Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
High-grade prostatic intraepithelial neoplasia (HGPIN) and atypical small acinar proliferation (ASAP) are intermediaries to distinctly benign or malignant diagnoses on prostate biopsy. HGPIN is a true pathologic entity and the only accepted precursor to prostate adenocarcinoma. ASAP on the other hand is not a defined pathologic entity, but instead represents diagnostic ambiguity with atypical findings suspicious but not sufficient for a diagnosis of malignancy. Given this distinction in pathologic classification, the management for ASAP has remained consistent over time while the management of HGPIN has changed over the past decade and a half due to evolving practice patterns with regards to PSA (prostate-specific antigen) screening and biopsy strategies, each of which influence the detection rates of HGPIN and prostate cancer. The following chapter reviews these two entities – their pathologic features, their prediction of subsequent cancer, and their current recommended management.
HGPIN is currently the only accepted precursor to prostate adenocarcinoma. It was initially described by John McNeal in 1969 by the term “intraductal dysplasia of the prostate,” but did not become widely accepted until its morphologic criteria were more precisely characterized by McNeal and David Bostwick in 1986. Initial PIN classification occurred on a scale of I–III; however, this was simplified to differentiation into low (I) and high (II, III) grades only. Low-grade PIN (LGPIN) is no longer reported by pathologists because of a lack of interobserver reproducibility due to its similarity to benign prostate, as well as because of its lack of clinical significance. In contrast, moderate to good interobserver agreement for the diagnosis of HGPIN has been demonstrated, particularly among genitourinary pathologists.
Historically, isolated HGPIN on sextant prostate biopsy was associated with high rates of concurrent prostate cancer and thus the recommendation for patients with this finding was for immediate repeat biopsy. Controversy regarding this guideline has evolved over the past two decades as initial biopsy strategies have increased sampling of the prostate through extended core biopsy schemes. The resulting identification of smaller foci of cancer on initial biopsy likely has contributed to decreased rates of cancer detection on repeat biopsy after HGPIN.
Autopsy and cystoprostatectomy series best estimate true HGPIN incidence, and in these studies the incidence ranges from 45% to 85%. Incidence varies based on age and ethnicity. PIN incidence increases with age. Sakr et al. reported a 9 and 20% incidence of LGPIN in the third and fourth decades of life, with HGPIN first found in the fifth decade. PIN in this study preceded the development of cancer by approximately 10 years. The frequency of HGPIN diagnosis by decade has been estimated to be 15, 24, 47, 58, and 70% in the fifth through ninth decades, respectively. When considering race, African-American men have been shown to have an increased frequency of HGPIN decade for decade in comparison to Caucasian men, similar to trends seen with prostate adenocarcinoma. Sakr et al. in a separate study examining 370 prostates from autopsy, 60% of which were from African-Americans, found HGPIN in the African-American cohort in 18, 31, 69, 78, and 86% in the fourth through eighth decades, respectively, compared to 14, 21, 38, 50, and 63% in prostates from Caucasian patients. This study also found that in patients under 60 years old, a higher proportion of African-American men were found to have extensive HGPIN on radical prostatectomy specimen when compared to Caucasian men (57% vs. 33%).
The incidence of HGPIN found on needle biopsy is more clinically relevant information but it underestimates the true incidence given sampling error. The incidence of HGPIN on needle biopsy reported in the literature is highly variable, ranging from 1.7% to 45% ( Table 6.1 ). Reported mean incidence is between 4% and 9%. Multiple potential explanations for this wide variation exist. Biopsy strategy may influence the frequency of a diagnosis of HGPIN, with saturation biopsies associated with higher rates of detection. Schoenfield et al. demonstrated a 22% incidence of HGPIN with initial 24-core saturation biopsy. Lane et al. found that with an initial saturation biopsy with a mean of 24 cores (range 20–33) the incidence of HGPIN was 45%. Differences in the composition of the study cohort also results in variation of HGPIN incidence. Bostwick and Cheng observed that lower likelihood of HGPIN diagnosis was seen in PSA screening populations when compared with studies reported from a urology practice. Finally, differences in pathologic processing and interpretation may also account for some of the variability. Use of nonstandard fixatives may enhance nuclear and nucleolar features resulting in higher rates of diagnosis whereas suboptimal thick sectioning may obscure nuclear detail and result in the opposite. A lack of standardized criteria regarding degree and extent of nucleolar enhancement and enlargement leaves room for subjective interpretation. While interobserver agreement is acceptably high among urologic pathologists, there is some decline in reproducibility with general pathologists. Laurila et al. in examining the diagnosis of HGPIN in the ERSPC cohort found significant country-to-country variability (incidence 0.8–7.6%) which the authors suggested was a result of interobserver variation given the lack of centralized pathologic review.
| Study | Year | N | Core number | HGPIN incidence (%) | ASAP incidence (%) |
|---|---|---|---|---|---|
| Kamoi et al. | 2000 | 611 | Not reported | 10.0 | 1.0 |
| O’dowd et al. | 2000 | 132,426 | Not reported | 3.7 | 2.5 |
| Borboroglu et al. | 2001 | 1,391 | Mean 7.7 ± 2.2 | 6.1 | 4.4 |
| Ouyang et al. | 2001 | 331 | Not reported | – | 6.3 |
| Iczkowski et al. | 2002 | 7,081 | Not reported | – | 2.6 |
| Lefkowitz et al. | 2002 | 1,223 | 12 | 9.7 | – |
| Abdel-Khalek et al. | 2004 | 3,081 | 6 | 2.7 | – |
| Brausi et al. | 2004 | 1,327 | Mean 9.5 (range 8–14) | 5.4 | |
| Naya et al. | 2004 | 1,086 | Not reported | 8.7 | 1.9 |
| Moore et al. | 2005 | 1,188 | ≥10 | 2.5 | 6.0 |
| Scattoni et al. | 2005 | 3,350 | 10–12 | – | 3.8 |
| Tan et al. | 2006 | 1,219 | 10 (range 6–18) | 4.6 | – |
| Amin et al. | 2007 | 2,265 | Median 10 (range 6–14) | 23.7 | 2.5 |
| Lopez | 2007 | 4,770 | 6–8 | 2.6 | 0.9 |
| Mancuso et al. | 2007 | 1,632 | Not reported | 6.2 | 1.9 |
| Schoenfield et al. | 2007 | 100 | 24 | 22.0 | 5.0 |
| Gallo et al. | 2008 | 1,223 | Mean 16.3 (range 12–20) | 11.4 | – |
| Lane et al. | 2008 | 257 | Median 24 (range 20–33) | 45.0 | 15.0 |
| De Nunzio et al. | 2009 | 650 | 12 | 22.0 | – |
| Merrimen et al. | 2009 | 12,304 | Not reported | 10.4 | 2.7 |
| Ploussard et al. | 2009 | 2,006 | 21 | 1.7 | 1.1 |
| Singh et al. | 2009 | 2,087 | Median 12 cores (range 8–14) | 4.2 | – |
| Laurila et al. | 2010 | 17,884 (HGPIN); 21,043 (ASAP) | Mostly 6, Finland 10–12 for >2002 | 2.8 | 2.4 |
| Lee et al. | 2010 | not reported | Not reported | 18.2 | – |
| Ryu et al. | 2010 | 3,130 | 6 cores if volume <30 bcc; 12 cores if ≥30 cm 3 | – | 7.8 |
| Koca et al. | 2011 | 2,433 | Mean 10.8 (range 8–12) | 4.7 | 2.4 |
| Garcia-Cruz et al. | 2012 | 1,000 | Not reported | 8.2 | – |
| El Shafei et al. | 2013 | 682 | 8–14 | 43.6 | 13.2 |
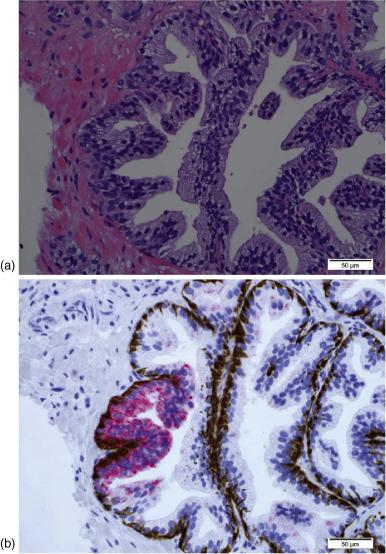
HGPIN is characterized by epithelial cell proliferation within architecturally normal prostate glands and ducts, accompanied by cellular changes that mimic cancer. These cytologic changes are primarily nuclear and include nuclear enlargement and hyperchromasia, as well as prominent nucleoli typically visible at 20× magnification. Similar to other types of malignancies, reversal of proliferative orientation is seen in HGPIN as growth at the luminal surface predominates, in contrast to proliferation in normal glands, which occurs at the level of the basal cells. As a premalignant lesion, HGPIN lacks invasion of the stroma, maintaining a basal cell layer, which may be continuous or disjointed. Using basal-cell-specific stains detecting the presence of P63, or high molecular weight cytokeratin via the 34βE12 antibody allows for differentiation of HGPIN from adenocarcinoma, which lacks a basal cell layer ( Figure 6.1 ).
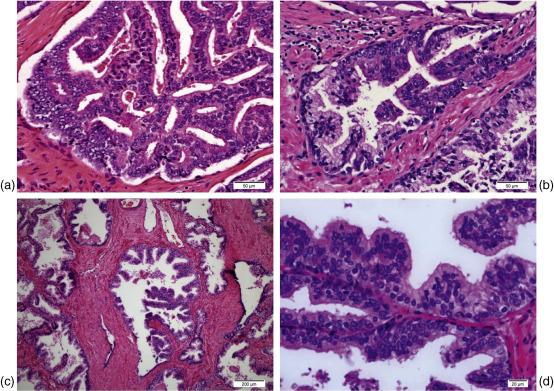
There are four main histologic subtypes of HGPIN: tufting, micropapillary, cribriform, and flat ( Figure 6.2 ). Typically multiple subtypes are present in a single case. The tufting pattern is the most common (in up to 97% of cases), resulting from variable degrees of hyperplasia within prostatic acini. Elongation of hyperplastic columns results in the micropapillary pattern. Flat HGPIN consists of a single layer of atypical cells. The cribriform variant must be distinguished from intraductal carcinoma of the prostate, which may be similar in appearance but is associated with high-grade, high-volume, infiltrative tumors. Rarer HGPIN variants described in the literature include signet ring cell, small cell neuroendocrine, mucinous, foamy gland (microvacuolated), hobnail (inverted), squamous, and desquamating apoptotic patterns Multiple studies have demonstrated a lack of variation in the degree of risk of subsequent diagnosis of prostate cancer based on the different histologic subtypes. Only one study has suggested that HGPIN variants may be used for risk stratification. Kronz et al. demonstrated that in patients requiring multiple biopsies for the diagnosis of cancer after an initial HGPIN diagnosis, predominant micropapillary or cribriform histology were associated with a higher rate of cancer on follow-up than predominant flat or tufting HGPIN (58.3% vs. 16.7%, p = 0.002). This study included a small number of patients however ( n = 144) and only 17% of them had predominant micropapillary/cribriform HGPIN.
Histologic evidence for HGPIN representing a premalignant state arises from its similarities to, and close association with, prostate cancer. HGPIN is frequently multifocal, predominantly located in the peripheral zone (75–80%), and less often found in the transitional zone (10–15%) and central zone (<5%), mirroring prostate cancer. Increasing grade of HGPIN has been shown to be associated with increasing fragmentation of the basal cell layer, suggesting a mechanism for early carcinogenesis via stromal invasion through basal cell layer disruptions. In prostatectomy and cystoprostatectomy series, HGPIN has been found in continuity with adenocarcinoma 60–100% of the time and its volume has been shown to increase with increasing Gleason grade and pathologic stage of disease. Interestingly, HGPIN volume has been shown in some studies to demonstrate an inverse relationship to tumor volume (i.e., glands with large tumors are associated with small amounts of HGPIN), which suggests that areas previously occupied by HGPIN may be replaced with adenocarcinoma. Direct conclusions based solely on histologic evaluation, however, are limited, given the static nature of the technique.
Further evidence for the clonal progression of HGPIN to prostate cancer is provided by the genetic and molecular similarities found between the two. Cytogenetic anomalies shared by HGPIN and prostate cancer include telomere shortening, rate of allelic imbalances, allelic loss of 8p12-21, c-myc amplification, loss of heterozygosity at chromosomes 6 and 8, and gain of chromosomes 7, 8, 10, and 12. Similar epigenetic changes between HGPIN and prostate cancer include hypermethylation of tumor suppressor genes APC and RARB2 , and higher global methylation rates when compared to benign prostatic tissue. Additional select shared molecular abnormalities include overexpression of p16, c-Met, and alpha-methylacyl-CoA racemase, as well as reduction of annexin I, and the presence of the TMPRSS2–ERG gene fusion. Together, these similarities in molecular and genetic derangements represent shared pathways leading to proliferative deregulation and development of an invasive phenotype via genetic instability supporting the model of progression from HGPIN to prostate adenocarcinoma.
Given HGPIN is a premalignant lesion, its ability to predict the presence of cancer as well as the potential for the development of cancer is of paramount clinical interest. The predictive value of isolated HGPIN is influenced by the extent of sampling on initial biopsy and the period of time to repeat biopsy. Cancer detection on immediate repeat biopsy likely indicates the presence of occult cancer missed at the time of the initial biopsy as opposed to development of cancer in the short interim between biopsies given on autopsy studies prostate cancer development occurs 5–10 years after initial diagnosis of PIN. A summary of recent studies examining cancer detection rates after an initial biopsy with HGPIN is provided in Table 6.2 . While sextant biopsy strategies were primarily being employed, increased sampling error resulted in prostate cancer detection rates of 22–79% on immediate repeat biopsy, with mean incidence around 30%, and thus the recommendation was for short interval repeat biopsy in patients with isolated HGPIN. With increasing utilization of extended core biopsies and the detection of smaller foci of prostate cancer initially, the rate of cancer detection on immediate repeat biopsy appears to have significantly decreased. Lefkowitz et al. demonstrated that after an extended 12-core biopsy the risk of cancer detection on biopsy done within a year occurred at a rate of 2.3%. Similarly, Moore et al. found that prostate cancer risk was just 4.5% when repeat biopsy was performed within 15 weeks of an initial biopsy involving greater than or equal to 10 cores. A study by Herawi et al. further highlights the effect of core number on the risk of prostate cancer within a year of HGPIN diagnosis. When six-core biopsy was used initially, the risk of cancer on repeat biopsy was significantly higher than eight or more cores that were obtained initially (20.8% vs. 13.3%, p = 0.01). In patients with an initial six-core biopsy, cancer detection rates after repeat biopsy with six cores and eight or more cores was 14.1 and 31.9%, respectively. In contrast, extended biopsy followed by a repeat extended biopsy resulted in only a 14.6% cancer detection rate within 1 year.
| Study | Year | N | Cores on initial biopsy | Cores on repeat biopsy | Time to rebiopsy | Overall CaP (prostate cancer) rate (%) | CaP first rebiopsy (%) | Time to second biopsy | CaP second rebiopsy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kamoi et al. | 1999 | 45 | 6 | Not reported | Within 1 year | 22.0 | 22.0 | – | – |
| O’dowd et al. | 2000 | 1306 | For all initial bx, ≤8 in 99.5% | For all repeat bx, ≤8 in 99.1% | <12 month | 22.6 | 22.6 | – | – |
| Borboroglu et al. | 2001 | 45 | Mean 7.7 ± 2.2 | Not reported | Mean 3.9 month | 44.0 | 44.0 | – | – |
| Kronz et al. | 2001 | 245 | Not reported | Not reported | Median 5.3 month | 32.2 | 24.5 | – | – |
| Lefkowitz et al. | 2001 | 43 | 12 | 12 | Within 1 year | 2.3 | 2.3 | – | – |
| Stewart et al. | 2001 | 64 | 6 | Mean 23 (range 14–45) | Median 2.4 year from initial to saturation bx | 30.7 | – | – | – |
| Lefkowitz et al. | 2002 | 31 | 12 | Not reported | 3 year | 25.8 | 25.8 | – | – |
| Abdel-Khalek et al. | 2004 | 83 | 6 | 11 | Not reported | 43.4 | 36.0 | Not reported | 19.0 |
| Bishara et al. | 2004 | 132 | 6 in 60% (more in rest) | 6 in 61% (more in rest) | Mean 7 month (range 1–33) | 28.8 | 20.5 | Not reported | 17.9 |
| Naya et al. | 2004 | 47 | Not reported | Not reported | Not reported | 10.6 | 10.6 | – | – |
| Gokden et al. | 2005 | 190 | Mean 6.6 (range 5–14) | Not reported | Not reported | 30.5 | 13.2 | Not reported | 13.7 |
| Moore et al. | 2005 | 22 | ≥10 | ≥10 | 15 weeks | 4.5 | 4.5 | Not reported | 0.0 |
| Schlesinger et al. | 2005 | 204 | At least 6, most 8–10 | Mean 9.9 | 6 month | 23.0 | 23.0 | – | – |
| Herawi et al. | 2006 | 791 | 332 with 6, 323 with ≥8 | 345 with 6–7, 426 with ≥8 | Mean 4.6 month | 17.6 | 17.6 | – | – |
| Netto et al. | 2006 | 41 | 10.6 (range 5–16) | 10.4 (range 6–16) | 11 month (range 1–41 month) | 39.0 | 39.0 | – | – |
| Tan et al. | 2006 | 29 | Median 10 (range 6–18) | Not reported | Mean 9 month (range 1–60 month) | 24.1 | 13.8 | – | – |
| Amin et al. | 2007 | 201 | 10 | Median 10 | Not reported | 21.9 | 15.9 | Not reported | 13.6 |
| Loeb et al. | 2007 | 96 | ≥6 in 91% | Not reported | 8 month (range 0–51 month) | 57.3 | 48.0 | – | – |
| Lopez JI | 2007 | 125 | 6–8 | 10–12 | Not reported | 16.8 | Not reported | – | – |
| Gallo et al. | 2008 | 65 | 12–20 | 12–20 | 3–12 month | 21.5 | 1.6 | 13–24 month | 11.6 |
| Merrimen et al. | 2009 | 564 | Not reported | Not reported | 0.91 year | 27.5 | – | – | – |
| Ploussard et al. | 2009 | 2006 | 21 | 21 | Mean 12.4 month (range 2–66) | 19.0 | 19.0 | – | – |
| Singh et al. | 2009 | 67 | Median 12 (range 8–14) | Mean 11 | Median 13.5 month | 41.8 | 35.8 | – | – |
| Laurilaet al. | 2010 | 626 | Mostly 6, Finland 10–12 far >2002 | Mostly 6, Finland 10–12 for >2002 | Within 6 weeks | 12.9 | Not reported | – | – |
| Lee et al. | 2010 | 328 | Mean 11.9 (range 6–26) | Mean 15.1 (range 6–32) | 1.31 year (±1.25 year) | 35.7 | 35.7 | – | – |
| Mitterberger et al. | 2010 | 104 | Not reported | Systematic 10 cores + targeted cores to hypervascular region (up to 5) | Within 6 months | 25.0 | 25.0 | – | – |
| Antonelli et al. | 2011 | 546 | 10.8 (range 6–23) | Not reported | Mean 7.8 month | 31.8 | 21.2 | – | – |
| Fleshner et al. | 2011 | 310 | Not reported | Not reported | 6, 12, 24, 36 month | 26.4 | – | – | – |
| Godoy et al. | 2011 | 112 | Not reported | Not reported | Median 34.4 month | 32.1 | 22.3 | 66.2 month from initial | 23.4 |
| Hailemariam et al. | 2011 | 66 | Not reported | Median 6 | Median 3 month (range 0.5–68) | 33.3 | 30.0 | 4 month from second bx (range 1–32) | 20.0 |
| Koca et al. | 2011 | 40 | Mean 10.8 (range 8–12) | Mean 10.8 (range 8–12) | Not reported | 0.0 | 0.0 | Not reported | 0.0 |
| Marshall et al. | 2011 | 269 | 10.5 placebo, 10.7 selenium | 9.7 placebo, 10.6 selenium | Not reported | 36.1 | – | – | – |
| Garcia-Cruz et al. | 2012 | 45 | 10 | 10 | 3–6 month | 22.2 | 22.2 | – | – |
| He et al. | 2012 | 94 | Mean 12 (range 6–20) | Not reported | Not reported | 38.3 | – | – | – |
| Roscigno et al. | 2012 | 262 | Median 12 (range 6–24) | Mean 22 (range 20–26) | Median 12 month (range 3–30) | 36.3 | 31.7 | Mean 14.5 month from last (range 3–29) | 12.9 |
| De Nunzio et al. | 2013 | 117 | 12 | 12 | 6 month | 31.6 | 18.8 | 6 months from last biopsy | 35.0 |
| El Shafei et al. | 2013 | 297 | 8–14 | Mean 15.7 (std deviation 4.6) | Mean 1.92 year | 32.7 | 32.7 | – | – |
When examining all studies with mean time to repeat biopsy of less than a year after an initial extended core biopsy (≥10 cores), observed cancer detection rates range from 2.3% to 39.0%. The majority of these studies demonstrated cancer detection rates of less than 23%, which is comparable to the average rate of 19% after benign biopsy reported by Epstein and Herawi. The two studies with over 30% cancer detection included patients who had repeat biopsies up to 3–4 years later, and one study included patients with widespread HGPIN only, and thus these studies likely overestimate actual short-term cancer risk.
While mandated short-term interval biopsy for isolated HGPIN after an extended core biopsy appears to be unnecessary based on these results, optimal long-term biopsy strategy is yet to be determined due to a dearth of data examining long-term risk. Overall incidence of prostate cancer in studies examining biopsies performed at a mean of greater than 1 year from initial biopsy ranges from 19% to 41.8%. Lefkowitz et al. found that on interval biopsy at 3 years after initial diagnosis of HGPIN, 25.8% of men had prostate cancer. A follow up study by the same group demonstrated a persistently elevated risk of prostate cancer 6 years after initial biopsy in those with a negative 3-year biopsy (positive in 23.4%), without significant differences found in PSA levels between benign and malignant biopsies. This led the authors to recommend that empiric biopsy be performed every 2–3 years after diagnosis of HGPIN. However, these findings are not universal. In a study by Gallo et al., overall risk of prostate cancer after a diagnosis of HGPIN on extended core biopsy (12–20 cores) was 21.5%, and diagnosis at less than 1, 1–2, 2–3, and 3–4 years after initial biopsy was only 1.6, 11.6, 5.7, and 6.1%, respectively.
Another consideration with regards to the utility and timing of long-term biopsy strategies after HGPIN diagnosis is the characteristics of cancer that is diagnosed. While bias exists given the increased screening these men undergo, it is important to note that for the most part cancer diagnosed after an initial biopsy with HGPIN demonstrates favorable characteristics. Based on radical prostatectomy specimens, Al-Hussain and Epstein demonstrated that patients with prior HGPIN diagnosis had small tumors (mean 0.3 mL), which were more often organ-confined in comparison to those diagnosed without a prior diagnosis of HGPIN (84% vs. 65%, p = 0.0007). Eminaga et al. found on examination of radical prostatectomy specimens that cancer associated with HGPIN was smaller, lower grade, and less advanced stage-wise than cancer not associated with HGPIN. Balaji et al. found no association between HGPIN diagnosis on biopsy and subsequent biochemical recurrence after radical prostatectomy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here