Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infectious complications are one of the leading causes of death in patients with severe burn injuries. Increases in the total body surface area and depth of burn injuries correlate with the excessive risk of infectious complications. Burn patients are commonly treated with many components such as fluid resuscitation, wound excision, grafting and coverage, infection control, and nutritional support. Progress in each treatment has contributed significantly to reduce mortality in severely burned patients. However mortality rates associated with infectious complications still remain high, and bacterial infections are a major cause of morbidity in burned patients.
Infections in severely burned patients frequently occur as opportunistic infections. In these patients, a majority of the causative infectious pathogens come from their own microbiota distributed in and on the skin, respiratory tract, and intestines. For example, staphylococci are found in 40% of wound isolates, and 14%–17% of burned patients become infected once they are colonized. Although antibiotics are useful to control infections, excessive antibiotic usage is directly related to the development of methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin is utilized clinically to treat MRSA; however, the management of invasive MRSA infection will become a serious problem if VISA/GISA strains spread widely. Currently, newer antibiotics such as linezolid (oxazolidinones) and quinupristin/dalfopristin (macrolides) are available to treat MRSA infection. In general, however, these agents are of limited usefulness because of their propensity to create antibiotic-resistant bacteria. These facts strongly indicate that a new strategy, apart from antibiotic therapy, is required to treat infections.
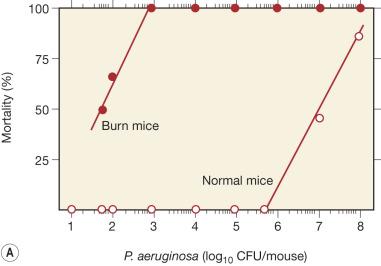
Sepsis stemming from wound infections is usually found in patients with severe thermal injuries. Topical antibacterials (silver sulfadiazine, silver nitrate, mafenide acetate, etc.) are very useful for controlling the colonization and multiplication of microorganisms on the surface of burn wounds. However, due to the burn-induced defects of the host's antibacterial defenses, very small amounts of Pseudomonas aeruginosa that escape from this treatment are enough to spread throughout the whole body. In fact, only 50 colony-forming units (CFU) of P. aeruginosa applied to burn wounds are sufficient to kill severely burned mice, whereas more than 1 × 10 8 CFU applied intradermally are required to kill noninjured mice. Similarly, burn-associated defects in host antibacterial defenses have been demonstrated against enteric bacteria in severely burned mice ( Fig. 20.1 ). In addition, polymicrobial sepsis frequently occurs in severely burned patients. Because such infections do not usually develop in healthy individuals, immune dysfunctions associated with burn injuries are a major reason for the increased susceptibility of severely burned patients to these infections.
The innate immune system is the first line of host defense against microbial invasion. The cells and molecules of innate immunity are rapidly activated by microbes or some signals induced by damaged tissues. The innate immune system consists of (1) soluble recognition molecules, (2) physical barriers, and (3) cellular components (myeloid cells and lymphoid cells). Soluble recognition molecules include natural antibodies (IgM and IgA), acute phase proteins, and the complement system. In heathy individuals, natural IgM is constitutively produced. However, reduced levels of antibodies are evident in severely burned patients. Burn-associated hypermetabolic responses stimulate the synthesis of acute phase proteins located in the liver and intestinal mucosa. Burn injury to tissues leads to complement activation with subsequent depletion of complement components, mainly C3, C4, and C5. As physical barriers, epithelial cells produce antimicrobial peptides (β-defensins and LL-37/hCAP-18), which are small molecular weight proteins with broad-spectrum antimicrobial activity. After injury or infection, epithelial cells produce alarmins such as thymic stromal lymphopoietin (TSLP), interleukin (IL)-25 and IL-33. This causes the initiation of type 2 immune responses. Although β-defensins are normally produced by epidermal keratinocytes, mRNA expression of these peptides is greatly decreased in the tissue around the burn wound when compared with normal skin in thermally injured patients. Decreased local production of antimicrobial peptides around burn wounds allows the local growth of bacteria, putting the patient at risk for wound-derived systemic infections. Studies in animal models of burn injury suggest that decreased antimicrobial peptide production by epidermal keratinocytes around burn wounds is due to the infiltration of suppressive myeloid and lymphoid cells that appear in response to burn injuries.
It is well established that burn injury initiates an early proinflammatory innate immune response followed by an adaptive counter-inflammatory response. The innate immune system is activated in response to either pathogen-associated or damage-associated molecular patterns. Both patterns are recognized by pattern recognition receptors (PRRs) on innate immune cells. Depending on the molecular patterns recognized, pro- or antiinflammatory soluble factors are rapidly released from these cells. Thus the development of burn-associated immunosuppression is initiated early after burn injury. Antiinflammatory responses are helpful for wound healing and the resolution of liver and intestinal inflammation in burn patients. However, dysregulated immunosuppression and persistent inflammation cause immunoparalysis, and patients with immunoparalysis become extremely susceptible to infections. This chapter will discuss cell populations and their functions involved in innate immunity as influenced by severe burn injuries.
Neutrophils are the most abundant white blood cells in the innate immune system. They are rapidly recruited to the injury or infection site, where they phagocytose and kill invading microorganisms. The maturation of neutrophils is under the control of transcription factors PU.1 and C/EBP. During maturation, the neutrophil goes through several stages, namely myeloblast, promyelocyte, myelocyte, metamyelocyte, band cell, and, finally, polymorphonuclear (segmented) cell. In the absence of infection or inflammation, mature neutrophils die within 15 hours by caspase 3-mediated spontaneous apoptosis. Inflammatory signals are capable of prolonging the life span of neutrophils by several days. However, even during inflammation, mature neutrophils die by apoptosis or NETosis (death during formation of neutrophil extracellular traps [NETs] for extracellular killing) while performing their antimicrobial function. These dead neutrophils are engulfed by macrophages.
Mature neutrophils leave the bone marrow and enter to the circulation. Getting neutrophils to the site of infection is of prime importance, and an elaborate series of adhesion events between neutrophils and the endothelium ensures that neutrophils leave the bloodstream only at the inflammatory site. This process includes tethering (capturing), rolling, adhesion, crawling, and transmigration. IL-8 production by activated neutrophils plays a central role in the recruitment of additional neutrophils to the infection site. After severe burn injuries, levels of IL-8 in plasma are approximately 60 times higher than those in plasma of healthy controls. An increase in peripheral blood neutrophils is seen in patients 2–5 days after burn injury. However, the chemotactic function and efficient migration of neutrophils are impaired in severely burned patients due to the decreased expression of CXCR2 (a receptor for IL-8). Migration speed of neutrophils toward the chemoattractant source is also impaired in severely burned patients. Such impairment of neutrophils starts as early as 24 hours after burn injury, reaches a maximum at 3 to 5 days after burn injury, and correlates to the size of the burn injury.
Neutrophils are efficient phagocytes that engulf microbes into phagosomes. The phagosome fuses with granules to produce a phagolysosome, in which microbes are exposed to many destructive enzymes, antimicrobial peptides, and reactive oxygen species. These components synergize and effectively kill microbes. Highly activated neutrophils kill extracellular microbes by releasing NETs, which consist of fibrils formed by active expulsion of DNA, chromatin, antimicrobial protein, and enzymes. The sticky DNA fibers of the NETs bind and immobilize pathogens and thus inhibit their further spread. NETs kill pathogens directly by means of antimicrobial histones and proteases. IL-8 is well-known to enhance NET formations of neutrophils. As described earlier, increased levels of IL-8 in the circulation of severely burned patients have been demonstrated. Increased levels of KC and MIP-2 have also been demonstrated in the sera and lungs of mice 2–8 hours after burn injury. KC and MIP-2 are functionally homologous to human IL-8. However, phagocytosis, oxidative metabolism, granular enzyme contents, and intracellular killing of neutrophils are greatly reduced in severely burned patients compared with those of neutrophils from healthy individuals. The attenuating CXCR2 expression on neutrophils is, at least in part, responsible for neutrophil dysfunction following burn injury.
Experimental studies in rodents have identified distinctive types of neutrophils that affect resistance to MRSA infections in burned mice ( Fig. 20.2 ). Neutrophils isolated from slightly burned (5% TBSA burn, MRSA-resistant hosts) or severely burned mice (25% TBSA burn, MRSA-susceptible hosts) are biologically and histologically different from the neutrophils isolated from naïve mice. These three neutrophil populations have unique cytokine/chemokine-producing patterns. Also, these three neutrophils express Toll-like receptors (TLRs) and surface CD49d/CD11b integrins differently. Neutrophils isolated from MRSA-resistant mice are proinflammatory IL-12 + CCL3 + cells (PMN 1 or N1), whereas neutrophils from MRSA-susceptible mice are an antiinflammatory IL-10 + CCL2 + cells (PMN 2 or N2). Neutrophils isolated from naïve mice are IL-12 − and IL-10 − cells. These neutrophils also differ in their promotion of macrophage differentiation. Quiescent macrophages from the lamina propria of normal mice convert to the M1 phenotype after cultivation with polymorphonuclear leukocyte (PMN) 1 in dual-chamber transwells supplemented with a mixture of TLR3/TLR9 agonists. In contrast, quiescent macrophages convert to the M2 phenotype after cultivation with PMN 2 in the same transwells. Neither M1 nor M2 macrophages appear even when quiescent macrophages are transwell-cultured with naïve PMN. Also, PMN 2 are shown to be inhibitory for macrophage conversion to the M1 phenotype under stimulation with TLR3/TLR9 agonists. Subsequently, IL-10 and CCL2 produced by PMN 2 are identified as inhibitors of TLR3/TLR9-induced macrophage polarization toward the M1 phenotype. Another distinguishing feature is their morphology. Although IL-12 − and IL-10 − neutrophils have a round nucleus, PMN 1 has a multilobular nucleus (indicative of mature neutrophils), and the nucleus of PMN 2 is ring-shaped. PMN 2 is predominantly demonstrated in severely burned mice.
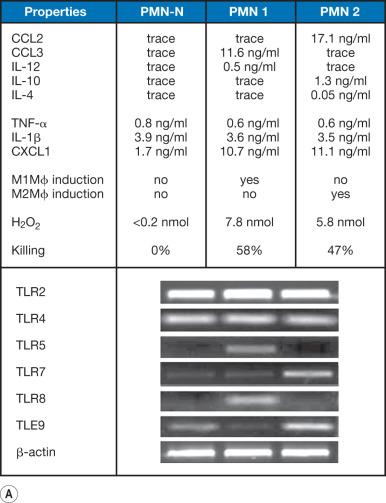
Furthermore the role of PMN 1 and PMN 2 on host anti-MRSA resistance has been examined in γ-irradiated NSG mice (lacking the functional immunocompetent cells) reconstituted with normal mouse macrophages (Mϕ-reconstituted γNSG mice). Mϕ-reconstituted γNSG mice become more resistant after inoculation with PMN 1, whereas the same mice become susceptible after inoculation with PMN 2. Additionally, γNSG mice are shown to be resistant against MRSA infection when they are inoculated with normal mouse macrophages previously cultured with PMN 1. Macrophages previously cultured with PMN 2 do not protect Mϕ-reconstituted γNSG mice infected with a lethal dose of MRSA. Similar results are obtained when Mϕ-reconstituted γNSG mice are orally infected with a lethal dose of Enterococcus faecalis . These results indicate that MRSA infection and E. faecalis translocation are not controlled in hosts predominantly bearing PMN 2. IL-10 and CCL2 released from PMN 2 are characterized as active cytokines that induce M2 macrophages from quiescent macrophages. Therefore these cytokines are targets for the macrophage polarization influenced by PMN 2. Infectious complications due to MRSA infection and enteric bacterial translocation have been successfully controlled in severely burned mice depleted of CCL2 or IL-10 by their antisense oligodeoxynucleotide (ODN). Thus antiinflammatory neutrophils appearing in hosts after severe burn injury suppress effective immune responses and increase susceptibility to infections.
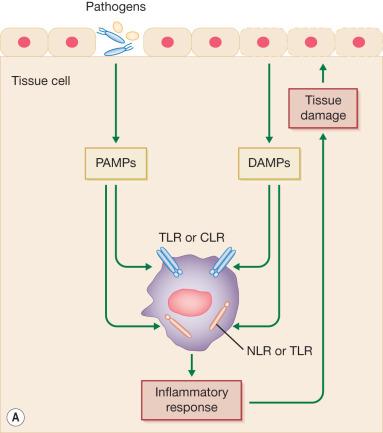
In microbial infection, pathogen-associated molecular patterns (PAMPs) present in diverse organisms but, when absent in the host, provide exogenous signals that alert the immune system to the presence of pathogens, thereby promoting immunity. For instance, bacterial lipopolysaccharide (LPS) is a PAMP and is recognized by TLR4 on the immune cell surface. Bacterial DNA that contains unmethylated CpG motifs is also a PAMP and is recognized by TLR9, which is located in the endosomal compartment of neutrophils. Both TLR4 and TLR9 are PRRs. In contrast, a variety of stressed, injured, or dying cells (including epithelial cells following burn injury) release damage-associated molecular patterns (DAMPs) as the endogenous danger signals that alert the innate immune system to unscheduled cell death and microbial invasion and in response to stress ( Fig. 20.3 ). HMGB1, hyaluronic acid, formyl peptides, heat-shock protein 70, S100A8, S100A9, and serum amyloid A are well-known DAMPs. These DAMPs are specifically recognized by several receptors ( Table 20.1 ). These ligand-specific interactions are able to induce either pro- or antiinflammatory effects. In severely burned patients, circulating mitochondrial DAMP levels are notably elevated (>10-fold) within 72 hours of burn injury. These mitochondrial DAMPs cause systemic inflammation, which is commonly characterized by fever, leukocytosis, increased secretion of adrenocorticotropic hormone and glucocorticoids, and alterations in plasma protein concentrations. Simultaneously, these DAMPs cause desensitization of chemokine receptors and formyl-peptide receptors on neutrophils. Neutrophils influenced by DAMPs lose their antimicrobial functions. Also, Clec12a (binding to uric acid crystals) mediates the antiinflammatory response of neutrophils influenced by DAMPs. Antiinflammatory neutrophils secrete high amounts of IL-10 and CCL2, potent antiinflammatory cytokines, and have been implicated in impaired host antibacterial immunity in severely burned patients.
| Receptor | PAMPs | DAMPs |
|---|---|---|
| TLR1/TLR2 | Lipopeptide | Serum amyloid A |
| TLR4 | LPS | Fatty acid Hyaluronic acid S100A8/A9 |
| NLRP3 | Uric acid | Uric acid ATP |
| RIG-1, MDA5, TLR7/8 | Viral RNA | Immune complex of snRNPs |
| TLR9 | Bacterial DNA | Self-DNA-containing immune complex Histone |
| RAGE | HMGB1 | |
| DAI, IFI16, AIM2, H2B, RNA polymerase III |
Bacterial DNA, viral DNA | Self-DNA |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here