Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter, the student should be able to answer the following questions:
What is steady-state balance, and, with water balance as an example, what are the elements needed to achieve steady-state balance?
What are the volumes of the body fluid compartments, and how do they change under various conditions?
How do the body fluid compartments differ with regard to their composition?
What determines the resting membrane potential of cells?
How do cells regulate their volume in isotonic, hypotonic, and hypertonic solutions?
What are the structural features of epithelial cells, how do they carry out vectorial transport, and what are the general mechanisms by which transport is regulated?
Normal cellular function requires that the intracellular composition—with regard to ions, small molecules, water, pH, and a host of other substances—be maintained within a narrow range. This is accomplished by the transport of many substances and water into and out of the cell via membrane transport proteins, as described in Chapter 1 . In addition, each day, food and water are ingested, and waste products are excreted from the body. In a healthy individual, these processes occur without significant changes in either the volume of the body fluid compartments or their composition. The maintenance of constant volume and composition of the body fluid compartments (and their temperature in warm-blooded animals and humans) is termed homeostasis . The human body has multiple systems designed to achieve homeostasis, the details of which are explained in the various chapters of this book. In this chapter, the basic principles that underlie the maintenance of homeostasis are outlined. In addition, the volume and composition of the various body fluid compartments are defined.
The human body is an “open system,” which means that substances are added to the body each day and, similarly, substances are lost from the body each day. The amounts added to or lost from the body can vary widely, depending on the environment, access to food and water, disease processes, and even cultural norms. In such an open system, homeostasis occurs through the process of steady-state balance .
To illustrate the concept of steady-state balance, consider a river on which a dam is built to create a man-made lake. Each day, water enters the lake from the various streams and rivers that feed it. In addition, water is added by underground springs, rain, and snow. At the same time, water is lost through the spillways of the dam and by the process of evaporation. For the level of the lake to remain constant (i.e., steady-state balance), the rate at which water is added, regardless of source, must be exactly matched by the amount of water lost, again regardless of route. Because the addition of water is not easily controlled and the loss by evaporation cannot be controlled, the only way to maintain a constant level of the lake is to regulate the amount that is lost through the spillways.
To understand steady-state balance as it applies to the human body, the following key concepts are important.
There must be a “set point” so that deviations from this baseline can be monitored (e.g., the level of the lake in the preceding example, or setting the temperature in a room by adjusting the thermostat).
The sensor or sensors that monitor deviations from the set point must generate “effector signals” that can lead to changes in either input or output, or both, to maintain the desired set point (e.g., electrical signals to adjust the spillway in the dam analogy, or electrical signals sent to either the furnace or air conditioner to maintain the proper room temperature).
“Effector organs” must respond in an appropriate way to the effector signals generated by the set point monitor (i.e., the spillway gates must operate, and the furnace or air conditioner must turn on).
The sensitivity of the system (i.e., how much of a deviation from the set point is tolerated) depends on several factors, including the nature of the sensor (i.e., how much of a deviation from the set point is needed for the sensor to detect the deviation), the time necessary for generation of the effector signals, and how rapidly the effector organs respond to the effector signals.
It is important to recognize that deviations from steady-state balance do occur. When input is greater than output, a state of positive balance exists. When input is less than output, a state of negative balance exists. Although transient periods of imbalance can be tolerated, prolonged states of positive or negative balance are generally incompatible with life.
Fig. 2.1 illustrates several important concepts for the maintenance of steady-state water balance (details related to the maintenance of steady-state water balance are presented in Chapter 35 ). As depicted in Fig. 2.1 , there are multiple inputs and outputs of water, many of which can vary but nevertheless cannot be regulated. For example, the amount of water lost through the lungs depends on the humidity of the air and the rate of respiration (e.g., low humidity and rapid breathing increase water loss from the lungs). Similarly, the amount of water lost as sweat varies according to ambient temperature and physical activity. Finally, water loss via the gastrointestinal tract can increase from a normal level of 100 to 200 mL/day to many liters with acute diarrhea. Of these inputs and outputs, the only two that can be regulated are increased ingestion of water in response to thirst and alterations in urine output by the kidneys (see Chapter 35 ).
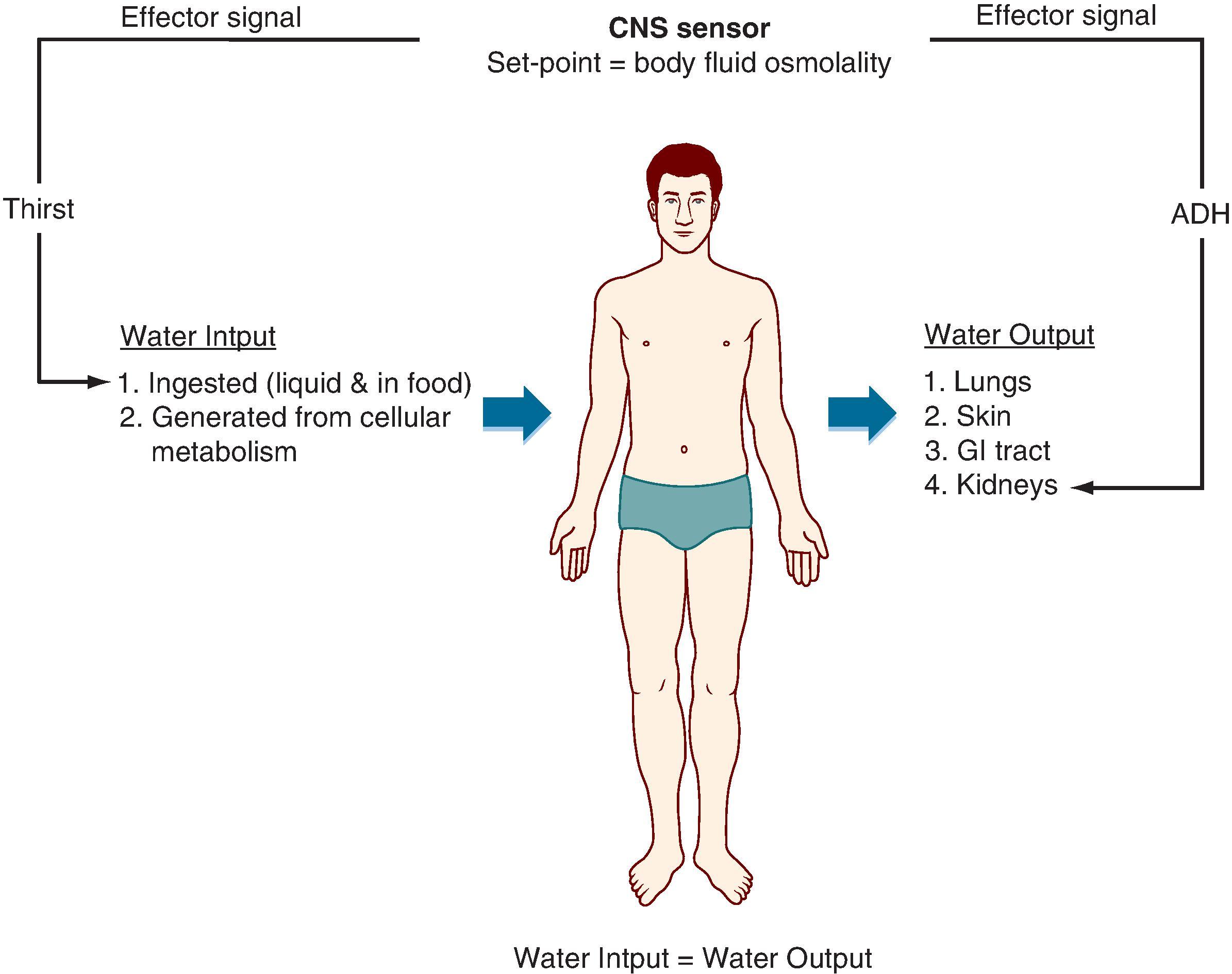
Water balance determines the osmolality of the body fluids. Cells within the hypothalamus of the brain monitor body fluid osmolality for deviations from the set point (normal range: 280–295 mOsm/kg H 2 O). When deviations are sensed, two effector signals are generated. One is neural and relates to the individual’s sensation of thirst. The other is hormonal (antidiuretic hormone, also called arginine vasopressin ), which regulates the amount of water excreted by the kidneys. With appropriate responses to these two signals, water input, water output, or both are adjusted to maintain balance and thereby keep body fluid osmolality at the set point.
Unicellular organisms maintain their volume and composition through exchanges with the environment they inhabit (e.g., sea water). The billions of cells that constitute the human body must maintain their volume and composition as well, but their task is much more difficult. This challenge, as well as its solution, was first articulated by the French physiologist Claude Bernard (1813–1878). He recognized that although cells within the body cannot maintain their volume and composition through exchanges with the environment, they can do so through exchanges with the fluid environment that surrounds them (i.e., the extracellular fluid). Bernard referred to the extracellular fluid as the milieu intérieur (“the environment within”). He also recognized that the organ systems of the body are designed and function to maintain a constant milieu interieur or a “constant internal environment.” This in turn allows all cells to maintain their volume and composition through exchanges with the extracellular fluid as a result of membrane transport (see Chapter 1 ).
Transport by the epithelial cells of the gastrointestinal tract, kidneys, and lungs is the body’s interface with the external environment and control both the intake and excretion of numerous substances, as well as water. The cardiovascular system delivers nutrients to and removes waste products from the cells and tissues and keeps the extracellular fluid well mixed. Finally, the nervous and endocrine systems provide regulation and integration of these important functions.
To provide background for the study of all organ systems, this chapter presents an overview of the normal volume and composition of the body fluid compartments and describes how cells maintain their intracellular composition and volume. Included is a presentation on how cells generate and maintain a membrane potential, which is fundamental for understanding the function of excitable cells (e.g., neurons and muscle cells). Finally, because epithelial cells are so central to the process of regulating the volume and composition of the body fluids, the principles of solute and water transport by epithelial cells are also reviewed.
Water makes up approximately 60% of the body’s weight; variability among individuals is a function of the amount of adipose tissue. Because the water content of adipose tissue is lower than that of other tissue, increased amounts of adipose tissue reduce the fraction of water in the total body as a percentage of weight. The percentage of body weight attributed to water also varies with age. In newborns, it is approximately 75%. This decreases to the adult value of 60% by the age of 1 year.
As illustrated in Fig. 2.2 , total body water is distributed between two major compartments, which are divided by the cell membrane. a
a In these and all subsequent calculations, it is assumed that 1 L of fluid (e.g., ICF and ECF) has a mass of 1 kg. Although 1 L of the ICF and ECF has a mass of slightly more than 1 kg, this simplification allows conversion from measurements of body weight to volume of body fluids.
The intracellular fluid (ICF) compartment is the larger compartment and contains approximately two-thirds of the total body water. The remaining third is contained in the extracellular fluid (ECF) compartment. The volumes of total body water, ICF, and ECF in liters are calculated as follows:
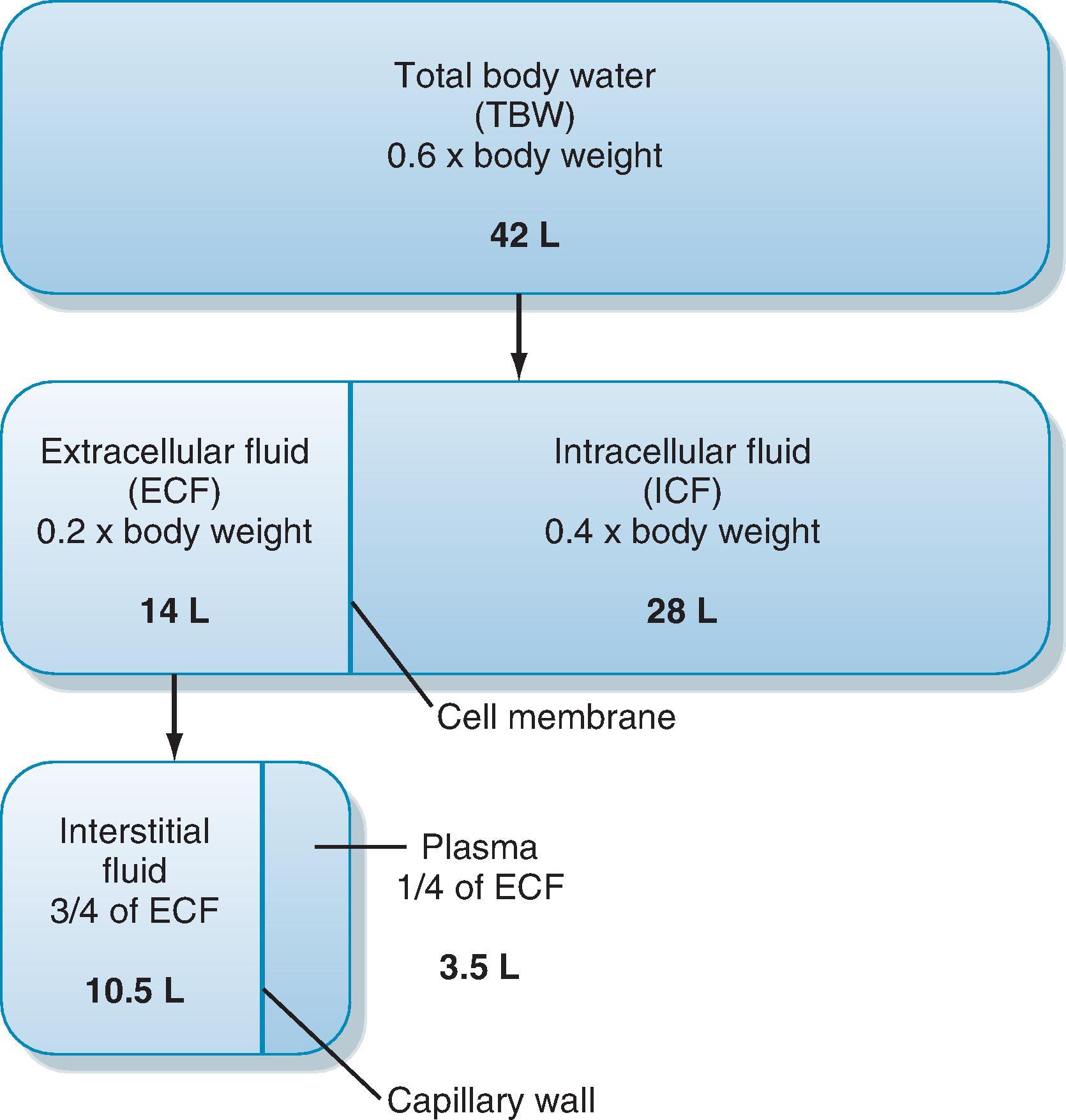
The ECF compartment is further subdivided into interstitial fluid and plasma. The ECF also includes fluid contained within bone and dense connective tissue, as well as the cerebrospinal fluid. The interstitial fluid surrounds the cells in the various tissues of the body and makes up three-fourths of the ECF volume. Plasma is contained within the vascular compartment and represents the remaining fourth of the ECF. In some pathological conditions, additional fluid may accumulate in what is referred to as a third space. Third-space collections of fluid are part of the ECF; an example is the accumulation of fluid in the peritoneal cavity (ascites) of individuals with liver disease.
As depicted in Fig. 2.2 , water moves between the ICF and ECF compartments across the plasma membranes of cells, and it moves between the vascular (plasma) and interstitial compartments across capillary walls. The pathways and driving forces for this water movement are different across cell membranes, in comparison to the capillary walls.
Movement of water between the ICF and ECF compartments, across cell membranes, occurs through aquaporins expressed in the plasma membrane (see Chapter 1 ). The driving force for this water movement is an osmotic pressure difference. The osmotic pressure of both the ICF and ECF is determined by the molecules/ions present in these fluids. For simplicity, these can be divided into (1) molecules of low molecular weight (e.g., glucose) and ions (e.g., Na + ) and (2) macromolecules (e.g., proteins). The osmotic pressures of both the ICF and ECF are in the range of 280 to 295 mOsm/kg H 2 O. For the ECF, the low-molecular-weight molecules and ions account for nearly all of this pressure because the osmotic pressure contributed by proteins is only 1 to 2 mOsm/kg H 2 O. The molecules/ions contributing to the osmotic pressure within the cell are less well understood, but they also include low-molecular-weight molecules (e.g., glucose), ions (e.g., Na + ), and macromolecules (e.g., proteins). The fact that cell volume remains constant when ECF osmolality is constant means that the osmotic pressure inside the cells is equal to that of the ECF. If an osmotic pressure difference did exist, the cells would either swell or shrink, as described in the section “Nonisotonic Cell Volume Regulation.”
Movement of water between the vascular (plasma) compartment and the interstitial fluid compartment occurs across the capillary wall. The amount of water that moves across the capillary wall and the mechanism of the water movement vary depending on the capillary. For example, in the capillary sinusoids of the liver, endothelial cells are often separated by large gaps (discontinuous capillary). As a result, water and all components of the plasma (and some cellular elements) can pass easily across the wall. Other capillaries are lined by endothelial cells that contain fenestrations that are up to 80 to 100 nm in diameter (e.g., in the kidneys). These fenestrations allow all components of the plasma (only cellular elements of blood cannot pass through the fenestrations) to move across the capillary wall. Some capillaries (e.g., in the brain) form a relatively tight barrier to water and small molecules and ions, and water movement occurs through small pores on the endothelial cell surface or through clefts between adjacent endothelial cells. These pores and clefts allow water and molecules smaller than 4 nm to pass. In addition, a small amount of water traverses the capillary wall via pinocytosis by endothelial cells.
The driving forces for fluid (water) movement across the capillary wall are hydrostatic pressure and oncotic pressure (i.e., osmotic pressure generated by proteins). Collectively, these are called the Starling forces . Capillary fluid movement is discussed in detail in Chapter 17 ; in brief, hydrostatic pressure within the capillary (as a result of the pumping of the heart and the effect of gravity on the column of blood in the vessels feeding a capillary) is a force that causes fluid to move out of the capillary. Hydrostatic pressure in the surrounding interstitial tissue opposes the effect of the capillary hydrostatic pressure. The oncotic pressure of the plasma in the capillary tends to draw fluid from the interstitium into the capillary. The oncotic pressure of the interstitial fluid opposes this. Depending on the capillary bed, proteins can cross the capillary wall to varying degrees. For example, very little protein crosses the wall of skeletal muscle capillaries and the capillaries in the glomerulus of the kidneys. In contrast, proteins readily cross the wall of the liver capillaries (i.e., sinusoids). The degree to which proteins cross the capillary wall is quantitated by a reflection coefficient (σ). If no protein crosses the capillary wall, σ = 1, and if proteins freely cross the capillary wall, σ = 0. Thus the amount of fluid moving across the wall of the capillary is determined as follows:
where
Q f = fluid movement
K f = filtration constant (measure of surface area + intrinsic permeability)
P c = capillary hydrostatic pressure
P i = interstitial fluid hydrostatic pressure
π c = capillary (plasma) oncotic pressure
π i = interstitial fluid oncotic pressure
σ = reflection coefficient for protein across the capillary wall.
Depending on the magnitude of these forces, fluid may move out of the capillary or into the capillary.
The compositions of the various body fluid compartments differ; however, as described later, the osmolalities of the fluid within these compartments are essentially identical. b
b Some exceptions do exist. The cerebrospinal fluid is part of the ECF, but its osmolality is slightly higher than that of the ECF elsewhere in the body. Also, regions within the kidney can have osmolalities that are either less than or greater than that of the ECF. However, these volumes are small (≈150 mL) in comparison with the total volume of the ECF (≥12 L).
Thus the compartments are in “osmotic equilibrium.” In addition, any change in the osmolality of one compartment quickly causes water to redistribute across all compartments, which brings them back into osmotic equilibrium. Because of this rapid redistribution of water, measuring the osmolality of plasma or serum, c
c Serum is derived from clotted blood. Thus serum differs from plasma by the absence of clotting factors. With regard to osmolality and the concentrations of other molecules and ions, the osmolality and concentrations in plasma and serum are virtually identical.
which is easy to do, reveals the osmolality of the other body fluid compartments (i.e., interstitial fluid and intracellular fluid).
As described later, Na + is a major constituent of the ECF. Because of its high concentration in comparison with other molecules and ions, Na + (and its attendant anions, primarily Cl − and HCO 3 − ) is the major determinant of the osmolality of this compartment. Accordingly, it is possible to obtain an approximate estimate of the ECF osmolality by simply doubling the sodium concentration [Na + ]. For example, if a blood sample is obtained from an individual, and the [Na + ] of the serum is 145 mEq/L, its osmolality can be estimated as follows:
In some clinical situations, it is possible to obtain a more accurate estimate of the serum osmolality, and thus the osmolalities of the ECF and ICF, by also considering the osmoles contributed by glucose and urea, as these are the next most abundant solutes in the ECF (the other components of the ECF contribute only a few additional milliosmoles). Accordingly, serum osmolality can be estimated as follows:
The glucose and urea concentrations are expressed in units of milligrams per deciliter (dividing by 18 for glucose and 2.8 for urea *
* The urea concentration in plasma is measured as the nitrogen in the urea molecule, or blood urea nitrogen (BUN).
allows conversion from the units of milligrams per deciliter to millimoles per liter and thus to milliosmoles per kilogram of H 2 O). This estimation of serum osmolality is especially useful in treating patients who have an elevated serum glucose concentration secondary to diabetes mellitus, and in patients with chronic renal failure, whose serum urea concentration is elevated because of reduced renal excretion.
As discussed in Chapter 1, the ability of a substance to cause water to move across the plasma membrane of a cell depends on whether the substance itself crosses the membrane. Recall Eq. 1.9 :
where Π ε = the effective osmotic pressure and σ = the reflection coefficient for the substance. For many cells, glucose and urea cross the cell membrane. Although they contribute to serum osmolality, as measured by a laboratory osmometer where all molecules are “effective osmoles,” they are ineffective osmoles for water movement across many, but not all, cell membranes. In contrast, Na + is an “effective osmole” for water movement across the plasma membrane of virtually all cells. Eq. 2.2 gives the best estimate of the effective osmolality of the serum.
In contrast to water, the movement of ions across cell membranes is more variable from cell to cell and depends on the presence of specific membrane transport proteins (see the section “Composition of Bo d y Fluid Compartments”). Consequently, in trying to understand the physiology of fluid shifts between body fluid compartments, it can be assumed that while water moves freely between the compartments, there is little net movement of solutes. For most situations, this is a reasonable assumption.
To illustrate the physiologic characteristics of fluid shifts, consider what happens when solutions containing various amounts of NaCl are added to the ECF. d
d Fluids are usually administered intravenously. When electrolyte solutions are infused by this route, equilibration between plasma and interstitial fluid is rapid (i.e., minutes) because of the high permeability of many capillary walls for water and electrolytes. Thus these fluids are essentially added to the entire ECF.
Addition of an isotonic NaCl solution (e.g., intravenous infusion of 0.9% NaCl: osmolality ≈ 290 mOsm/kg H 2 O) e
e A 0.9% NaCl solution (0.9 g NaCl/100 mL) contains 154 mmol/L of NaCl. Because NaCl does not dissociate completely in solution (i.e., 1.88 Osm/mol), the osmolality of this solution is 290 mOsm/kg H 2 O, which is very similar to that of normal ECF.
to the ECF increases the volume of this compartment by the volume of fluid administered. Because this fluid has the same osmolality as does the ECF, and therefore the ICF, there is no driving force for fluid movement between these compartments, and the volume of the ICF remains unchanged. Although Na + can cross cell membranes, it is effectively restricted to the ECF by the activity of the Na + ,K + -ATPase, which is present in the plasma membrane of all cells (see the section “Ionic Composition of Cells”). Therefore, there is no net movement of the infused isotonic NaCl solution into cells.
Neurosurgical procedures and cerebrovascular accidents (strokes) often result in the accumulation of interstitial fluid in the brain (i.e., edema) and swelling of the neurons. Because the brain is enclosed within the skull, edema can raise intracranial pressure and thereby disrupt neuronal function, which leads to coma and death. The blood-brain barrier, which separates the cerebrospinal fluid and brain interstitial fluid from blood, can be permeated freely by water but not by most other substances. As a result, excess fluid in brain tissue can be removed by imposing an osmotic gradient across the blood-brain barrier. Mannitol can be used for this purpose. Mannitol is a sugar (molecular weight, 182 g/mol) that does not readily cross the blood-brain barrier and membranes of cells (neurons and other cells in the body). Therefore, mannitol is an effective osmole, and intravenous infusion results in the movement of interstitial fluid out of the brain by osmosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here