in serum. Moreover, it will need to be sufficiently durable at high levels to provide sterilizing immunity. Thus, the bar for an antibody-based vaccine is exceedingly high. Additionally, these data suggest that a successful vaccine will need to target not only the induction of bnAbs, but also the induction of FcR-mediated antiviral activity, as well as antiviral innate and T-cell immunity. , ,
Chronic HIV-1 Infection . Chronic HIV-1 infection begins in Fiebig stage VI ( Fig. 31.4 ) after transmission since CD8 T-cell and other cellular responses begin to select virus escape mutants in T/F virus progeny early in AHI ( Fig. 31.10 ). The chronic stage of HIV-1 infection is characterized by a long (average 8–10 years) asymptomatic period of clinical latency with the progressive loss of CD4 T-cells at varying rates that, in the absence of antiretroviral treatment, leads to clinical AIDS.
The plasma viral load, which peaks during the acute phase of infection at values of 10 6 to greater than 10 7 viral RNA copies per milliliter, declines and remains at a relatively stable plateau (the set-point ) during chronic infection. The set-point differs from patient to patient and serves as an indicator of disease progression. It remains high in rapid progressors, who rapidly progress to AIDS, whereas it falls to relatively low values in long-term nonprogressors, who contain viremia spontaneously and progress to disease slowly. Thus, patients with more than 10 5 HIV RNA copies per milliliter of plasma at set-point are 10-fold more likely to progress to AIDS during the following 5 years than those with less than 10 5 copies/mL. In a few seropositive persons, called elite controllers or elite suppressors, viral loads spontaneously fall below the limit of detection by standard clinical assays (<50–75 viral RNA copies/mL). ,
The asymptomatic phase of HIV-1 infection can last from a few months to more than 25 years, after which symptoms occur, following onset of opportunistic infections. At times, the infection can also manifest itself as a purely cachectic disease, to which the name slim disease has been given in Africa. It can also cause a neurological syndrome such as acute encephalitis or progressive dementia (the “AIDS dementia complex”). The symptomatic phase of the disease is accompanied by a return of gag p24 antigenemia, an accelerated decrease in the number of CD4 + T-cells, and a greatly increased virus load. The number of peripheral T-cells producing HIV-1 can reach 1 in 10, with production of up to 10 10 virions per day. ,
Chronic HIV-1 on Antiretroviral Therapy . Antiretroviral therapy (ART) has dramatically converted HIV-1 infection into a chronic disease with a near-normal life span. Treatment with ART can achieve the goal of maintaining optimal health for the individual and elimination of HIV-1 transmission through rapid and durable viral suppression in all persons with HIV-1. Starting ART as soon as possible after transmission has been shown to reduce the size of the latent reservoir of CD4 T-cells and to prevent immune system damage. The use of long-acting ART combinations such as monthly i.m. Rilpivirine and Cabotegravir can maintain remission and potentially increase ART adherence. In general, early administration of ART to HIV-1–infected individuals results in multiple beneficial effects on the immune system, both prevention of loss of immune cell function and restoration of any damaged immune tissues. In June 2020, it was estimated that 26 million people world wide are living with HIV-1 on ART, but this number represents only 67% of people living with AIDS (UNAIDS). Thus, treating every HIV-1–infected individual worldwide has not been achieved, indicating the continued need of a successful HIV-1 vaccine.
Several modalities can reduce HIV-1 infection rates in persons at risk of exposure, including screening of blood donors, awareness and counseling campaigns, the use of condoms, expanded treatment of sexually transmitted infections, and male circumcision. The use of a tenofovir vaginal gel, before and after sexual intercourse, has also been found to lower HIV-1 incidence rates by 39% among sexually active South African women over 2.5 years of use, with efficacy reaching 54% among highly exposed women who used the microbicide as instructed. Pre-exposure prophylaxis (PrEP) using oral antiretrovirals such as a combination of tenofovir and emtricitabine demonstrated substantial efficacy in reducing the risk of acquiring HIV in high-risk individuals, including men who have sex with men (MSM). Immediate postexposure ART can also prevent transmission. , Antiviral treatment of HIV-1–infected adults (treatment as prevention) was associated with an overall 93% reduction in HIV transmission to their uninfected partner.
Thus, HIV-1 is fundamentally a preventable disease. While antiretroviral treatment as prevention, PrEP, microbicides, voluntary medical male circumcision, use of condoms and prevention of mother-to-child transmission are all potentially effective preventive measures, when an effective and safe HIV-1 vaccine becomes available, it will be a cornerstone of an integrated HIV prevention strategy, serving to prevent infections when other preventive methods fail. ,
There are three types of potentially protective adaptive immune responses that a successful HIV-1 vaccine could induce: non-neutralizing antibodies that protect by targeting virus-infected cells and causing cell death by FcR-mediated antiviral effector functions, neutralizing antibodies that broadly react with a wide variety of HIV-1 strains and prevent virus transmission, and CD8 T-cells that broadly recognize and kill HIV-1–infected cells. , , In addition, innate immune responses induced by a vaccine or vaccine adjuvant will likely be essential for successful vaccine development.
Non-neutralizing antibody protection is weaker than neutralizing antibody protection and will likely need to be paired with other types of immune responses such as CD8 killing of infected CD4 T-cells and optimally, bnAbs, to be effective. , Of the seven HIV-1 efficacy trials that have been completed, only one (the RV144 trial in Thailand) showed protection, estimated at 31.2%, while the others showed no efficacy ( Table 31.1 ), , discussed below in the section “ Vaccines to Induce Non-neutralizing Antibodies. ” There are two additional non - neutralizing antibody trials (HVTN 705 and HVTN 706) that are testing a recombinant Adenovirus Type 26 vector (Ad26) that expresses mosaic genes for anti-HIV-1 CD4 and CD8 T-cell induction coupled with Envs for non-neutralizing antiviral antibody induction. HVTN 705 has ended and showed no efficacy, and HVTN 706 will be reported out in 2024 ( Table 31.1 ).
Neutralizing antibodies are the correlate of protection for most currently approved vaccines. However, a major reason that we do not have a broadly reactive and potent HIV-1 vaccine is that, unlike current vaccines such as measles, mumps, and SARS-CoV-2 that easily induce neutralizing antibodies, HIV-1 Env does not readily induce potent and broad neutralizing antibodies. The reasons for difficulty in inducing a broadly protective HIV-1 vaccine bnAbs are listed in Table 31.2 . A number of Phase I clinical trials are now beginning with immunogens specifically designed to target bnAb precursors to expand precursor B cell numbers and then test sequential Envs for their ability to guide neutralizing antibody bnAb lineages to affinity mature ( Table 31.3 ), discussed below in Section “ Strategies to Induce HIV-1 Broadly Neutralizing Antibodies .”
| HIV-1 is a rapidly mutating RNA retrovirus that integrates into the host genome ∼72 hours after infection, and once integrated, forms a latent reservoir of infected cells that resists immune system elimination. |
| A successful HIV-1 vaccine must have long-lived, high levels of protective immunity that effects sterilizing immunity and completely prevents infection—a high bar that no other vaccine has had to reach. |
| HIV-1 uses immune molecules (CD4 and CCR5) as receptors, leading to infection and deletion of key immune cells. |
| Over 50% of the molecular mass of the HIV-1 envelope (Env), the target of neutralizing antibodies, is comprised of glycans that mask neutralizing determinants. |
| The Env has evolved to mimic host proteins and glycans, resulting in minimal recognition of neutralizing epitopes by the immune system. |
| When broadly neutralizing antibodies are made, they may have unusual traits such as long heavy chain complementarity determining region-3s (HCDR-3s) and autoreactivity, leading to control of bnAb precursors by immune tolerance mechanisms. |
| Broadly neutralizing antibodies have unusually high numbers of rare somatic mutations, leading to infrequent neutralizing antibody maturation. |
| The HIV-1 Env contains variable loops that can be immunogenic, leading to high levels of diverting or “off-target” antibodies that out-compete neutralizing antibodies. |
| While conserved, the neutralizing epitopes of HIV-1 Env mutate to escape neutralizing antibodies—thus a polyclonal antibody response to multiple neutralizing epitopes is needed to avoid virus escape. |
| Immunogen | Adjuvant | bnAb Target | |
|---|---|---|---|
| HVTN 300 | CH505 TF chTrimer (Clade A BG505 backbone) | 3M-052-AF + Alum | Germline targeting CD4bs CH103 lineage |
| HVTN 301 | 426c Core NP (prime) + HxB2 Core NP (boost) | Adjuplex | Germline targeting CD4bs germline |
| HVTN 302 | mRNA BG505 MD39.3 Trimer (three versions: soluble, membrane bound, and membrane bound with CD4KO) | N/A | mRNA Trimer |
| HVTN 303 | Fusion Protein + Trimer 4571 (prime or boost) + Trimer 6931 (boost) | Adjuplex | Fusion Protein |
| HVTN 3XX | sD-BG505 MD39 Trimer (prime) + Trimer 4571 (boost) | pIL-12 (DNA), Alum (protein) | DNA trimer prime/ Protein trimer boost |
| HVTN 3XX | sD-eOD-GT8 (prime) + Trimer 4571 (boost) | pIL-12 (DNA), Alum (protein) | CD4bs germline (VRC01) |
| HVTN 3XX | N332-GT5 Trimer (fractionated dosing prime + bolus boost) | SMNP | V3 BG18 germline bnAb lineage |
| HVTN 3XX | Clade C 16055 Gly4 NFL Trimer (prime) +/- membrane bound Ad4 Clade C 1086C NFL Trimer (boost) + Trimer 4571 (boost) | TBD | CD4bs bnAb germline |
| HVTN 307 | V3G CH848 Pr-NP1 (Protein NP prime) + V3G CH848 mRNA-Tr2 (mRNA Trimer boost) | 3M-052-AF + Alum | V3 DH270 bnAb lineage |
| HVTN 306 | V3G CH848 mRNA-gp160-1 (mRNA NP prime) + V3G CH848 mRNA-Tr2 (mRNA Trimer boost) | 3M-052-AF + Alum | V3 DH270 bnAb lineage |
| HVTN 309 | CH505M5 Pr-NP1 (prime) + CD4bs CH505 Pr-NP2 (boost) | TBD | CD4bs CH235 bnAb lineage |
| HVTN 3XX | Man9 V3 glycopeptide STAR polymer (NP) | 3M-052-AF + Alum | FAB dimerized broad NAbs |
The third type of protective immune responses to prevent HIV-1 transmission is CD8 cytolytic T-cells. HLA Class 1a-specific cytotoxic T-cells (CTL) can mediate control of HIV-1 infection in HLA B57 or HLA B7 elite controllers that spontaneously control HIV-1 viral load and are asymptomatic. In the setting of acute HIV-1 infection, HLA-class 1a-specific responses rapidly select HIV-1 escape mutants but fail to eradicate the virus. Vaccine-induced CD8 T-cell responses induced by Adenovirus Type 5 expressing HIV-1 genes failed to protect against infection, in spite of induction of CD8 T-cells (HVTN502, HVTN503) ( Table 31.1 ). , CD8 T-cells selected escape viral mutants but analyses of acquisition rates in the trials showed a nonsignificant increase in HIV-1 acquisition in vaccines. , Thus, HLA Class 1a-restricted CTL clearly have some degree of anti-HIV-1 activity but have not yet protected against infection in the setting of vaccination, nor are able to clear infection in the setting of acute HIV-1 infection. However, recent data suggest that anti-HIV-1 CD8 CTL restricted by MHC class 1b (HLA-E) can eradicate SIV from ∼50% of SIV-challenged monkeys.
Thus, unlike most vaccines approved for clinical use that did not require in-depth insight into host-virus interactions, the way forward to develop an HIV-1 vaccine requires a deep understanding of both host-HIV-1 interactions and the immunobiology of protective immune responses. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here