Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
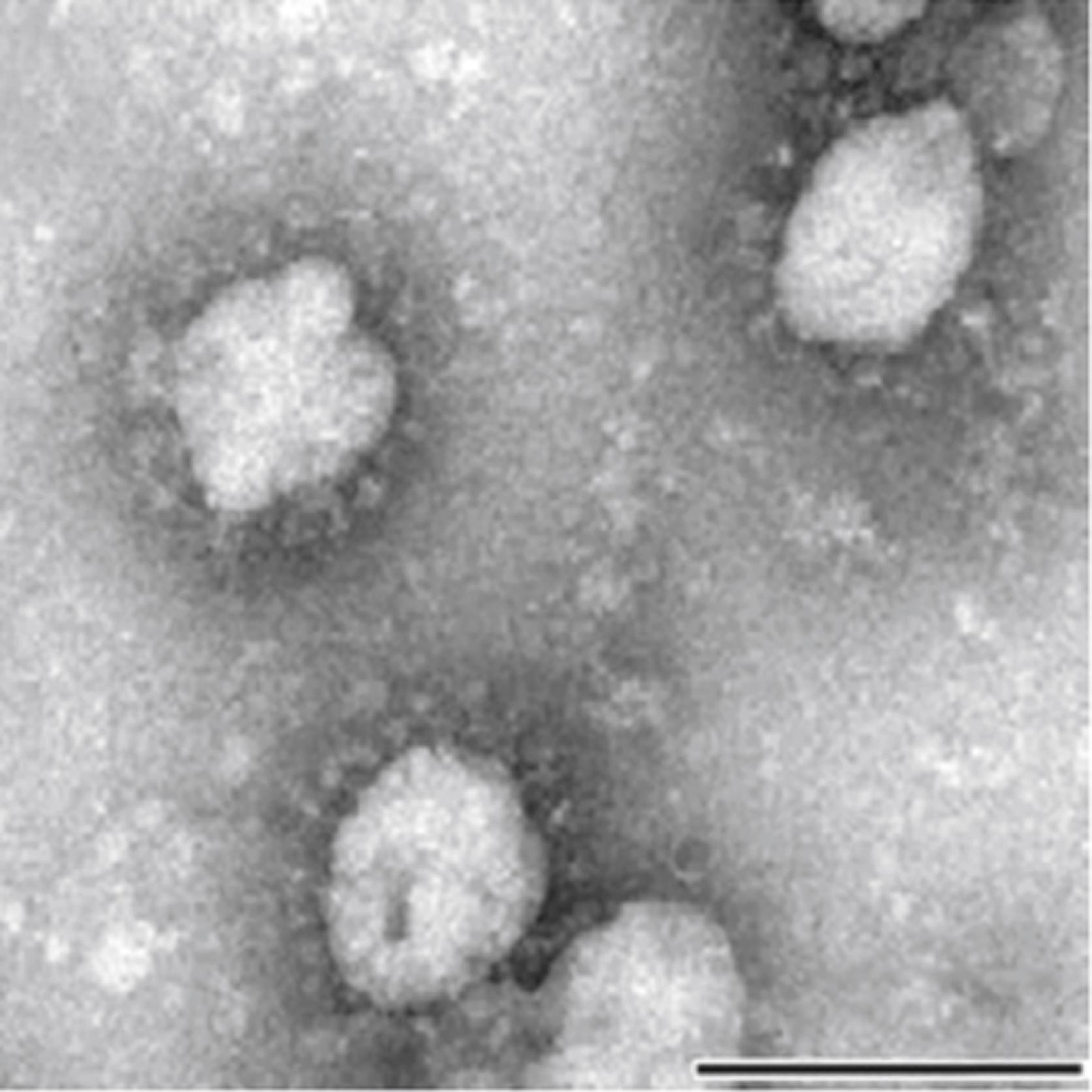
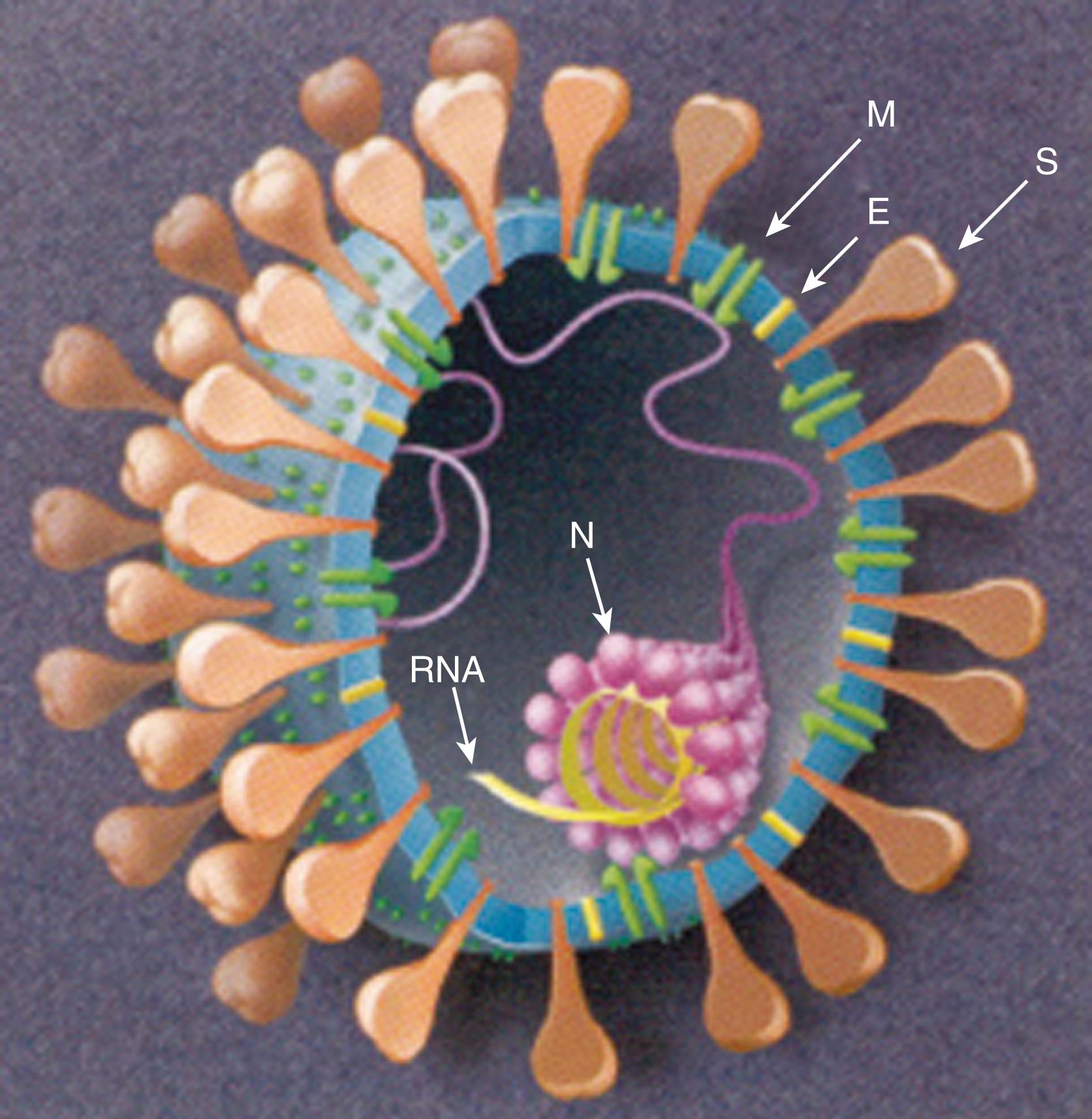
Coronaviruses are included in the Coronaviridae family under the order Nidovirales. They are enveloped, non-segmented, single-stranded, positive-sense RNA viruses named after their corona-like or crown-like surface projections seen on electron microscopy that correspond to large surface spike proteins ( Figs. 222.1 and 222.2 ). Four genera of coronaviruses have been described. Coronaviruses are host-specific and can infect humans and a variety of animals. Human coronaviruses (HCoVs) emerged from animal coronaviruses in independent events. , Recognized HCoVs are part of the Alphacoronavirus and Betacoronavirus genera ( Table 222.1 ). ,
| Genus | Subgenus | Species | Host | Associated Diseases |
|---|---|---|---|---|
| Alphacoronavirus | — | HCoV-229E | Human | Common cold |
| HCoV-NL63 | Human | Common cold, croup | ||
| Betacoronavirus | Embecovirus | HCoV-OC43 a | Human | Common cold |
| HCoV-HKU1 | Human | Common cold, gastroenteritis | ||
| Sarbecovirus | SARS-CoV-1 | Human | SARS | |
| SARS-CoV-2 | Human | COVID-19, MIS, post-COVID-19 condition | ||
| Merbecovirus | MERS-CoV | Human | MERS | |
| Nobecovirus | No HCoV identified | — | — | |
| Hibecovirus | No HCoV identified | — | — | |
| Gammacoronavirus | — | No HCoV identified | — | — |
| Deltacoronavirus | — | No HCoV identified | — | — |
a This species has been abolished according to the International Committee on Taxonomy of Viruses. It is now considered part of the species betacoronavirus 1 . However, because the name HCoV-OC43 is still commonly used, the species is referred to as HCoV-OC43 in this chapter.
The first recognized HCoV strains include the endemic HCoVs, HCoV-229E and HCoV-OC43, described in the 1960s as causes of upper respiratory tract infections. Genetic analysis suggests HCoV-229E and HCoV-OC43 evolved from bat and rodent coronaviruses, with camelids and cattle acting as possible intermediate hosts, in the 1800s and 1900s, respectively. Strains such as HCoV-B814, HCoV-OC16, HCoV-OC37, and HCoV-OC48 were also described in the 1960s, and coronavirus-like particles were detected in stool in the 1980s, primarily in infants with gastroenteritis and necrotizing enterocolitis, but further characterization was not completed.
In 2003, severe acute respiratory syndrome (SARS)-CoV-1 was identified as a novel respiratory pathogen responsible for a global outbreak of SARS with a high case fatality rate. Emerging in 2002 in China, the outbreak lasted 9 months and resulted in 8098 infected people and 774 deaths before SARS-CoV-1 was eradicated through infection control and public health measures. Data suggest that SARS-CoV-1 evolved in 2002 from SARS-CoV–1-like viruses in horseshoe bats, with civet cats and other wild market animals serving as intermediate hosts.
Renewed interest in coronavirus research led to the discovery of two novel endemic HCoVs in 2004 from patients with upper respiratory tract infections: HCoV-NL63 (also known as HCoV-NL and HCoV-NH) and HCoV-HKU1. Genetic analysis suggest that HCoV-NL63 and HCoV-HKU1 evolved from bat and rodent coronaviruses with not-yet-identified intermediate hosts in the 1200–1400s and 1950s respectively. It is unclear how HCoV-NL63 and HCoV-HKU1 relate to the HCoV strains originally described in the 1960s (i.e., HCoV-B814, HCoV-OC16, HCoV-OC37, and HCoV-OC48) or to the enteric coronavirus-like particles detected in stool in the 1980s.
In 2012, the Middle East respiratory syndrome (MERS)-CoV (also called hCoV-EMC) was identified as a novel HCoV responsible for a cluster of severe respiratory illnesses with high case fatality in the Kingdom of Saudi Arabia. , Since then, MERS-CoV has been identified in 27 countries and has been associated with 2574 cases and 886 deaths, the majority of which continue to be in Saudi Arabia. Data suggest that MERS-CoV likely evolved from a bat coronavirus in 2012, with camels acting as intermediate hosts. MERS-CoV remains endemic in camels, with primary human infections typically being associated with direct or indirect contact with camels and secondary cases resulting from human-to-human transmission among close contacts.
In 2019, SARS-CoV-2 was identified as the cause of a global pandemic of a respiratory illness called COVID-19 (“CO” stands for corona, “VI” for virus, and “D” for disease) that first arose in China and that has affected 196,553,009 people and killed 4,200,412 people as of July 2021. , SARS-CoV-2 is thought to have evolved from a bat coronavirus with a not-yet-identified intermediate host, but other possibilities have been considered. , The case fatality rate of SARS-CoV-2 infection is higher than that of the endemic HCoVs but lower than that of SARS-CoV-1 and MERS-CoV. However, SARS-CoV-2 has had the greatest recognized impact globally of all known HCoVs to date given the susceptibility of the human population, the associated severity of illness, the challenges of sustaining control attained through infection control and public health measures, and variant forms threatening to evade control through natural or vaccine-mediated immunity. Whether the current endemic HCoVs had comparable severe pandemics at the time of their emergence is not known.
HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 are found worldwide and cause disease, predominantly in the winter and spring months in temperate climates. , Seroprevalence data suggest that exposure is common in early childhood.
Modes of transmission for endemic HCoVs are not well studied. Based on studies of other respiratory viruses and limited studies of HCoV-229E and HCoV-OC43, transmission likely occurs primarily by a combination of spread by droplets and direct and indirect contact with fomite transmission more likely to occur when organic material is also present. , The possible role of aerosol spread needs further study. HCoV-229E and HCoV-OC43 are most likely transmitted during the first few days of illness, when symptoms and respiratory tract viral loads are maximal. , Further study is needed to confirm if this is true for HCoV-NL63 and HCoV-HKU1.
The incubation period for HCoV-229E is 2–5 days (median, 3 days). , Further study is needed to confirm the incubation periods for HCoV-OC43, HCoV-NL63, and HCoV-HKU1.
The pathogenesis of endemic HCoVs is best described for HCoV-229E, and it is not clear how well this correlates with the pathogenesis of the other endemic HCoVs. HCoV-229E infections are initiated through inoculation of respiratory tract mucosal surfaces using aminopeptidase N (also known as CD13) for cell entry , while NL63 uses angiotensin-converting enzyme 2 (ACE2) and OC43 and HKU1 use 9-O-acetylsialic acids as receptors.
For HCoV-229E, nasal mucosal plasma exudation and increased interferon γ (IFNγ) levels in nasal lavage specimens correlate with symptom severity. , Upper respiratory tract viral loads peak within the first 3 days after infection and drop off dramatically at 1 week correlating with detection of antibodies. , , Antibodies reach maximal levels approximately 1 week later and decline thereafter.
Immunity is not complete, and reinfection is common for the endemic HCoVs. , Higher circulating antibody levels, especially levels of specific IgA anti-HCoV, correlate with reduced symptoms and reduced virus shedding on re-exposure experiments using HCoV-229E. ,
HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 infections typically present as the common cold, with endemic HCoVs being the second most common cause of the common cold after rhinoviruses. , Typical symptoms including rhinorrhea, nasal congestion, sore throat, sneezing, and cough that may be associated with fever. , , , Based on data for HCoV-229E, symptoms typically peak on day 3 or 4 of illness correlating with peak viral loads and are self-limited. ,
Endemic HCoVs are also be associated with acute otitis media or exacerbations of asthma. , , , Less frequently, these viruses are associated with lower respiratory tract infections, including bronchiolitis and pneumonia, primarily in infants and immunocompromised children and adults. , , ,
HCoV-HKU1 infections also can be associated with symptoms of gastroenteritis, including vomiting and diarrhea, which typically occur in conjunction with respiratory symptoms. , , HCoV-HKU1 also appears to be more frequently associated with febrile seizures compared with other HCoVs. ,
HCoV-NL63 infections also can cause croup; HCoV-NL63 is the second most common cause of croup after parainfluenza virus type 1. , A purported association of HCoV-NL63 with Kawasaki disease has not been substantiated, and a possible association of HCoV-229E with Kawasaki disease has not been reproduced.
Some laboratories offer comprehensive real-time polymerase chain reaction (RT-PCR) for detection of HCoVs 229E, OC43, NL54, and HKU1. Upper respiratory tract specimens, such as nasopharyngeal samples or mid-turbinate swabs, are the most appropriate samples for virus detection when testing is available. , , Stool samples can be positive during some HCoV-HKU1 infections. , For cases of HCoV-299E and HCoV-OC43 infection, specimens are most likely to be positive during the first few days of illness; whether this also is true for HCoV-NL63 and HCoV-HKU1 needs further study.
Because of the self-limited nature of infection with HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, few treatment studies have been performed. Care typically is supportive.
Practicing good hand and respiratory hygiene is the most useful control measure to curb the spread of all respiratory viruses, including HCoVs. , Standard disinfectants should be used to clean and disinfect environmental surfaces that are frequently touched by infected persons to reduce the potential for indirect transmission of HCoVs by fomites. ,
Prophylactic intranasal IFNα has reduced the duration and severity of HCoV-229E infection in research settings but has not been used clinically. , A proprietary extract of the roots of North American ginseng ( Panax quinquefolium ) can reduce the number of colds and the severity and duration of cold symptoms in adults when taken daily, presumably due to immune stimulation. Efficacy for a decrease in the number of colds specifically due to HCoVs has not been studied.
Healthcare personnel should use a gown, gloves, mask, and eye protection for the duration of illness when caring for children hospitalized with signs and symptoms of a respiratory tract infection.
SARS-CoV-1 has been eradicated. The last SARS-CoV-1 infections were reported in 2004, when 13 cases linked to laboratory biosafety practice breaches and 4 sporadic community-acquired cases of SARS were identified in Southeast Asia. During the 2002–2003 global outbreak, a large proportion of SARS-CoV-1 transmission occurred within hospitals from patients with unrecognized illness. ,
Droplet spread and direct contact are the most common modes of transmission for SARS-CoV-1, although evidence for spread by indirect contact and aerosol also exists. , SARS-CoV-1 is most likely transmitted during the second week of illness, when symptoms and viral load in the respiratory tract peak, and when nosocomial super-shedding events have been described.
The incubation period for SARS-CoV-1 is 2–10 days (median, 4 days).
Most evidence regarding the pathogenesis and immunity of SARS-CoV-1 is from infections in adults because few children were affected by the 2002–2003 outbreak. SARS-CoV-1 infection is initiated through inoculation of the respiratory tract mucosa using ACE2 as the functional receptor for cell entry. Viremia and replication in the lung and gastrointestinal tract follow. , Replication at other sites likely occurs given the wide distribution of SARS-CoV-1 in tissues examined at autopsy. ,
Peak viral loads in nasopharyngeal specimens are detected during the second week of symptoms, with higher viral loads being associated with worse outcomes. , , A rise in SARS CoV-1–specific antibodies and a decrease in SARS-CoV-1 viral load occur during the second and third week, typically corresponding to symptomatic improvement, , but paradoxically, clinical deterioration is observed in some patients. Elevated levels of IFNγ, inflammatory cytokines interleukin-1 (IL-1), IL-6, and IL-12; neutrophil chemokine IL-8; monocyte chemoattractant protein 1; and IFNγ-inducible protein-10 have been detected, with levels of IL-6 correlating with severity of disease, , suggesting that host immune responses likely contribute to clinical deterioration in these patients.
SARS-CoV-1 is primarily associated with severe symptoms, but asymptomatic and mild cases have been described. SARS-CoV-1 disproportionately affects adults, who typically manifest fever, myalgia, headache, malaise, and chills, followed by a nonproductive cough and dyspnea 3–5 days later. Approximately 25% develop watery diarrhea. Respiratory distress progresses, leading to intubation and ventilation in 25% of cases. The overall associated mortality rate is approximately 10%, with most deaths occurring in the third week of illness. The case-fatality rate for persons >60 years of age approaches 50%. Typical laboratory abnormalities include lymphopenia and increased serum lactate dehydrogenase (LDH) and creatine kinase levels. , Most patients have progressive unilateral or bilateral, ill-defined airspace infiltrates on chest imaging. , Pneumothoraces and other signs of barotrauma are common in patients receiving mechanical ventilation.
Infants and children <12 years of age who develop SARS typically have fever, cough, rhinorrhea, and milder symptoms compared with adolescents and adults. Associated lymphopenia is less severe, and radiographic changes are milder and usually resolve more quickly than in adolescents and adults. No infants or children died of SARS-CoV-1 infection in the 2002–2003 outbreak. , Adolescents who developed SARS had clinical courses more closely resembling that of adults, including fever, myalgia, headache, and chills, and they were more likely to have dyspnea, hypoxemia, and worsening chest radiographic findings.
Women infected with SARS-CoV-1 during pregnancy who survive have an increased risk of spontaneous miscarriage, preterm delivery, and intrauterine growth restriction. There is no evidence of vertical transmission of SARS-CoV-1. , Two neonates born to mothers with SARS-CoV-2 in the 2002–2003 outbreak developed gastrointestinal complications (e.g., jejunal perforation, necrotizing enterocolitis with ileal perforation) soon after birth, but neither had clinical evidence of SARS-CoV-1 infection. It is unclear whether these findings related to complications of maternal SARS or treatments for SARS, such as ribavirin and corticosteroids, used during pregnancy.
Public health laboratories offer RT-PCR and antibody testing for SARS-CoV-1. Upper and lower respiratory tract specimens, stool samples, and serum should be considered for RT-PCR testing. , , , Serum samples for RT-PCR testing are most likely to be positive in the first week of illness, , whereas respiratory and stool specimens may not be positive until the second week of illness when symptoms and viral loads peak. , Infants and children with SARS-CoV-1 infections are less likely to have positive specimens, consistent with the milder symptoms and presumed correspondingly lower viral loads in children. ,
Laboratory guidance for SARS-CoV-1 diagnostic testing is available on the Centers for Disease Control and Prevention (CDC) website. Because of the potential for false-positive results and the associated public health implications, testing for SARS-CoV-1 in the absence of known person-to-person transmission should be done only in consultation with public health officials and when there is a high degree of clinical suspicion.
Corticosteroids, type 1 IFN agents, convalescent plasma, ribavirin, and lopinavir or ritonavir were used to treat SARS. , For many of these agents, anecdotal reports suggest benefit, and in vitro assays and animal models offer supportive data, , , with the exception of ribavirin, for which in vitro studies do not support efficacy. However, controlled trials with these agents were not performed.
Since the SARS-CoV-1 outbreak, remdesivir, chloroquine, viral entry- and protease-inhibiting agents, RNA-interfering agents, and glycyrrhizin have been tested in vitro with supportive data. , However, no definitive conclusions can be drawn. If SARS-CoV-1 re-emerges, these treatments should be studied through controlled clinical trials to determine their effectiveness.
Good hand and respiratory hygiene along with disinfection of frequently touched surfaces should be followed to reduce the transmission of SARS-CoV-1. A gown, gloves, an N95 respirator (if available, or else a face mask), eye protection, and negative-pressure isolation (if available) are recommended for patients with SARS-CoV-1 infection treated by healthcare personnel for the duration of illness or 10 days after resolution of fever, provided respiratory symptoms are absent or improving.
Human coronaviruses (HCoVs) 229E, OC43, NL63, and HKU1 are endemic worldwide and emerged from animal coronavirus in independent events between the 13th and 15th century to the 1950s.
Exposure is common in early childhood; in temperate climates, infections primarily occur in the winter and spring months.
The incubation period for HCoV-229E is 2–5 days (median, 3 days); further study is needed to confirm the incubation periods for HCoV-OC43, HCoV-NL64, and HCoV-HKU1.
HCoV strains 229E, OC43, NL63, and HKU1 are associated with the common cold, acute otitis media, asthma exacerbations, and, less frequently, bronchiolitis and pneumonia.
HCoV-HKU1 may also be associated with vomiting and diarrhea and appears to be associated more frequently with febrile seizures compared with other HCoVs.
HCoV-NL63 is also associated with croup.
Upper and lower respiratory tract specimens can be tested for endemic HCoV by real-time polymerase chain reaction.
Stool samples have been positive for some patients with HCoV-HKU1 infection.
Infections with HCoV strains 229E, OC43, NL63, and HKU1 require supportive care.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here