Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nearly 1 million hernias are repaired annually in the United States, making hernia repair one of the most common operations performed by general surgeons. Despite the frequency of this procedure, no surgeon has ideal results, and complications such as postoperative pain, nerve injury, and surgical site infection remain. The chance of recurrence, especially after ventral hernia repair, generally increases with time, suggesting that hernia is a chronic disease process affecting patients over their lifetime.
General surgeons care for many diseases affecting the abdominal wall, including intrinsic diseases (hernia, diastasis, athletic pubalgia/core muscle injury, benign and malignant tumors) and extrinsic diseases (prosthetic- and intervention-related complications, benign and malignant tumors). The field of Abdominal Core Health has been recognized as encompassing the stability, function, and quality of life involving the abdominal core. The abdominal core is defined as the circumferential soft tissues of the diaphragm superiorly, the pelvic floor inferiorly, and the abdominal wall and flank anterolaterally, excluding the abdominopelvic viscera. Maintenance of abdominal core health may include exercise, physical therapy, medical therapy (including compression garment or truss use), alternative medical therapies (including acupuncture or yoga), surgical intervention, and measures to prevent disease (e.g., hernia prophylaxis). Rebranding this area of surgery is reflective of the care actually provided to patients and may provide new avenues for clinical care and research that are badly needed to help determine optimal treatments and to identify methods of prevention. The management of hernia is an integral component of maintaining abdominal core health.
Hernia is derived from the Latin word for rupture. A hernia is defined as an abnormal protrusion of an organ or tissue through a defect in its surrounding walls. Although a hernia can occur at various sites of the body, these defects most commonly involve the abdominal wall, particularly the inguinal region. Abdominal wall hernias occur only at sites at which the aponeurosis and fascia are not covered by striated muscle ( Box 45.1 ). These sites most commonly include the inguinal, femoral, and umbilical areas; linea alba; lower portion of the semilunar line; and sites of prior incisions ( Fig. 45.1 ). The so-called neck or orifice of a hernia is located at the innermost musculoaponeurotic layer, whereas the hernia sac is lined by peritoneum and protrudes from the neck. There is no consistent relationship between the area of a hernia defect and the size of a hernia sac.
A hernia is reducible when its contents can be replaced within the surrounding musculature, and it is irreducible or incarcerated when it cannot be reduced. A strangulated hernia has compromised blood supply to its contents, which is a serious and potentially fatal complication. Strangulation occurs more often in large hernias that have small orifices. In this situation, the small neck of the hernia obstructs arterial blood flow, venous drainage, or both to the contents of the hernia sac. Adhesions between the contents of the hernia and peritoneal lining of the sac can provide a tethering point that entraps the hernia contents and predisposes to intestinal obstruction and strangulation. A more unusual type of strangulation is a Richter hernia. In a Richter hernia, a small portion of the antimesenteric wall of the intestine is trapped within the hernia, and strangulation can occur without the presence of intestinal obstruction.
An external hernia protrudes through all layers of the abdominal wall, whereas an internal hernia is a protrusion of intestine through a defect in the peritoneal cavity. An interparietal hernia occurs when the hernia sac is contained within a musculoaponeurotic layer of the abdominal wall. In broad terms, most abdominal wall hernias can be separated into inguinal and ventral hernias. This chapter focuses on the specific aspects of each of these conditions individually.
Inguinal hernias are classified as direct or indirect. The sac of an indirect inguinal hernia passes from the internal inguinal ring obliquely toward the external inguinal ring and ultimately into the scrotum. In contrast, the sac of a direct inguinal hernia protrudes outward and forward and is medial to the internal inguinal ring and inferior epigastric vessels. As indirect hernias enlarge, it sometimes can be difficult to distinguish between indirect and direct inguinal hernias. This distinction is of little importance because the operative repair of these types of hernias is similar. A pantaloon-type hernia occurs when there is both an indirect and a direct hernia component.
Hernias are a common problem; however, their true incidence is unknown. It is estimated that 5% of the population will develop an abdominal wall hernia, but the prevalence may be even higher. About 75% of all hernias occur in the inguinal region. Two-thirds of these are indirect and the remainder are direct inguinal hernias. Femoral hernias represent only 3% of all groin hernias.
Men are 25 times more likely to have a groin hernia than women. An indirect inguinal hernia is the most common hernia, regardless of gender. In men, indirect hernias predominate over direct hernias at a ratio of 2:1. Indirect hernias are by far the most common type of hernia in women. The female-to-male ratio for femoral and umbilical hernias, however, is about 10:1 and 2:1, respectively. Although femoral hernias occur more frequently in women than in men, inguinal hernias remain the most common hernia in women. Femoral hernias are rare in men. Ten percent of women and 50% of men who have a femoral hernia have or will develop an inguinal hernia.
Indirect inguinal and femoral hernias occur more commonly on the right side. This is attributed to a delay in atrophy of the processus vaginalis after the normal slower descent of the right testis to the scrotum during fetal development. The predominance of right-sided femoral hernias is thought to be caused by the tamponading effect of the sigmoid colon on the left femoral canal.
The prevalence of hernias increases with age, particularly for inguinal, umbilical, and femoral hernias. The likelihood of strangulation and need for hospitalization also increase with aging. Strangulation, the most common serious complication of a hernia, occurs in only 1% to 3% of groin hernias and is more common at the extremes of life. Most strangulated hernias are indirect inguinal hernias; however, femoral hernias have the highest rate of strangulation (15%–20%) of all hernias, and it is therefore recommended that all femoral hernias be repaired at the time of discovery.
The surgeon must have a comprehensive understanding of the anatomy of the groin to select and to use various options for hernia repair properly. In addition, the relationships of muscles, aponeuroses, fascia, nerves, blood vessels, and spermatic cord structures in the inguinal region must be completely understood to obtain the lowest incidence of recurrence and to avoid complications. These anatomic considerations must be understood from the anterior and posterior approaches because both are useful in different situations ( Figs. 45.2 and 45.3 ).
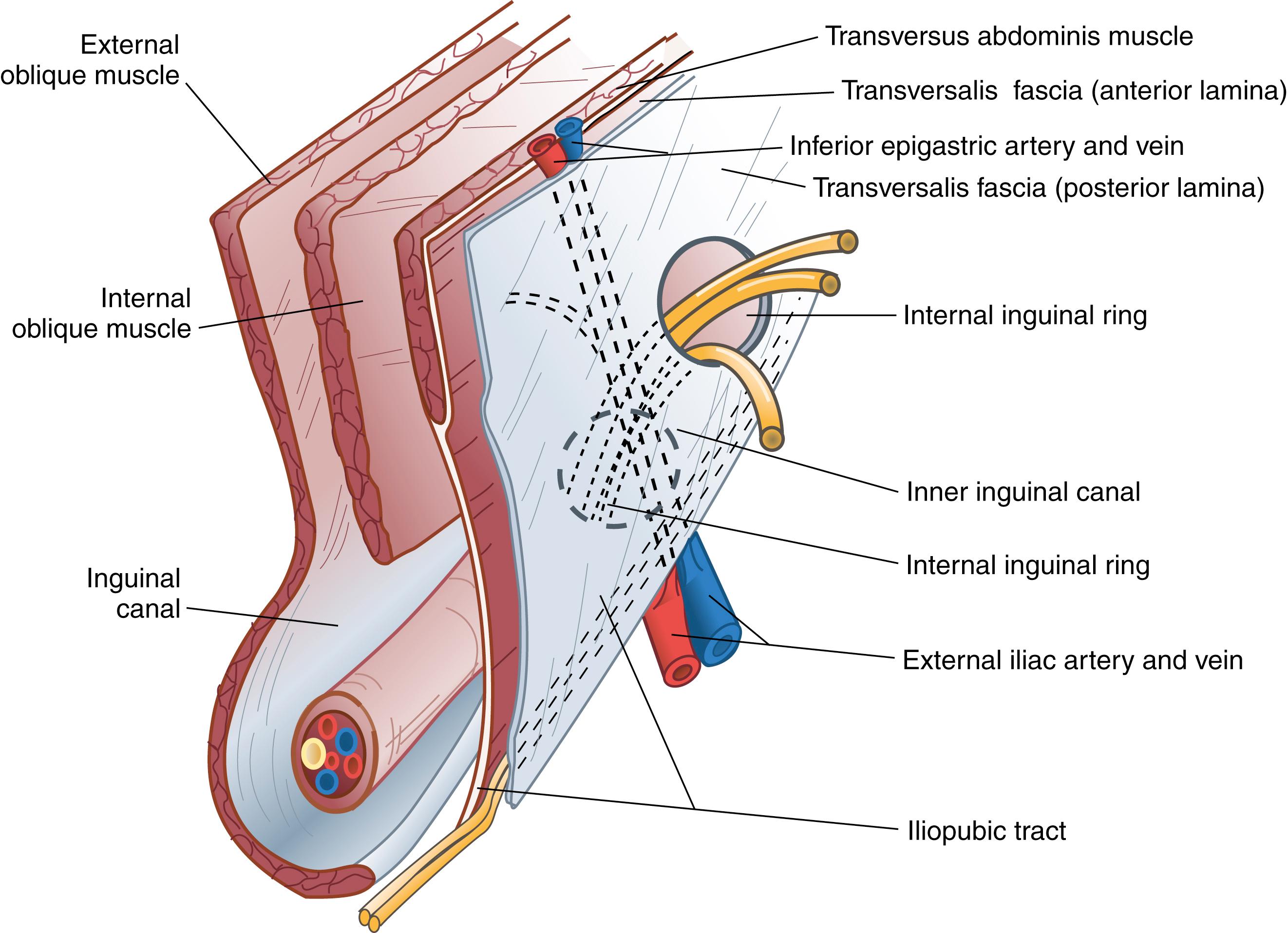
From anterior to posterior, the groin anatomy includes the skin and subcutaneous tissues, below which are the superficial circumflex iliac, superficial epigastric, and external pudendal arteries and accompanying veins. These vessels arise from and drain to the proximal femoral artery and vein, respectively, and are directed superiorly. If encountered during operation, these vessels can be retracted or even divided when necessary.
The external oblique muscle is the most superficial of the lateral abdominal wall muscles; its fibers are directed inferiorly and medially and lie deep to the subcutaneous tissues. The aponeurosis of the external oblique muscle is formed by a superficial and deep layer. This aponeurosis, along with the bilaminar aponeuroses of the internal oblique and transversus abdominis, forms the anterior rectus sheath and, finally, the linea alba by linear decussation. The external oblique aponeurosis serves as the superficial boundary of the inguinal canal. The inguinal ligament (Poupart ligament) is the inferior edge of the external oblique aponeurosis and extends from the anterior superior iliac spine to the pubic tubercle, turning posteriorly to form a shelving edge. The lacunar ligament is the fan-shaped medial expansion of the inguinal ligament, which inserts into the pubis and forms the medial border of the femoral space. The external (superficial) inguinal ring is an ovoid opening of the external oblique aponeurosis that is positioned superiorly and slightly laterally to the pubic tubercle. The spermatic cord exits the inguinal canal through the external inguinal ring.
The internal oblique muscle forms the middle layer of the lateral abdominal musculoaponeurotic complex. The fibers of the internal oblique are directed superiorly and laterally in the upper abdomen; however, they run in a slightly inferior direction in the inguinal region. The internal oblique muscle serves as the cephalad (or superior) border of the inguinal canal. The medial aspect of the internal oblique aponeurosis fuses with fibers from the transversus abdominis aponeurosis to form a conjoined tendon. This structure actually is present in only 5% to 10% of patients and is most evident at the insertion of these muscles on the pubic tubercle. The cremaster muscle fibers arise from the internal oblique, encompass the spermatic cord, and attach to the tunica vaginalis of the testis. These muscle fibers should be minimally disrupted during open inguinal hernia repair to help reduce chronic groin pain.
The transversus abdominis muscle layer is oriented horizontally throughout most of its area; in the inguinal region, these fibers course in a slightly oblique downward direction. The strength and continuity of this muscle and aponeurosis are important for the prevention and treatment of inguinal hernia.
The aponeurosis of the transversus abdominis covers anterior and posterior surfaces. The lower margin of the transversus abdominis arches along with the internal oblique muscle over the internal inguinal ring to form the transversus abdominis aponeurotic arch. The transversalis fascia is the connective tissue layer that underlies the abdominal wall musculature. The transversalis fascia, sometimes referred to as the endoabdominal fascia, is a component of the inguinal floor. It tends to be denser in this area but still remains relatively thin.
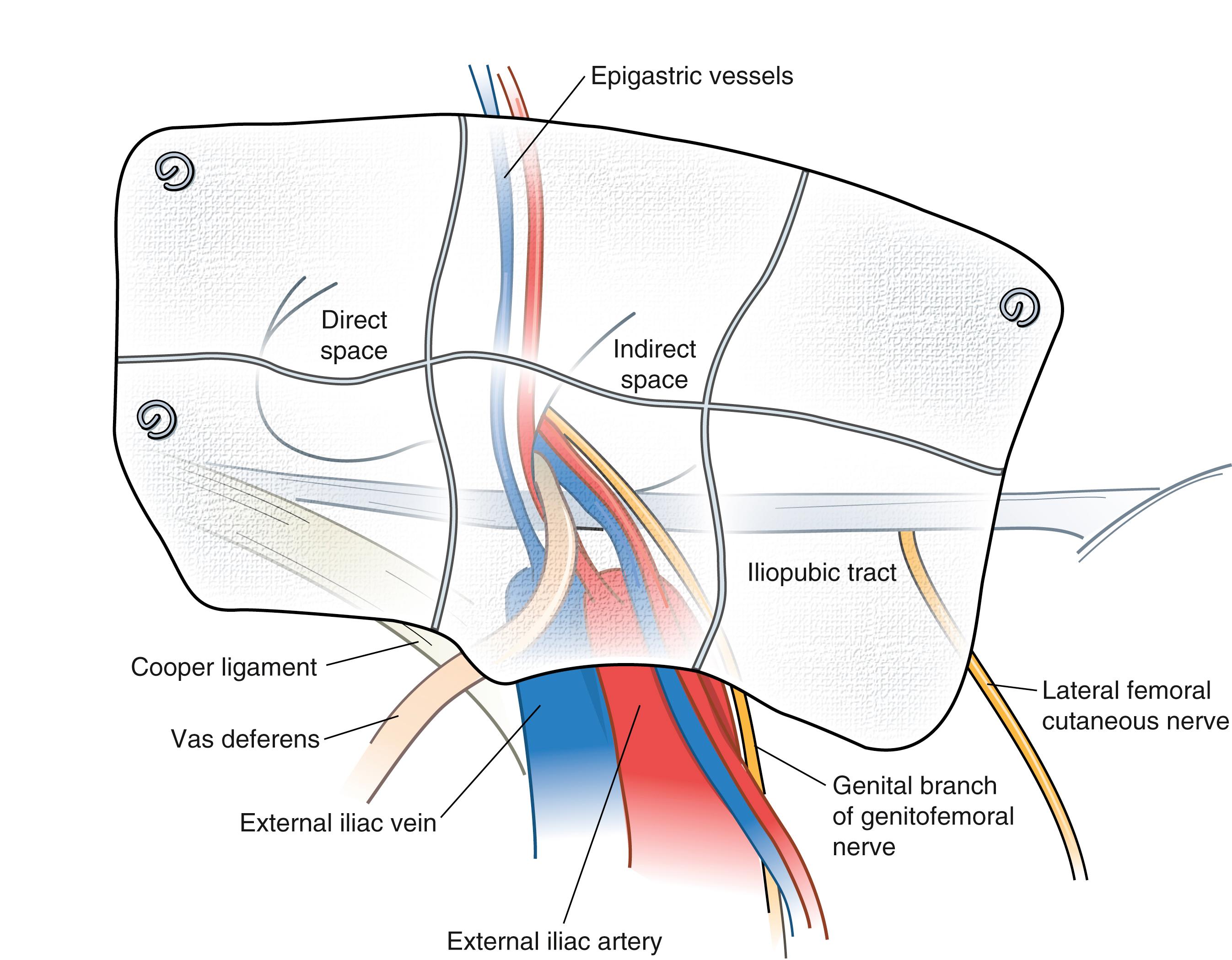
The iliopubic tract is an aponeurotic band that is formed by the transversalis fascia and transversus abdominis aponeurosis and fascia. The iliopubic tract is located posterior to the inguinal ligament and crosses over the femoral vessels and inserts on the anterior superior iliac spine and inner lip of the wing of the ilium.
The inferior crus of the deep inguinal ring is composed of the iliopubic tract; the superior crus of the deep ring is formed by the transversus abdominis aponeurotic arch. The lateral border of the internal ring is connected to the transversus abdominis muscle, which forms a shutter mechanism to limit the development of an indirect hernia.
The iliopubic tract is an extremely important structure in the repair of hernias from the anterior and posterior approaches. It composes the inferior margin of most anterior repairs. The portion of the iliopubic tract lateral to the internal inguinal ring serves as the inferior border below which staples or tacks are not placed during a laparoscopic repair because the femoral, lateral femoral cutaneous, and genitofemoral nerves are located inferior to the iliopubic tract. Although it cannot always be visualized during posterior repairs, if the tacking device cannot be palpated on the anterior abdominal wall, one must assume it is below the iliopubic tract.
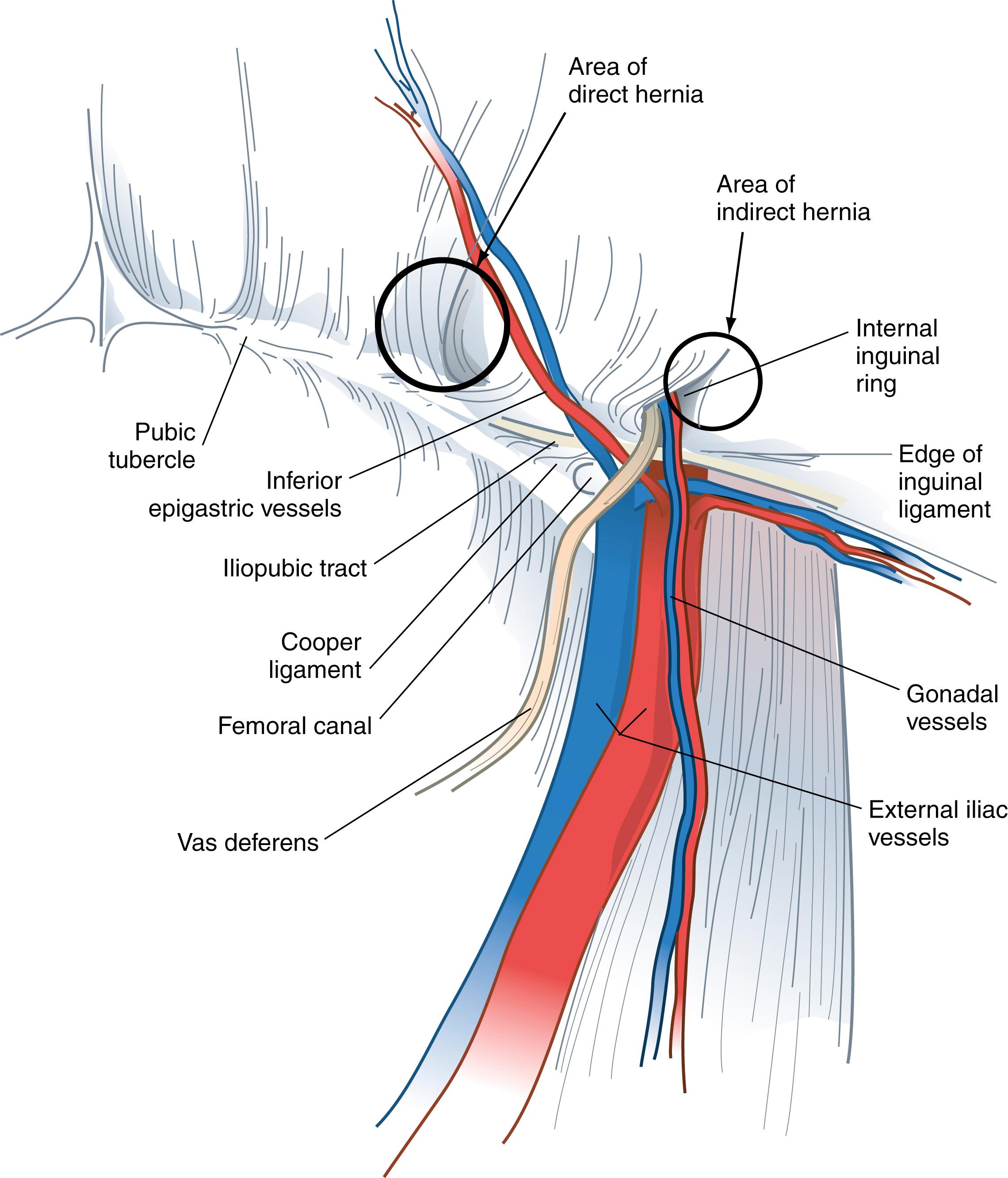
The pectineal (Cooper) ligament is formed by the periosteum and aponeurotic tissues along the superior ramus of the pubis. This structure is posterior to the iliopubic tract and forms the posterior border of the femoral canal. In approximately 75% of patients, there will be a vessel that crosses the lateral border of Cooper ligament that is a branch of the obturator artery. If this vessel is injured, troublesome bleeding can result. Cooper ligament is an important landmark for open and laparoscopic repairs and is a useful anchoring structure, particularly in laparoscopic repairs.
The inguinal canal is about 4 cm in length and is located just cephalad to the inguinal ligament. The canal extends between the internal (deep) inguinal and external (superficial) inguinal rings. The inguinal canal contains the spermatic cord in men and the round ligament of the uterus in women.
The spermatic cord is composed of the cremaster muscle fibers, testicular artery and accompanying veins, genital branch of the genitofemoral nerve, vas deferens, cremasteric vessels, lymphatics, and processus vaginalis. These structures enter the cord at the internal inguinal ring, and vessels and vas deferens exit the external inguinal ring. The cremaster muscle arises from the lowermost fibers of the internal oblique muscle and encompasses the spermatic cord in the inguinal canal. The cremasteric vessels are branches of the inferior epigastric vessels and pass through the posterior wall of the inguinal canal through their own foramen.
The inguinal canal is bounded superficially by the external oblique aponeurosis. The internal oblique and transversus abdominis musculoaponeuroses form the cephalad wall of the inguinal canal. The inferior wall of the inguinal canal is formed by the inguinal ligament and lacunar ligament. The posterior wall, or floor of the inguinal canal, is formed by the aponeurosis of the transversus abdominis muscle and transversalis fascia.
Hesselbach triangle refers to the margins of the floor of the inguinal canal. The inferior epigastric vessels serve as its superolateral border, the rectus sheath as the medial border, and the inguinal ligament and pectineal ligament as the inferior border. Direct hernias occur within Hesselbach triangle, whereas indirect inguinal hernias arise lateral to the triangle. It is not uncommon, however, for medium and large indirect inguinal hernias to involve the floor of the inguinal canal as they enlarge.
The iliohypogastric and ilioinguinal nerves and genital branch of the genitofemoral nerve are the important sensory nerves in the groin area ( Fig. 45.4 ). The iliohypogastric and ilioinguinal nerves provide sensation to the skin of the groin, base of the penis, and ipsilateral upper medial thigh. The iliohypogastric and ilioinguinal nerves lie beneath the internal oblique muscle to a point just medial and superior to the anterior superior iliac spine, where they penetrate the internal oblique muscle and course beneath the external oblique aponeurosis. The main trunk of the iliohypogastric nerve runs on the anterior surface of the internal oblique muscle and aponeurosis medial and superior to the internal ring. The iliohypogastric nerve may provide an inguinal branch that joins the ilioinguinal nerve. The ilioinguinal nerve runs anterior to the spermatic cord in the inguinal canal and branches at the superficial inguinal ring. The genital branch of the genitofemoral nerve innervates the cremaster muscle and skin on the lateral side of the scrotum and labia. This nerve lies on the iliopubic tract and accompanies the cremaster vessels to form a neurovascular bundle. In women, this branch generally follows the course of the round ligament.
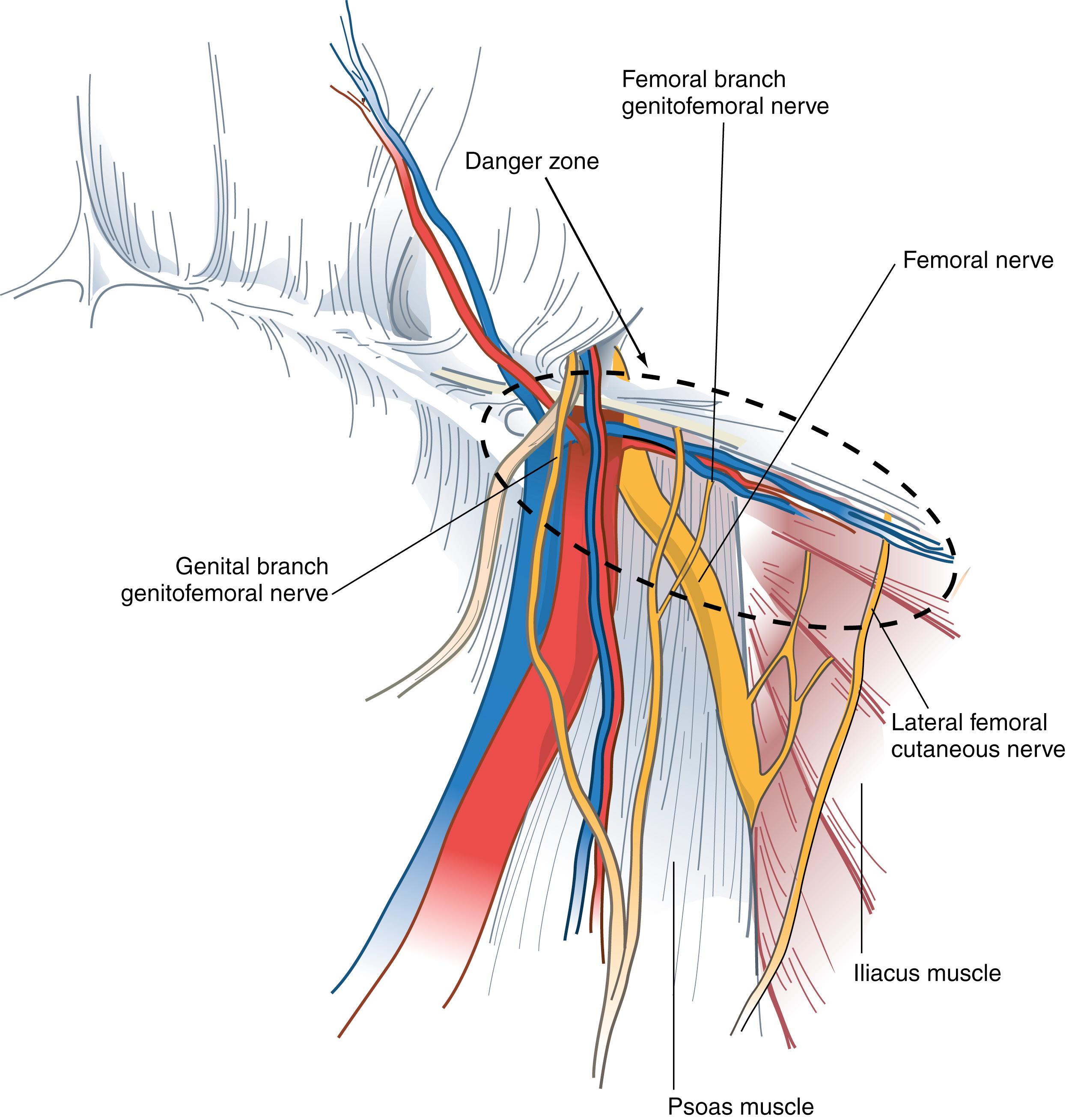
The preperitoneal space contains adipose tissue, lymphatics, blood vessels, and nerves. The nerves of the preperitoneal space of specific concern to the surgeon include the lateral femoral cutaneous nerve and genitofemoral nerve. The lateral femoral cutaneous nerve originates as a root of L2 and L3 and is occasionally a direct branch of the femoral nerve. This nerve courses along the anterior surface of the iliac muscle beneath the iliac fascia and passes under or through the lateral attachment of the inguinal ligament at the anterior superior iliac spine. This nerve runs beneath or occasionally through the iliopubic tract, lateral to the internal inguinal ring.
The genitofemoral nerve usually arises from the L2 or L1–L2 nerve roots. It divides into genital and femoral branches on the anterior surface of the psoas muscle. The genital branch enters the inguinal canal through the deep ring, whereas the femoral branch enters the femoral sheath lateral to the artery.
The inferior epigastric artery and vein are branches of the external iliac vessels and are important landmarks for laparoscopic hernia repair. These vessels course medial to the internal inguinal ring and eventually lie beneath the rectus abdominis muscle, immediately superficial to the transversalis fascia. The inferior epigastric vessels serve to define the types of inguinal hernia. Indirect inguinal hernias occur lateral to the inferior epigastric vessels, whereas direct hernias occur medial to these vessels.
The deep circumflex iliac artery and vein are located below the lateral portion of the iliopubic tract in the preperitoneal space. These vessels are branches of the inferior epigastric or external iliac artery and vein. It is important to dissect only above the iliopubic tract during a laparoscopic hernia repair to avoid injury to these vessels.
The vas deferens courses through the preperitoneal space from caudad to cephalad and medial to lateral to join the spermatic cord at the deep inguinal ring.
The boundaries of the femoral canal are the iliopubic tract anteriorly, Cooper ligament posteriorly, and femoral vein laterally. The pubic tubercle forms the apex of the femoral canal triangle. This canal usually contains connective tissue and lymphatic tissue. A femoral hernia occurs through this space and is medial to the femoral vessels.
A bulge in the inguinal region is the main diagnostic finding in most groin hernias. Most patients will have associated pain or vague discomfort in the region, but one-third of patients will have no symptoms. Groin hernias are usually not extremely painful unless incarceration or strangulation has occurred. In the absence of physical findings, alternative causes for pain need to be considered. On occasion, patients may experience paresthesias related to compression or irritation of the inguinal nerves by the hernia. Masses other than hernias can occur in the groin region. Physical examination alone often differentiates between a groin hernia and these masses ( Box 45.2 ).
Inguinal hernia
Hydrocele
Varicocele
Ectopic testis
Epididymitis
Testicular torsion
Lipoma
Hematoma
Sebaceous cyst
Hidradenitis of inguinal apocrine glands
Inguinal lymphadenopathy
Lymphoma
Metastatic neoplasm
Femoral hernia
Femoral lymphadenopathy
Femoral artery aneurysm or pseudoaneurysm
The inguinal region is examined with the patient in the supine and standing positions. The examiner visually inspects and palpates the inguinal region, looking for asymmetry, bulges, or a mass. Having the patient cough or perform a Valsalva maneuver can facilitate identification of a hernia. The examiner places a fingertip over the inguinal canal and repeats the examination. Finally, a fingertip is placed into the external inguinal ring by invaginating the scrotum to detect a small hernia. A bulge moving lateral to medial in the inguinal canal suggests an indirect hernia. If a bulge progresses from deep to superficial through the inguinal floor, a direct hernia is suspected. This distinction is not critical because repair is approached the same way, regardless of the type of hernia. A bulge identified below the inguinal ligament is consistent with a femoral hernia.
A bulge of the groin described by the patient that is not demonstrated on examination presents a dilemma. Having the patient stand or ambulate for a time may allow an undiagnosed hernia to become visible or palpable. If a hernia is strongly suspected but undetectable, repeated examination at another time may be helpful.
Ultrasonography also can aid in the diagnosis. There is a high degree of sensitivity and specificity for ultrasound in the detection of occult direct, indirect, and femoral hernias. On occasion, laparoscopy can be diagnostic and therapeutic for particularly challenging cases.
There are numerous classification systems for groin hernias. One simple and widely used system is the Nyhus classification ( Box 45.3 ). The European Hernia Society Groin Hernia Classification has gained acceptance as a simple scheme that is easy to apply clinically. Hernias are characterized as femoral hernias, or hernias that are medial or lateral to the epigastric vessels. Each of these three possibilities is then further characterized according to the size of the orifice (1 for <1.5 cm, 2 for 1.5 cm to 3 cm, 3 for >3 cm). Though their purpose is to promote a common language and understanding for communication of physicians and to allow appropriate comparisons of therapeutic options, these classifications are incomplete and contentious. Most surgeons continue to describe hernias by their type, location, and volume of the hernia sac.
Indirect inguinal hernia: internal inguinal ring normal (e.g., pediatric hernia)
Indirect inguinal hernia: internal inguinal ring dilated but posterior inguinal wall intact; inferior deep epigastric vessels not displaced
Posterior wall defect
Direct inguinal hernia
Indirect inguinal hernia: internal inguinal ring dilated, medially encroaching on or destroying the transversalis fascia of Hesselbach triangle (e.g., scrotal, sliding, or pantaloon hernia)
Femoral hernia
Recurrent hernia
Direct
Indirect
Femoral
Combined
Most surgeons recommend operation on discovery of a symptomatic inguinal hernia because the natural history of a groin hernia is that of progressive enlargement and weakening, with a small potential for incarceration and strangulation. However, in patients with minimal symptoms, the clinician is often faced with balancing the risk for hernia-related complications, such as incarceration and bowel strangulation, with the potential for complications in the short and long term. Fitzgibbons and colleagues reported a prospective randomized trial of a watchful waiting strategy for men with asymptomatic or minimally symptomatic inguinal hernias. These investigators randomized more than 700 men to a watchful waiting or open tension-free hernia repair. At 2 years of follow-up, there were no deaths attributed to the study, and the risk for hernia incarceration in the watchful waiting group was extremely low, 0.3% of study participants or 1.8 events/1000 patient-years. Almost 25% of patients assigned to watchful waiting crossed over to the surgical group, usually for pain related to the hernia that limited activity. In a later report, the crossover rate had increased to 68% at 10 years, with nearly 80% of men older than 65 years having an operation. Patients who later had surgery did not have increased surgical site infections or higher recurrence rates than those who were initially assigned to early repair. These studies provide conclusive evidence that a strategy of watchful waiting is safe for older patients with asymptomatic or minimally symptomatic inguinal hernias and that even though most patients eventually undergo repair, when they do, the operative risks and complication rates are no different from those of patients undergoing immediate repair. Watchful waiting can be a cost-effective management strategy for selected patients with no or minimal symptoms or who have suboptimal risk for operation. These results should not be applied to women, as women have not been included in these studies, or to patients with femoral hernias, which have a greater risk of strangulation than inguinal hernias.
Patients electing nonoperative management can occasionally have symptomatic improvement with the use of a truss. This approach is more commonly used in Europe. Spring trusses are more versatile than elastic ones, although most information on their use has been anecdotal. Correct measurement and fitting are important. Symptom control has been reported in about 30% of patients. Complications associated with the use of a truss include testicular atrophy, ilioinguinal or femoral neuritis, and hernia incarceration.
It is generally agreed that nonoperative management is not used for femoral hernias because of the high incidence of associated complications, particularly strangulation.
Anterior repairs are the most common operative approach for inguinal hernias. Tension-free repairs are now standard, and there are a variety of different types. Older tissue types of repair are rarely indicated, except for patients with simultaneous contamination or concomitant bowel resection, when placement of a mesh prosthesis may be contraindicated.
There are some technical aspects of the operation common to all anterior repairs. Open hernia repair is begun by making a transversely oriented linear or slightly curvilinear incision above the inguinal ligament and a fingerbreadth below the internal inguinal ring. The internal inguinal ring is located topographically at the midpoint between the anterior superior iliac spine and ipsilateral pubic tubercle. Dissection is continued through the subcutaneous tissues and Scarpa fascia. The external oblique fascia and external inguinal ring are identified. The external oblique fascia is incised through the superficial inguinal ring to expose the inguinal canal. The genital branch of the genitofemoral nerve and the ilioinguinal and iliohypogastric nerves are identified and avoided or mobilized to prevent transection and entrapment. The spermatic cord is mobilized at the pubic tubercle by a combination of blunt and sharp dissection. Improper mobilization of the spermatic cord too lateral to the pubic tubercle can cause confusion in the identification of tissue planes and essential structures and may result in injury to the spermatic cord structures or disruption of the floor of the inguinal canal.
The cremaster muscle of the mobilized spermatic cord is separated parallel to its fibers from the underlying cord structures. The cremaster artery and vein, which join the cremaster muscle near the inguinal ring, can usually be avoided but may need to be cauterized or ligated and divided. In general, minimal disruption of the cremasteric fibers and vasculature are recommended to minimize chronic groin pain. When an indirect hernia is present, the hernia sac is located deep to the cremaster muscle and anterior and superior to the spermatic cord structures. Incising the cremaster muscle in a longitudinal direction usually suffices to expose an indirect sac. The hernia sac is carefully separated from adjacent cord structures and dissected to the level of the internal inguinal ring. The sac is opened and examined for visceral contents if it is large; however, this step is unnecessary in small hernias. The sac can be mobilized and placed within the preperitoneal space, or the neck of the sac can be ligated at the level of the internal ring and any excess sac excised. If a large hernia sac is present, it can be divided with use of electrocautery to facilitate ligation. It is not necessary to excise the distal portion of the sac. If the sac is broad based, it may be easier to displace it into the peritoneal cavity rather than to ligate it. Direct hernia sacs protrude through the floor of the inguinal canal and can be reduced below the transversalis fascia before repair. To accomplish this, the weakened floor (transversalis fascia) is incised, exposing the preperitoneal fat below. This fat is mobilized from the neck of the direct defect and any other component of the direct hernia is reduced. The redundant transversalis fascia is excised and the floor can often be reapproximated using a running absorbable suture. A “lipoma” of the cord actually represents retroperitoneal fat that has herniated through the deep inguinal ring; this should be suture ligated and removed.
A sliding hernia presents a special challenge in handling the hernia sac. With a sliding hernia, a portion of the sac is composed of visceral peritoneum covering part of a retroperitoneal organ, usually the colon or bladder. In this situation, the grossly redundant portion of the sac (if present) is excised and the peritoneum reclosed. The organ and sac then can be reduced below the transversalis fascia, similar to the procedure for a direct hernia.
Although tissue repairs have largely been abandoned because of unacceptably high recurrence rates, they remain useful in certain situations. In strangulated hernias, for which bowel resection is necessary, mesh prostheses are contraindicated and a tissue repair is necessary. Available options for tissue repair include iliopubic tract, Shouldice, Bassini, and McVay repairs.
The iliopubic tract repair approximates the transversus abdominis aponeurotic arch to the iliopubic tract with the use of interrupted sutures ( Fig. 45.5 ). The repair begins at the pubic tubercle and extends laterally past the internal inguinal ring. This repair was initially described using a relaxing incision (see later); however, many surgeons who use this repair do not perform a relaxing incision.
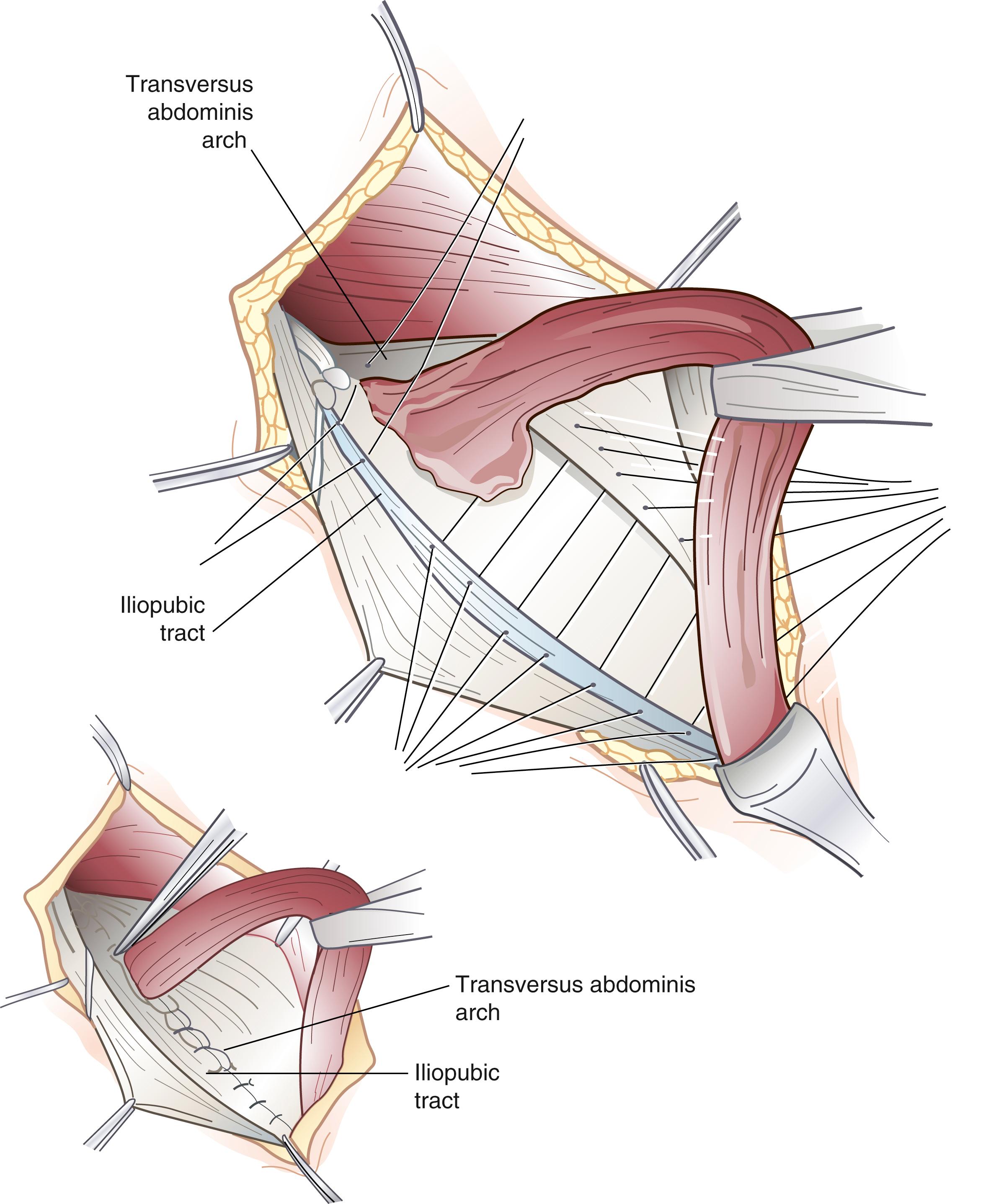
The Shouldice repair emphasizes a multilayer imbricated repair of the posterior wall of the inguinal canal with a continuous running suture technique. After completion of the dissection, the posterior wall of the inguinal canal is reconstructed by superimposing running suture lines progressing from deep to more superficial layers. The initial suture line secures the transversus abdominis aponeurotic arch to the iliopubic tract. Next, the internal oblique and transversus abdominis muscles and aponeuroses are sutured to the inguinal ligament. The Shouldice repair is associated with a very low recurrence rate and a high degree of patient satisfaction in highly selected patients.
The Bassini repair is performed by suturing the transversus abdominis and internal oblique musculoaponeurotic arches or conjoined tendon (when present) to the inguinal ligament. This once popular technique is the basic approach to nonanatomic hernia repairs and was the most popular type of repair done before the advent of tension-free repairs.
Cooper ligament repair, also known as the McVay repair, has traditionally been popular for the correction of direct inguinal hernias, large indirect hernias, recurrent hernias, and femoral hernias. Interrupted nonabsorbable sutures are used to approximate the edge of the transversus abdominis aponeurosis to Cooper ligament. When the medial aspect of the femoral canal is reached, a transition suture is placed to incorporate Cooper ligament and the iliopubic tract. Lateral to this transition stitch, the transversus abdominis aponeurosis is secured to the iliopubic tract. An important principle of this repair is the need for a relaxing incision. This incision is made by reflecting the external oblique aponeurosis cephalad and medial to expose the anterior rectus sheath. An incision is then made in a curvilinear direction, beginning 1 cm above the pubic tubercle throughout the extent of the anterior sheath to near its lateral border. This relieves tension on the suture line and results in decreased postoperative pain and hernia recurrence. The fascial defect is covered by the body of the rectus muscle, which prevents herniation at the relaxing incision site. The McVay repair is particularly suited for strangulated femoral hernias because it provides obliteration of the femoral space without the use of mesh.
Tension-free repair has become the dominant method of inguinal hernia repair ( Fig. 45.6 ). Recognizing that tension in a repair is the principal cause of recurrence, current practices in hernia management use a synthetic mesh prosthesis to bridge the defect, a concept popularized by Lichtenstein. There are several options for placement of mesh during anterior inguinal herniorrhaphy, including the Lichtenstein approach, plug and patch technique, and sandwich technique, with both an anterior and preperitoneal piece of mesh.
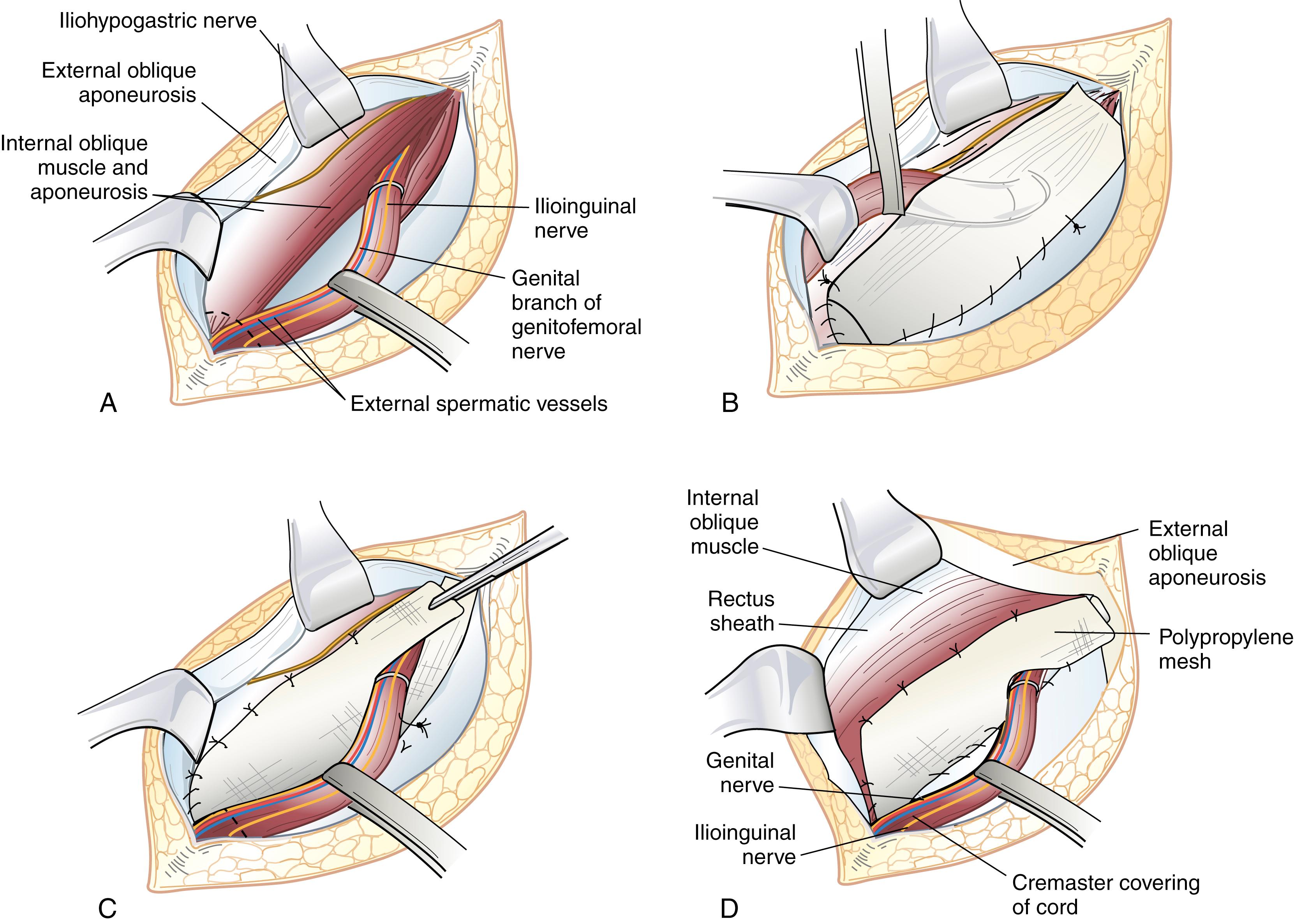
In the Lichtenstein repair, a piece of prosthetic nonabsorbable mesh is fashioned to fit the canal. A slit is cut into the distal lateral edge of the mesh to accommodate the spermatic cord. There are various preformed, commercially available prostheses available for use. The periosteum overlying the pubic tubercle is exposed and this dissection is extended medially toward the midline of the pubis for at least 15 to 20 mm. Fixation of mesh to the pubic tubercle itself should be avoided to minimize the risk of chronic groin pain. The inferolateral edge of the mesh is sutured to the shelving edge of the inguinal ligament starting just adjacent (but not into) the pubic tubercle) using a nonabsorbable suture. The medial aspect of the mesh is allowed to generously overlap the pubic tubercle by at least 15 mm. This suture is taken to a point just lateral and superior to the internal inguinal ring and tied. Interrupted sutures are then placed affixing the superomedial aspect of the mesh to the conjoined tendon. Great care is taken to visualize the ilioinguinal and iliohypogastric nerves to avoid injury or entrapment. In addition, adequate medial overlap of the mesh medial to the pubic tubercle is ensured. At this point, the tails created by the slit are sutured together around the spermatic cord, snugly forming a new internal inguinal ring. It is important to protect the ilioinguinal nerve and genital branch of the genitofemoral nerve from entrapment by placing them with the cord structures as they are passed through this newly fashioned internal inguinal ring or avoiding their enclosure in the repair.
Adapting the principles of tension-free repair, Gilbert has reported using a cone-shaped plug of polypropylene mesh that, when inserted into the internal inguinal ring, would deploy like an upside-down umbrella and occlude the hernia. This plug is sewn to the surrounding tissues and held in place by an additional overlying mesh patch. This patch may not need to be secured by sutures; however, to do so requires dissection to create a sufficient space between the external and internal oblique muscles for the patch to lie flat over the inguinal canal. This so-called plug and patch repair, an extension of the Lichtenstein original mesh repair, has now become the most commonly performed primary anterior inguinal hernia repair. Although this repair can be done without suture fixation by some experienced surgeons, most secure plug and patch with several monofilament nonabsorbable sutures, especially for very weak inguinal floors or large defects.
The sandwich technique involves a bilayered device, with three polypropylene components. An underlay patch provides a posterior repair similar to that of the laparoscopic approach, a connector functions similar to a plug, and an onlay patch covers the posterior inguinal floor. The use of interrupted fixating sutures is not mandatory, but most surgeons place three or four fixation sutures in this repair.
Another option for a tension-free mesh repair involves a preperitoneal approach using a self-expanding polypropylene patch. A pocket is created in the preperitoneal space by blunt dissection, and then a preformed mesh patch is inserted into the hernia defect, which expands to cover the direct, indirect, and femoral spaces. The patch lies parallel to the inguinal ligament. It can remain without suture fixation, or a tacking suture can be placed.
The Stoppa-Rives repair uses a subumbilical midline incision to place a large mesh prosthesis into the preperitoneal space. Blunt dissection is used to create an extraperitoneal space that extends into the prevesical space, beyond the obturator foramen, and posterolateral to the pelvic brim. This technique has the advantage of distributing the natural intra abdominal pressure across a broad area to retain the mesh in a proper location. The Stoppa-Rives technique is particularly useful for large, recurrent, or bilateral hernias.
The open preperitoneal approach is useful for the repair of recurrent inguinal hernias, sliding hernias, femoral hernias, and some strangulated hernias. A transverse skin incision is made 2 cm above the internal inguinal ring and is directed to the medial border of the rectus sheath. The muscles of the anterior abdominal wall are incised transversely, and the preperitoneal space is identified. If further exposure is needed, the anterior rectus sheath can be incised and the rectus muscle retracted medially. The preperitoneal tissues are retracted cephalad to visualize the posterior inguinal wall and the site of herniation. The inferior epigastric artery and veins are generally beneath the midportion of the posterior rectus sheath and usually do not need to be divided. This approach avoids mobilization of the spermatic cord and injury to the sensory nerves of the inguinal canal, which is particularly important for hernias previously repaired through an anterior approach. If the peritoneum is incised, it is sutured closed to avoid the evisceration of intraperitoneal contents into the operative field. The transversalis fascia and transversus abdominis aponeurosis are identified and sutured to the iliopubic tract with permanent sutures. Femoral hernias repaired by this approach require closure of the femoral canal by securing the repair to Cooper ligament. A mesh prosthesis is frequently used to obliterate the defect in the femoral canal, particularly with large hernias.
Laparoscopic inguinal hernia repair is another method of tension-free mesh repair based on a preperitoneal approach. The laparoscopic approach provides the mechanical advantage of placing a large piece of mesh behind the defect, covering the myopectineal orifice, and using the natural forces of the abdominal wall to disperse intra abdominal pressure over a larger area to support the mesh in place. Proponents have touted quicker recovery, less pain, better visualization of anatomy, and usefulness for fixing all inguinal hernia defects. Critics have emphasized longer operative times, technical challenges, increased risk of recurrence, and increased cost. Laparoscopic repair is also associated with an approximately 0.3% risk of visceral or vascular injury. Although controversy exists about the usefulness of laparoscopic repair for primary unilateral inguinal hernias, most agree that this approach has advantages for patients having bilateral or recurrent hernia repairs. Adopting practice guidelines for the performance of laparoscopic hernia repairs could help control costs.
When considering the laparoscopic approach for repair of inguinal hernias, the surgeon has several options. The most popular techniques are totally extraperitoneal (TEP) and transabdominal preperitoneal (TAPP) approaches. The main difference between these two techniques is the sequence of gaining access to the preperitoneal space. In the TEP approach, the dissection begins in the preperitoneal space using a balloon dissector. With the TAPP repair, the preperitoneal space is accessed after initially entering the peritoneal cavity. Each approach has its merits. With the TEP approach, the preperitoneal dissection is quicker, and the potential risk for intraperitoneal visceral damage is minimized. However, the use of dissection balloons is costly, the working space is more limited, and it may not be possible to create a working space if the patient has had a prior preperitoneal operation. Also, if a large tear in the peritoneum is created during a TEP approach; the potential working space can become obliterated, necessitating conversion to a TAPP approach. For these reasons, knowledge of the transabdominal technique is essential in performing laparoscopic inguinal hernia repairs. The transabdominal approach allows identification of the groin anatomy before extensive dissection and disruption of natural tissue planes. The larger working space of the peritoneal cavity can make early experience with the laparoscopic approach easier.
There are no absolute contraindications to laparoscopic inguinal hernia repair other than the patient’s inability to tolerate general anesthesia. Patients who have had extensive prior lower abdominal surgery can require significant adhesiolysis and may be best approached anteriorly. In particular, in patients who have had a radical retropubic prostatectomy with the preperitoneal space previously dissected, accurate and safe dissection can be challenging.
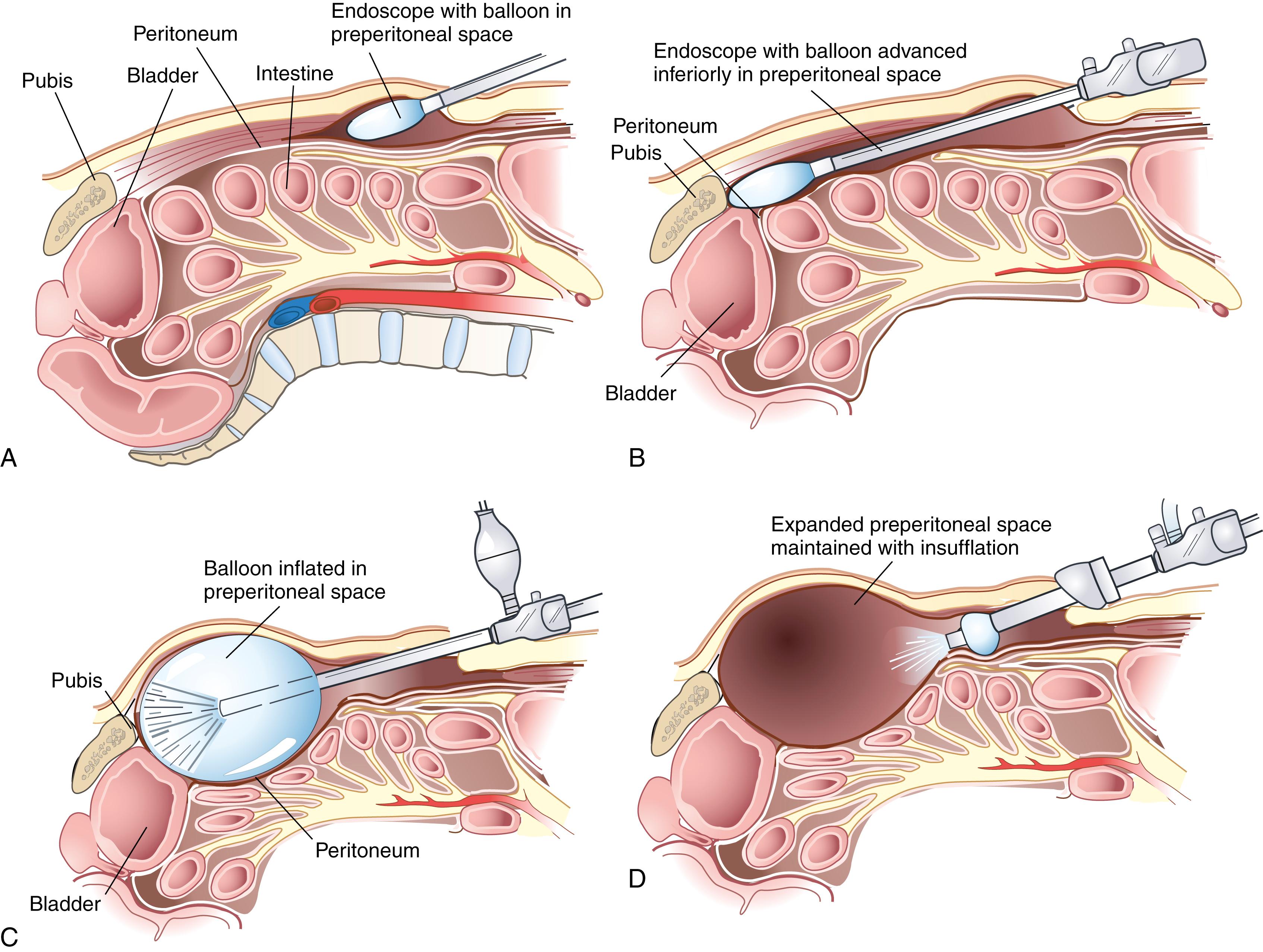
In the TEP approach, an infraumbilical incision is used. The anterior rectus sheath is incised, the ipsilateral rectus abdominis muscle is retracted laterally, and blunt dissection is used to create a space beneath the rectus. A dissecting balloon is inserted deep to the posterior rectus sheath, advanced to the pubic symphysis, and inflated under direct laparoscopic vision ( Fig. 45.7 ). After it is opened, the space is insufflated, and additional trocars are placed. A 30-degree laparoscope provides the best visualization of the inguinal region (see Fig. 45.3 ). The inferior epigastric vessels are identified along the lower portion of the rectus muscle and serve as a useful landmark. Cooper ligament must be cleared from the pubic symphysis medially to the level of the external iliac vein. The iliopubic tract is also identified. Care must be taken to avoid injury to the femoral branch of the genitofemoral nerve and lateral femoral cutaneous nerve, which are located lateral to and below the iliopubic tract (see Fig. 45.4 ). Lateral dissection is carried out to the anterior superior iliac spine. Finally, the testicular vessels and vas deferens are identified and mobilized away from the peritoneum.
In the TAPP approach, an infraumbilical incision is used to gain access to the peritoneal cavity directly. Two 5-mm ports are placed lateral to the inferior epigastric vessels at the level of the umbilicus. A peritoneal flap is created high on the anterior abdominal wall, extending from the median umbilical fold to the anterior superior iliac spine. The remainder of the operation proceeds similar to a TEP procedure.
A direct hernia sac and associated preperitoneal fat are gently reduced by traction if not already reduced by balloon expansion of the peritoneal space. A small, indirect hernia sac is mobilized from the cord structures and reduced into the peritoneal cavity. A large sac may be difficult to reduce. In this case, the sac is divided with cautery near the internal inguinal ring, leaving the distal sac in situ. The proximal peritoneal sac is closed with a loop ligature or clips to prevent pneumoperitoneum from occurring. After all hernias are reduced, a 12 × 14-cm piece of polypropylene mesh is inserted through a trocar and unfolded. It covers the direct, indirect, and femoral spaces and rests over the cord structures. It is imperative that the peritoneum be dissected at least 4 cm off the cord structures to prevent the peritoneum from encroaching beneath the mesh, which can lead to recurrence. The mesh is carefully secured with a fixation device to Cooper ligament medially, anteriorly to the posterior rectus musculature and transversus abdominis aponeurotic arch at least 2 cm above the hernia defect, and laterally to the iliopubic tract. The mesh extends beyond the pubic symphysis and below the spermatic cord and peritoneum ( Fig. 45.8 ). The mesh is not fixed in this area and tacks are not placed inferior to the iliopubic tract beyond the external iliac artery. Fixation placed in this area may injure the femoral branch of the genitofemoral nerve or lateral femoral cutaneous nerve. Fixation is also avoided in the so-called triangle of doom, bounded by the ductus deferens medially and spermatic vessels laterally, to avoid injury to the external iliac vessels and femoral nerve. As long as one can palpate the tip of the tacking device, these structures are not likely to be injured.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here