Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Histology is the study of the microscopic structure of biological material and the ways in which individual components are structurally and functionally related.
Histology is central to biological and medical sciences, standing at the crossroads between a range of disciplines.
Anatomy: sometimes referred to as microscopic anatomy or microanatomy, histology places gross anatomy into context, enabling the understanding of cell and tissue microstructure and how it relates to function.
Physiological and life sciences: physiology, pharmacology, cell biology and biochemistry, among others; histology links them together, providing a point of reference that is between gross anatomy and these disciplines and explains the structure underpinning function and treatment.
Pathology: knowledge of histology makes it easier to predict, understand and treat the functional deficits that are brought about by structural changes resulting from physiological adaptations or disease.
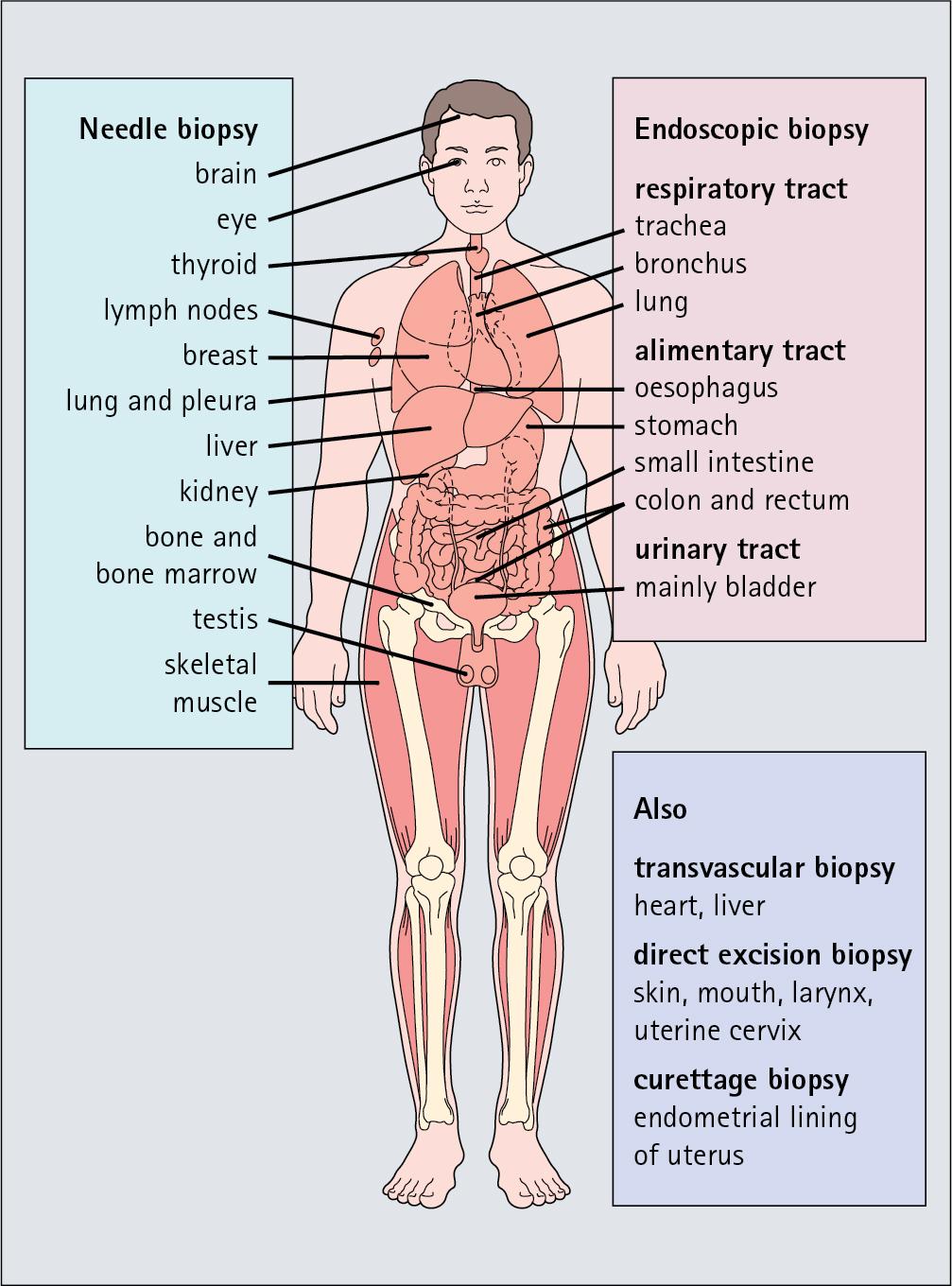
Knowledge of normal histological appearance is essential if abnormal or diseased structures are to be recognized and to understand how abnormal biochemical and physiological processes result in disease. Samples of human biological material can be obtained from many areas of the body by quick, safe biopsy techniques ( Fig. 1.1 ), using instruments such as:
Scalpels for directly accessible tissues such as the skin, mouth and nose.
Needles into solid organs.
Endoscopic tubes into the alimentary tract or body cavities.
Special flexible cannulae inside blood vessels.
In modern medicine, despite sophisticated imaging and genetic testing, a histological diagnosis is still the mainstay, or the ‘gold standard’, of clinical practice. This is illustrated via two case studies in Fig. 1.2 .
Histology is a dynamic and evolving science.
Modern investigative techniques have revolutionized our understanding of cells and tissues. The techniques of electron and confocal microscopy, cloning of cells in culture, protein sequencing and molecular genetics have also given unprecedented insight into the working of cells. In the past 10 years, the application of single-cell RNA sequencing (scRNA-seq) has allowed new cell types to be discovered in tissues and new functions to be ascribed to known cell types. This research has been translated into clinical practice, enabling significant advances in diagnostic pathology and in developing new and targeted treatment (for an example see Advanced Concept: Tissue Microarray, page 8).
These studies are giving new insights into normal tissue function and how these are altered in disease. An understanding of the dynamics of cell populations in the respiratory system and the kidney is stimulating research into new targets for treatment. In tumour biology, there is a shift to personalized medicine in the understanding that even similar-looking tumours are unique: two breast tumours can have different expression of oncogenes and markedly different receptor expression (see Fig. 18.27 ). Knowing the detailed cellular characteristics of a tumour enables targeted treatment of individuals in a bespoke manner, appropriate to their tumour biology, rather than treating all tumours of an organ as one disease. Knowledge of normal tissues, including through histology, is the starting point for wider applications in both research and diagnosis.
Some histological terminology persists from a time before cell and tissue function was understood.
The first microscopic investigations of biological material were carried out by Robert Hooke in his seminal publication Micrographia, just over 300 years ago. The study of histology began with the development of simple light microscopes and techniques for preparing thin slices of biological material to make them suitable for examination.
Such studies led Virchow to propound his cellular theory of the structure of living organisms that established the cell as the basic building block of most biological material. Each cell was considered an individual unit surrounded by a wall called the cell membrane and containing within it all the machinery for its function. Early microscopists had limited understanding of physiology and function and named features by their appearance or eponymously; for example, the microscopic connections running through bone and linking osteocyte cells are called canaliculi and the macrophage-like cells in the liver Küppfer cells. This can be frustrating, as there is a lot of vocabulary to learn and the system does not easily accommodate new information; however, it is slowly evolving.
The cell is the basic unit of structure of most living organisms, and cells vary considerably. Although all cells in the human body are ultimately derived from a single fertilized ovum, each cell develops structural attributes to suit its function through the process of differentiation. Cell biology has shown that cells of diverse morphological appearance can be grouped together because of common functional attributes or interactions.
The general structural and biological properties of cells are discussed in Chapter 2 .
Some cells are adaptable. It has also become apparent that even in adults, there are populations of highly adaptable, uncommitted stem cells, which can modify both their structure and their functional activity to adapt to changing environmental demands. This facility is of vital importance in adaptation to internal or external stress and is seen in normal and disease processes, for example, enlargement (hypertrophy) of a skeletal muscle cell in response to exercise, a reduction in size (atrophy) caused by inactivity or increasing numbers of cells (hyperplasia) in a gland because of excess stimulation. Some cells are constantly being replaced by this population of stem cells and are able to repopulate tissues after injury, for example, the outer layers of skin. Other cells cannot regenerate in a meaningful way; loss of such cells leads to the formation of a replacement tissue that fills the defect but is non-functional (e.g. replacement of damaged heart muscle by strong fibrous tissue after a heart attack).
Case Study 1 A 20-year-old student develops kidney failure, and the cause is not apparent from blood tests or imaging findings. The renal physician therefore removes a piece of kidney via a needle biopsy so that the diagnosis can be made by histological examination, immunohistochemical staining and electron microscopy. Special staining methods highlight subtle structural abnormalities by light microscopy (see Fig. 1.2 ). Electron microscopy provides valuable information about abnormalities at a subcellular level, and immunohistochemical staining allows the detection of immune complex deposition contributing to the pathogenesis of the disease. Based on the abnormalities that are revealed, an accurate histological diagnosis is determined and the renal physician can institute appropriate treatment. Clinical management of this patient’s condition requires knowledge of the microanatomy of the kidney. Assessment of progress and the effectiveness of treatment can be monitored by repeat biopsy.
Case Study 2 A 15-year-old girl has swollen lymph nodes in her neck. A surgeon removes one so that it can be examined histologically, and microscopy reveals that the swelling is caused by a form of cancer. The classification of tumours is determined by histology and immunohistochemistry. Accurate histological assessment of tumours is the cornerstone of modern cancer treatment, because the treatment given to this girl depends on the histological type of the tumour (i.e. whether it is derived from muscle, lymphoid cells or endocrine cells). Thus the pathologist’s report, which depends on the histological assessment of that specific tumour being accurate, will determine the type of chemotherapy deemed to be most efficacious for each cancer patient’s treatment protocol. Increasingly, an initial histological diagnosis is the gateway to further molecular analysis of the tumour, which can give prognostic or therapeutic information used in patient management.
Cells are classified according to their main function.
The following groupings are used in this book: epithelial cells, support cells, contractile cells, nerve cells, germ cells, blood cells, immune cells and hormone-secreting cells ( Fig. 1.3 ). It is important, however, to recognize that a cell may have several functions and be a member of more than one cell group. For example:
Many of the hormone-producing cells are also epithelial in type, being tightly bound together by specialized junctions to form a gland.
Many immune cells are also blood cells.
Some support cells are also contractile.
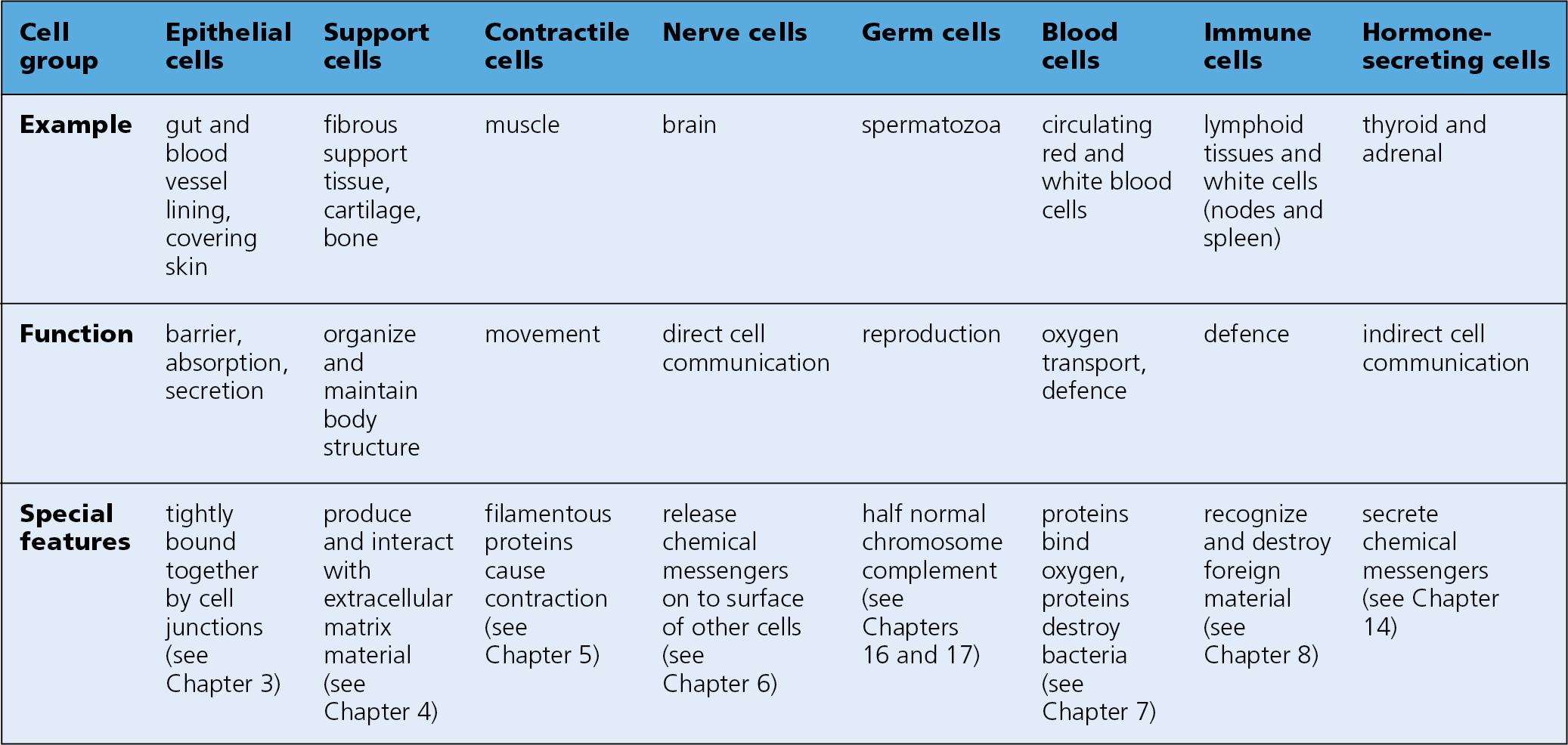
The structural and functional specializations delineating each type of cell group are broadly outlined in Chapter 3, Chapter 4, Chapter 5, Chapter 6, Chapter 7, Chapter 8 and are discussed in more detail throughout the book.
Tissues are functional arrangements of cells.
Tissues are discrete, organized collections of cells having similar morphological characteristics. These were traditionally subdivided into four types:
Epithelial tissues, or cells that cover surfaces, line body cavities or form solid glands such as salivary glands.
Muscular tissues, or cells with contractile properties.
Nervous tissues referred to cells forming the brain, spinal cord and nerves.
Connective tissue, a term used widely to describe a wide range of living material characterized by a dominant extracellular matrix component and the associated cells that produce the matrix. In theory, its function is to act as a supporting stroma, serving more highly specialized cell types.
The original group of ‘connective tissues’ included cell/matrix combinations, such as bone, cartilage, tendon, fibrous tissue, adipose tissue, bone marrow and blood. It has also been traditional to use the term loose areolar connective tissue to describe tissue that is partly made up of support cells that produce an extracellular matrix but which also contains cells belonging to the immune system (e.g. lymphocytes and macrophages), nerve cells and blood vessels.
In this book, the term connective tissue has been avoided because it underemphasizes the structural organization involved in this group of highly developed tissues. Instead, the concept of support cells is used, which emphasizes the importance of interactions between extracellular matrix and cells.
Support cells and their specializations are discussed in Chapter 4 , and bone, cartilage, tendons and ligaments are discussed in Chapter 13 .
In some cases, the cells forming a tissue are all of the same structure, forming simple tissues ; for example, fat cells forming adipose tissue. However, most apparently distinct tissues contain a mixture of cells with different functions, which may be termed compound tissues ( Fig. 1.4 ). For example, ‘nervous tissue’ contains nerve cells (neurons), support cells (astrocytes), immune cells (microglia) and epithelial cells (ependyma).
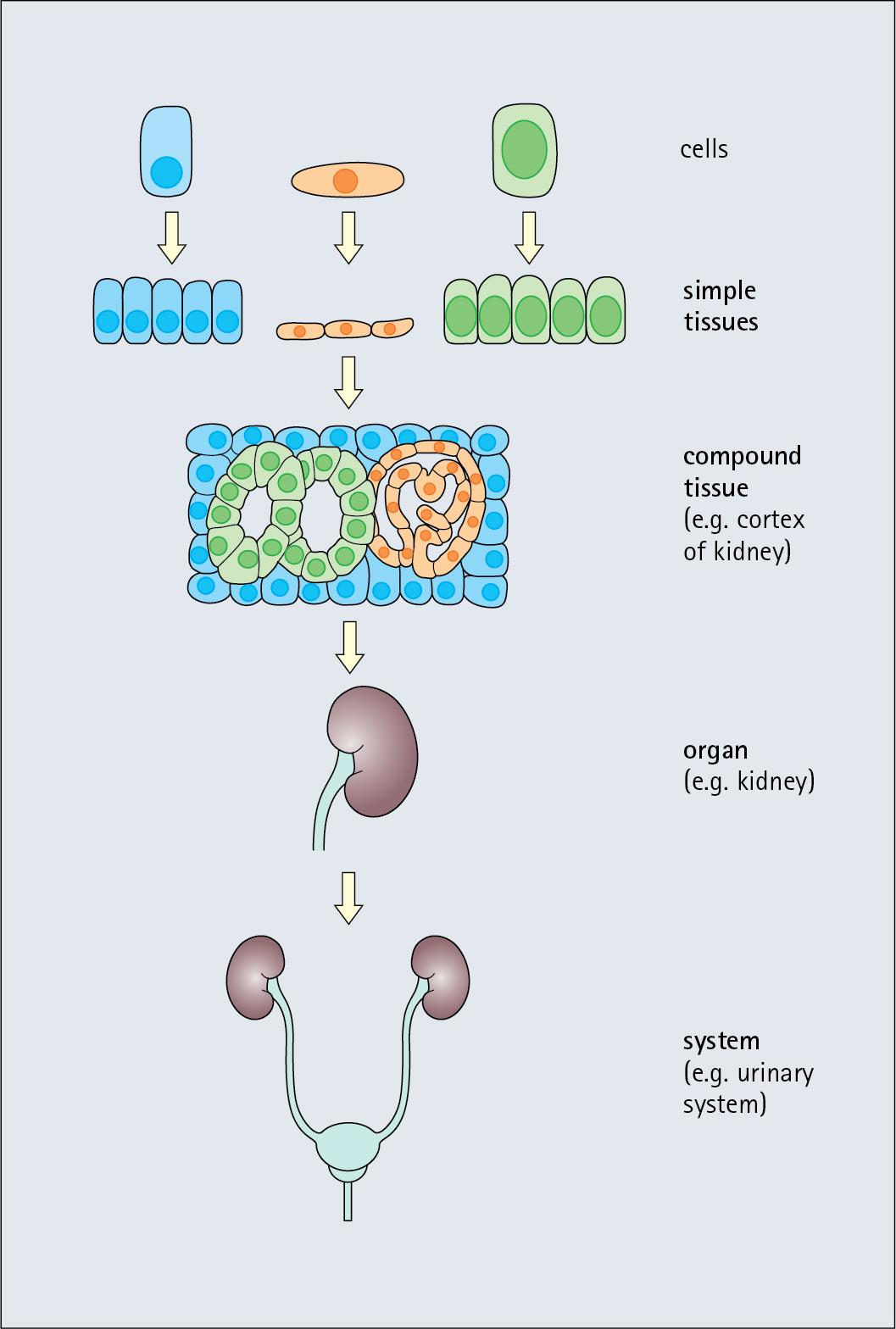
The concept of simple and compound tissues is useful in descriptive histology, but for brevity, the unqualified term tissue is used to imply either type.
Tissues form organs and systems.
An organ – for example, the heart, liver or kidney – is an anatomically distinct group of tissues, usually of several types, that perform specific functions. For example, in the organ skin, the tissue types involved are an outer epithelium – the epidermis – overlying the dermis, made of supporting connective tissue and containing the mechanical, immunological and nutrient support, and nerve endings, and housing the skin appendages. Deep to this is the subcutis, which is composed primarily of adipose tissue. In the intestines of the gastrointestinal (GI) tract, these organs are composed of layers of tissues from the lumen outward, including a lining epithelium, layers of underlying support tissue followed by external smooth muscle arranged to facilitate peristalsis. Peristalsis (the coordinated muscle contractions propelling food along the intestine) and glandular secretions are controlled by a range of nerve plexi.
The term system can be used to:
Describe cells with a similar function but widely distributed in several anatomical sites.
Describe a group of organs that have similar or related functional roles.
The specialized hormone-producing cells scattered in the gut and lung (diffuse endocrine system) cannot be an organ, as they do not form an anatomically distinct mass, whereas the tongue, oesophagus, stomach, intestines, exocrine pancreas and rectum are components of the ‘alimentary system’, and the kidney, pelvicalyceal system, ureters and bladder are part of the ‘urinary system’.
The relationships between cells, tissues, organs and systems are shown in Fig. 1.4 .
The most common way to study cells is by light microscopy. Tissues are mounted on glass slides as thin preparations, stained with appropriate dyes, illuminated by light and viewed using glass lenses. This is called histology , or histopathology if biopsy material from diseased tissue is being investigated. The analysis of the fine structure of cells – for example, blood cells or those obtained via cervical smear – by light microscopy is referred to as cytology .
There is a limit to the detail that can be resolved using light microscopy, with very small structures within cells being invisible. Electron microscopy uses a beam of electrons instead of light, which greatly increases resolution, allowing the subcellular composition of cells to be defined.
These techniques are complemented by the increasing use of immunohistochemical methods. Antibodies are applied to specific cell constituents to visualize details within cells at the light microscopic level that are not visible by other techniques. For example, the location of specific proteins or subcellular components can now be defined in light microscopic preparations by using immunohistochemical staining techniques. Complementary to this, immunofluorescence techniques enable molecules to be visualized using antibodies linked to fluorescent probes. Use of a fluorescence microscope with the appropriate filters to produce a wavelength of light that excites the fluorescently tagged antibody facilitates viewing of a range of dyes, and the advent of confocal microscopy has greatly enhanced the resolution possible with light microscopy ( Fig. 1.5 ) It is also possible to demonstrate specific DNA and RNA sequences by the technique of in situ hybridization, thereby gaining fundamental insight into the molecular working of cells.
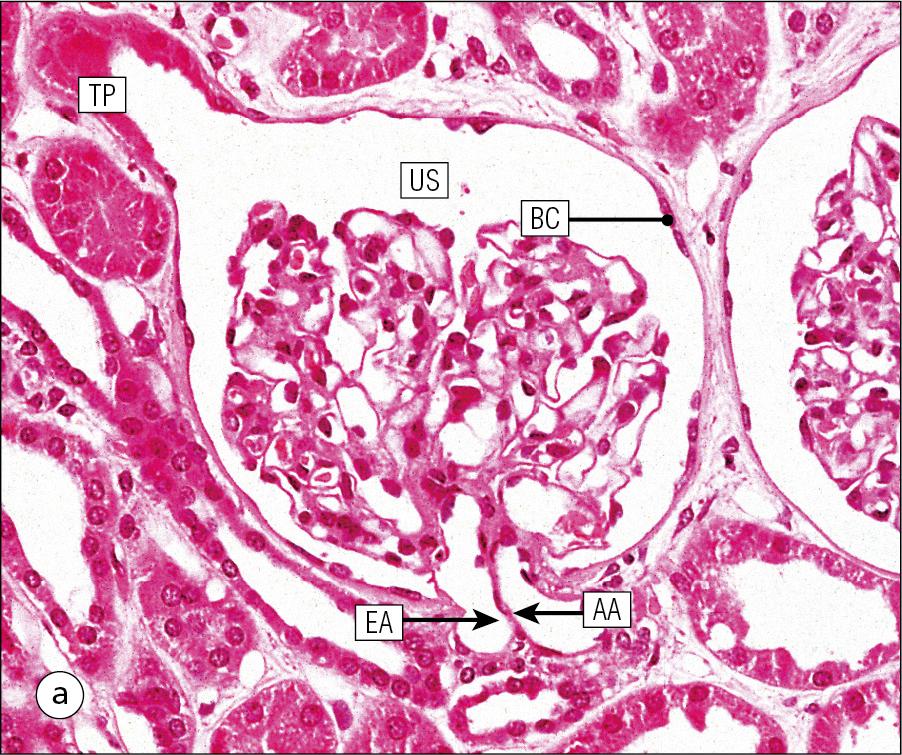
Light microscopy using wax-embedded sections is the main technique used in histology.
Routine light microscopy uses thin sections of tissue to study cell morphology. Resolution of structures by light microscopy is of the order of 0.2 μm, but in routine practice with paraffin sections, resolution seldom exceeds 0.6 μm. Sections are usually obtained from paraffin wax-impregnated tissues (see box on p. 6 for detail) using a cutting instrument called a microtome . Sections are mounted on a glass microscope slide for staining. In routine laboratory practice, it generally takes 24 hours to produce a wax section for histology.
In some cases (e.g. diagnostic biopsies), it is necessary to look at fresh tissues that have not been exposed to protein cross-linking in fixation. In this situation, tissue is made sufficiently firm to cut by freezing, a technique referred to as preparing a frozen section (see box on p. 6 for detail).
Paraffin embedding is the standard method of preparing thin sections of biological material for histological examination by light microscopy. It is cheap, comparatively simple and lends itself to automation.
The sample is preserved by immersion in a fixative, usually in an aqueous formalin-based fixative solution, which cross-links or precipitates proteins and prevents degradation of the tissue. It is then progressively dehydrated by passage through a series of alcohol solutions (e.g. 60%, 70%, 90% and 100%) until all water (intrinsic tissue water and fixative water) has been removed and the specimen is thoroughly permeated with absolute alcohol. The alcohol is then replaced by an organic solvent (e.g. xylene), which is miscible both with alcohol and with molten liquid paraffin wax (alcohol is not miscible with paraffin wax). The resulting specimen is immersed in paraffin wax at a temperature just above the melting point of the wax, which is solid at normal working room temperature. When the biological material is thoroughly permeated by the molten wax, it is allowed to cool so that the wax solidifies. The wax acts as a physical support to the sample, allowing thin sections (2–8 μm) to be cut using a microtome, without deformation of the cellular structure and architecture.
The process of fixation and embedding of biological material in paraffin and other media may destroy certain components, particularly enzymes, fats and some antigenic sites. If frozen water is used as the supporting medium, these components are better preserved and can be demonstrated by suitable techniques. Fresh (unfixed) material is rapidly frozen to −150°C to −170°C by immersion in, for example, liquid nitrogen, so that it hardens to a solid mass owing to freezing of tissue water. Thin sections (5–10 μm) are then cut on a special microtome housed in a refrigerated cabinet (a cryostat) and stained without exposure to alcohol or other organic solvents.
Frozen sections are used to demonstrate the cellular localization of enzymes and soluble lipids and in the identification of substances using immunofluorescence and immunocytochemical methods. There are obvious advantages to preparing frozen sections, including speed and retention of antigenicity, although without appropriate consideration, ice crystal damage can damage the tissue, thereby reducing resolution.
Further use is made of frozen sections in diagnostic histopathology, when an urgent tissue diagnosis is required of, for example a suspected tumour while the patient is still on the operating table. In skilled hands, a frozen section of a sample of human tissue stained with haematoxylin and eosin (H&E) can be prepared and examined under the microscope within 5 minutes of its removal from the body. In this way, a rapid and accurate histological diagnosis can be established while the patient is in the operating theatre, enabling the appropriate surgical room procedure to be performed.
To see tissue detail, it is necessary to stain the tissue components in a histological section.
Cells are virtually colourless, and thus sections need to be stained for light microscopy. To enable staining, the wax is removed from the sections with an organic solvent before the section is rehydrated through increasing dilutions of alcohol in water. When fully rehydrated, the sections are stained with any of a number of stains, some of which are listed here. There are four main types of staining:
Empirical
Histochemical
Enzyme histochemical
Immunohistochemical
Empirical stains are widely used and form the basis of most routine stains in histology and histopathology.
Many of the stains have been discovered by trial and error over a period of 100 years or more, and many methods use dyes and principles (e.g. use of mordants to fix a dye in a material) that were developed by the textile industry. In many cases, the precise details of the mechanism of the specific linkage between dye and tissue is not fully understood: sometimes it appears to be related to the sizes of the dye molecules used and sometimes the result of ionic charges on the dye molecules. The most common empirical stain is haematoxylin and eosin (H&E), a simple, reliable and inexpensive method of producing sections with blue nuclei and pink/red cytoplasm. Most of the micrographs in this book are stained with H&E. Other empirical stains include van Gieson’s and trichrome methods (see Advanced Concept box on p. 7).
In histochemical methods, specific chemical compounds within the tissue can be localized.
In some cases, staining is the result of a specific chemical reaction between a tissue component and a component of the stain solution; these methods are called histochemical methods. A commonly used example of a simple histochemical staining method is the periodic acid–Schiff (PAS) reaction, which demonstrates a wide range of tissue carbohydrates, including cytoplasmic glycogen and complex carbohydrate–containing substances, such as epithelial mucins. The rationale of the method is that carbon-to-carbon bonds in 1,2-glycols are cleaved using an oxidative agent, periodic acid. This produces dialdehydes, which then react with the colourless Schiff’s reagent (fuchsin–sulphurous acid) to produce a vivid magenta-coloured compound.
The combination of the two dyes, haematoxylin (blue) and eosin (red), is the most useful stain for the examination of biological material; the staining is simple to perform, reliable, inexpensive and informative. Cell nuclei stain blue (depending on section thickness and the formulation of haematoxylin used), and most components of the cell cytoplasm stain pink/red.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here