Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The hereditary optic neuropathies comprise a group of disorders in which the cause of optic nerve dysfunction appears to be hereditary, based on familial expression or genetic analysis. Clinical variability, both within and among families with the same disease, often makes recognition and classification difficult. Inherited optic neuropathies are often classified by pattern of transmission, most commonly autosomal dominant, autosomal recessive, and maternal (via mitochondrial DNA [mtDNA]). The same genetic defect may not be responsible for all pedigrees with optic neuropathy inherited in a similar fashion. Similarly, different genetic defects may cause identical or similar phenotypes – some inherited in the same manner, others not. Alternatively, the same genetic defect may result in different clinical expression, although the pattern of inheritance should be consistent. Single cases are often presumed or proven to be caused by inherited genetic defects, making the pattern of familial transmission unavailable as an aid to classification.
The inherited optic neuropathies typically manifest as symmetric, bilateral, painless, central visual loss. Neuroimaging must be performed prior to considering inherited optic neuropathy as a cause of optic atrophy to exclude an underlying mass lesion. In many of the inherited optic neuropathies the papillomacular nerve fiber bundle is affected, with resultant central or cecocentral scotomas. The exact location of initial pathology along the ganglion cell and its axon, and the pathophysiologic mechanisms of optic nerve injury, remain unknown, but mitochondrial dysfunction plays a central role in the pathogenesis of most, if not all, of the inherited optic neuropathies. Optic nerve damage is usually permanent and may be progressive. Once optic atrophy is observed, substantial nerve injury has already occurred.
In some of the hereditary optic neuropathies, optic nerve dysfunction is isolated. In others, various neurologic and systemic abnormalities are regularly observed and inherited diseases with primarily neurologic or systemic manifestations, such as the multisystem degenerations, can include optic atrophy. In this chapter, the hereditary optic neuropathies are classified into three major groups:
Those that occur primarily without associated neurologic or systemic signs.
Those that frequently have associated neurologic or systemic signs.
Those in which optic neuropathy is usually recognized as secondary in the disease process.
As more specific genetic defects are discovered, our concept of the phenotypes of these disorders will likely change, as will our classification.
Leber hereditary optic neuropathy (LHON) was one of the first diseases to be etiologically linked to specific mtDNA defects. It has a minimum point prevalence of 1 in 31,000 in the northeast of England, 1 in 39,000 in the Netherlands, and 1 in 50,000 in Finland. Age of onset is typically between 15 and 35 years, but may range from 1 to 87 years. LHON is expressed predominantly in males, a feature not expected from mtDNA inheritance. Between 25% and 50% of male and 5% and 10% of female LHON carriers will have vision loss during their lifetime. Visual loss is bilateral, painless, central, and usually subacute in onset. In about 50% of cases, the vision loss is recognized as occurring sequentially, with one eye affected weeks to months before the second eye. Within 1 year, both eyes are invariably affected. Over weeks or months, vision worsens in each eye to acuities of 20/200 or worse. Color vision is affected early and severely, and visual fields typically show central or cecocentral defects. Funduscopic abnormalities may be seen in patients with LHON and in their asymptomatic maternal relatives. Especially during the acute phase of visual loss, there may be hyperemia of the optic nerve head (pseudoedema), dilation and tortuosity of vessels, and circumpapillary telangiectatic microangiopathy ( Fig. 54.1 ). Eventually, there is optic atrophy with nerve fiber layer dropout, especially in the papillomacular bundle, sometimes with non-glaucomatous cupping of the disc. In patients who are known to harbor deleterious LHON mutations, serial observation reveals that visual acuity and visual fields may show a subclinical decline prior to the patient noting vision loss which is accompanied by an increase in retinal nerve fiber layer thickness on OCT. In most LHON patients, visual loss is the only manifestation of the disease. Some pedigrees have family members with associated cardiac conduction abnormalities, especially pre-excitation syndromes, minor neurologic and skeletal abnormalities, and even multiple sclerosis-like disease.
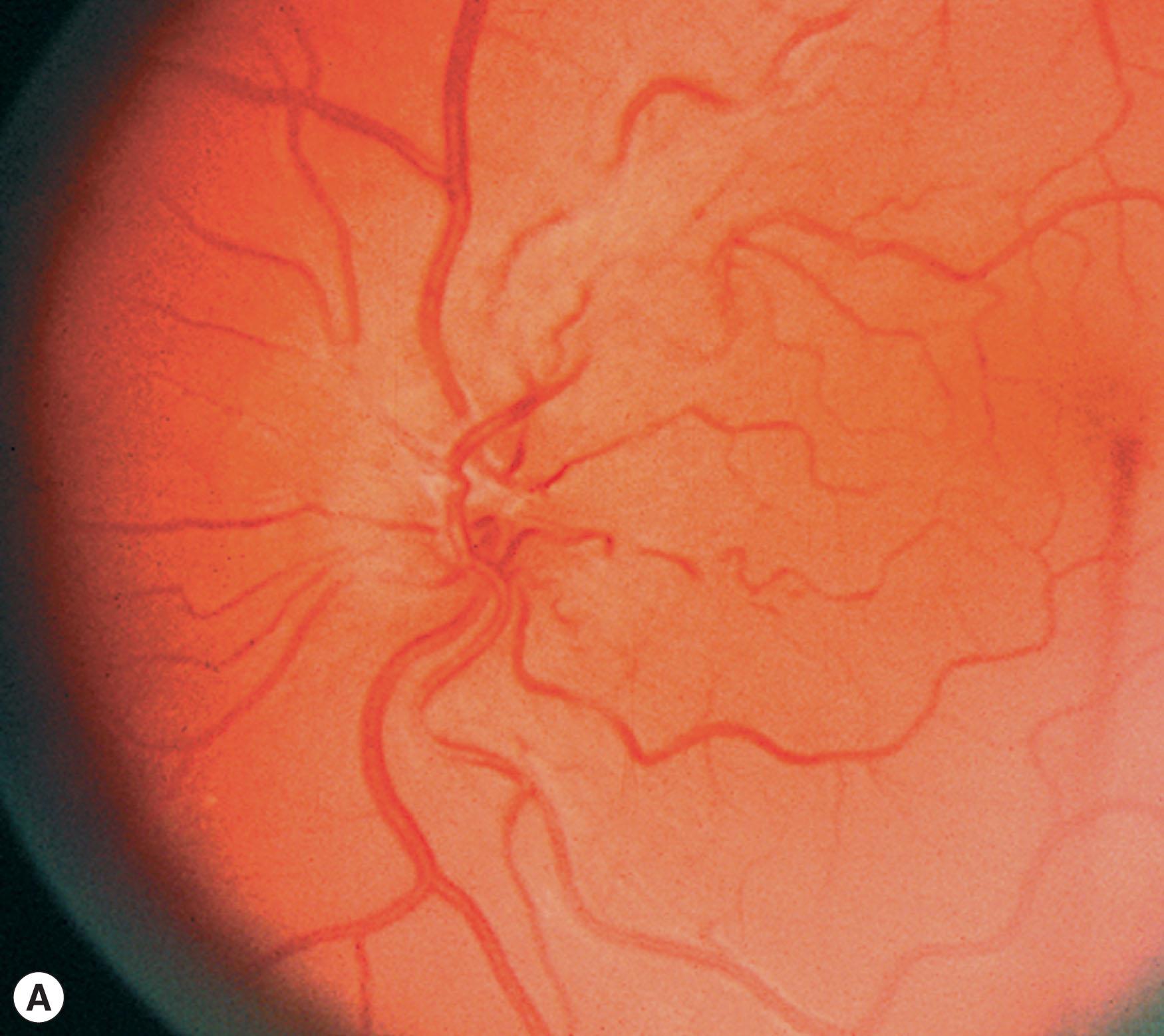
Ancillary tests in LHON are of limited clinical value. Fluorescein angiography may help distinguish the LHON optic disc from true disc edema as the optic disc will demonstrate leakage in true disc edema, but not in LHON. Electrocardiograms may show cardiac conduction abnormalities. Optical coherence tomography has demonstrated initial thickening of the peripapillary nerve fiber layer, followed by thinning as optic atrophy ensues. Visual evoked responses are predictably abnormal when there is visual loss. Standard flash electroretinograms, electroencephalograms, cerebrospinal fluid (CSF), and brain computed tomography (CT) and magnetic resonance imaging (MRI) are generally normal. Rarely, MRIs show anterior visual pathway enhancement acutely in affected LHON patients and bright T2 lesions in the late phases.
LHON pedigrees follow a maternal inheritance pattern, and the disease has been linked to point mutations in the mtDNA ( Fig. 54.2 ). Three point mutations in the mtDNA, known as the “primary LHON mutations,” cause about 90% of cases of LHON worldwide. They are located at mtDNA nucleotide positions 11778 (69% of cases), 3460 (13% of cases), and 14484 (14% of cases) (see Fig. 54.2 ). Other point mutations have been found with a greater frequency in LHON patients than controls. Some of these mutations may be true primary mutations in a few pedigrees. Others are of unknown significance. Screening for LHON in a patient with visual loss should begin with the three primary mutations. In those primary mutation-negative patients in whom suspicion remains high, mitochondrial genome sequencing can be considered, but should be interpreted by someone familiar with the complexities of mitochondrial genetics. If mitochondrial genome sequencing is normal in patients with high suspicion for LHON, whole exome sequencing can be considered with the assistance of a geneticist or genetic counselor to evaluate for nuclear genome abnormalities expressed in the mitochondria.
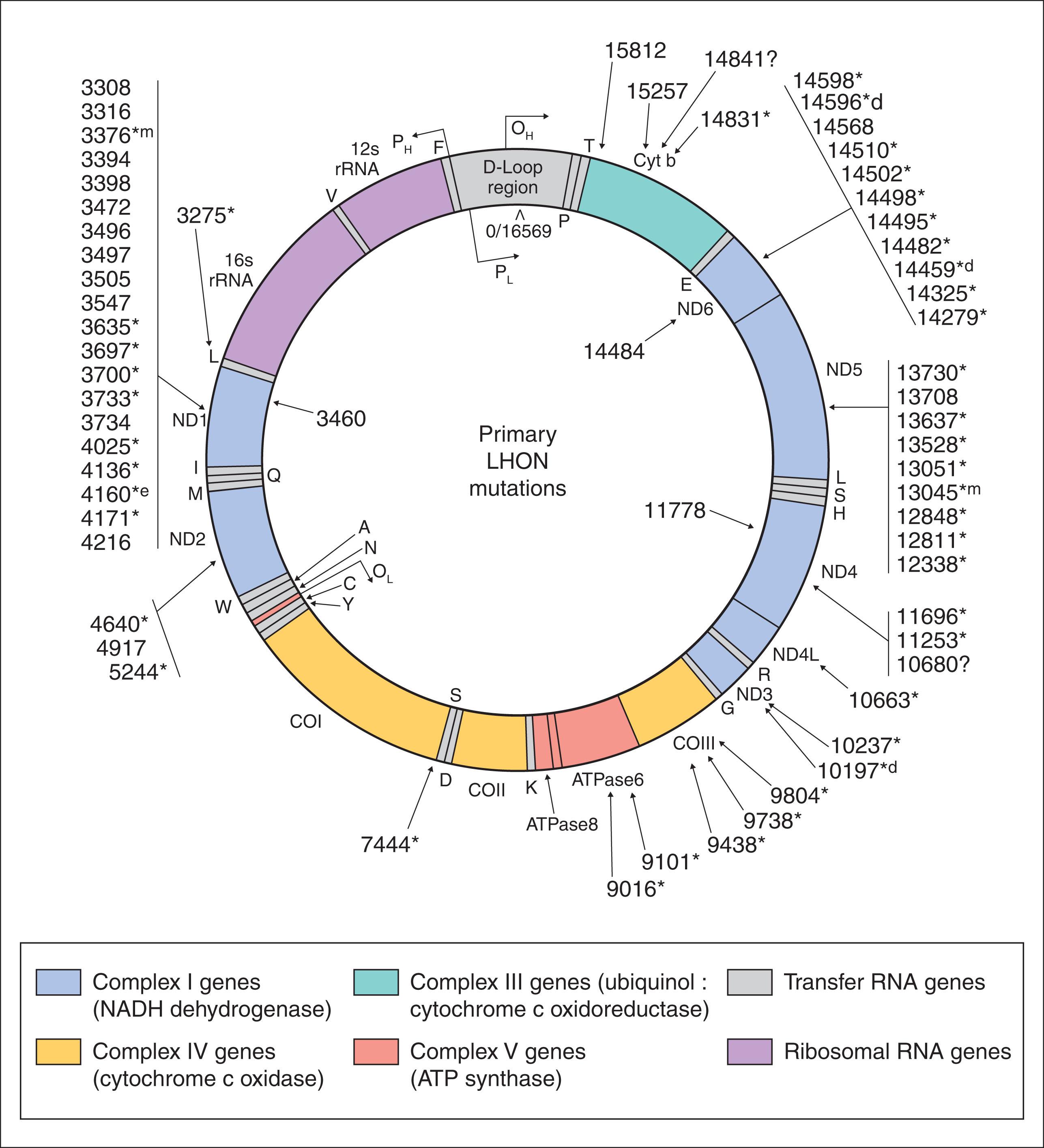
Among the primary mutations, the LHON clinical phenotype is remarkably similar. The only consistent differentiating feature is the better prognosis for visual outcome in those patients with the 14484 mutation. Up to 70% of patients with the 14484 mutation will have some degree of visual improvement compared to no more than 20% of patients with the 11778 mutation. Patients with the 3460 mutation probably have the same chance of visual recovery as those with the 11778 mutation, but the numbers of patients are too small for meaningful analysis. Patients with an age of onset of visual loss of less than 20 years, especially less than 10 years, have a much better visual prognosis.
The genetic defects, however, cannot fully explain the determinants of expression in this disease. The presence of a mtDNA mutation is necessary for phenotypic expression, but it is not sufficient. The presence of heteroplasmy (the co-existence of both mutant and normal mtDNA) may be a factor in expression. Other mitochondrial or nuclear DNA factors may modify expression of the disease, including X-linkage as an explanation for the prominence of male expression. Various environmental triggers for the development of visual loss in LHON have been suggested. Systemic illnesses, nutritional deficiencies, and medications or toxins that stress the organism's mitochondrial energy production might be detrimental to those individuals already genetically at risk for mitochondrial energy deficiency. Smoking, probably through its deleterious effect on mitochondrial function, has been associated with a higher expression of visual loss among carriers of the LHON primary mutation; only heavy alcohol intake has been similarly associated.
The pathophysiology of LHON remains unknown. It may involve abnormal oxidative phosphorylation and deficient generation of adenosine triphosphate (ATP), either directly or indirectly related to free radical production, resulting in irreversible damage to the ganglion cells and their axons. The reason these mechanisms result in selective damage to the optic nerve remains uncertain. Histochemical studies of the optic nerve in animals have shown a high degree of mitochondrial respiratory activity within the unmyelinated, prelaminar portion of the optic nerve, suggesting a particularly high requirement for mitochondrial function in this region. Of great interest is the identification of genetically induced rodent models of complex I deficiency that show histopathological features of optic nerve degeneration similar to those seen in LHON patients.
Therapies tried in the treatment of LHON include coenzyme Q10, idebenone, l -carnitine, succinate, dichloroacetate, elamipretide, EPI-743, curcumin, cyclosporine, near-infrared light, vitamin K 1 , vitamin K 3 , vitamin C, thiamine, vitamin B 2 , and vitamin E. A randomized, controlled study of idebenone in the treatment of LHON patients within 5 years of visual loss suggested that patients with a disparity in their visual function between the two eyes (i.e. those likely earlier in the course of their disease) may have a better visual outcome with treatment. Treatment effect persisted following cessation of therapy at 30-month follow-up. Idebenone has been approved by the European Medicine Agency to be used under exceptional circumstances due to the rarity and severity of LHON. However, irreversible damage makes optic atrophy an unlikely candidate for a good response to any therapy. When used, idebenone has been recommended for those with disease duration of less than one year at a dosage of 300 mg 3 times per day with meals. Avoiding agents that might stress mitochondrial energy production is a non-specific recommendation with no proven benefit. However, we suggest that LHON patients and maternal relatives at risk avoid tobacco, excessive alcohol, and environmental toxins. Symptomatic therapies include pacemakers in those patients with serious cardiac conduction defects and low-vision aids for patients with severe visual loss. Directed therapies are being created to replace or bypass specific genetic or metabolic deficiencies in patients with LHON, and may eventually be used to prevent vision loss in patients and in their relatives who are at risk. Rodent models of induced LHON with the G11778A mutation have been phenotypically rescued by allotopically expressed wild-type ND4. Several phase I, II, and III trials are underway at various stages to evaluate AAV2-ND4 allotypic rescue for LHON associated with the G11778A mutation. Thus far allotypic rescue with adenoviral vectors appears to have an acceptable safety profile, and early results suggest that some cases may have visual acuity improvement, even bilaterally after unilateral injection, although more studies are necessary. Genetic counseling regarding maternal inheritance among family members is essential.
Autosomal dominant (“Kjer type”) optic atrophy (DOA) is the most common of the autosomal hereditary optic neuropathies, with an estimated disease prevalence in the northeast of England of 1 in 35,000 individuals, and as high as 1 in 12,000 in Denmark.
Although it is difficult for patients to identify a precise onset of reduced vision, the majority of affected patients date the onset of visual symptoms to between 4 and 10 years of age, with 58%−84% of patients reporting visual impairment by age 11 years. Rarely, severely affected individuals have visual difficulties and sensory nystagmus prior to schooling. Many patients are unaware of a visual problem and are discovered to have optic atrophy as a direct consequence of examination of other affected family members. Visual acuity is usually reduced to the same mild extent in both eyes. Acuities range from 20/20 to 20/800. Only about 15% of patients have vision of 20/200 or worse. Hand motion or light perception vision is extremely rare. There is considerable interfamilial and intrafamilial variation in acuities. Although not as rapid and devastating as LHON, DOA results in visual impairment severe enough to preclude driving in about 50% of affected adults. Blue–yellow color defects are classic in patients with dominant optic atrophy, but a mixed color deficit is most common. Visual fields in patients with dominant optic atrophy characteristically show central, paracentral, or cecocentral scotomas. A bitemporal depression may mimic chiasmal compression. The optic atrophy in patients with dominantly inherited optic neuropathy may be subtle, temporal only, or involve the entire disc ( Fig. 54.3 ). The most characteristic change is a striking triangular excavation of the temporal portion of the disc, sometimes leading to the erroneous diagnosis of glaucoma. Insidious progressive decline in visual function occurs in about 50%−75% of patients.
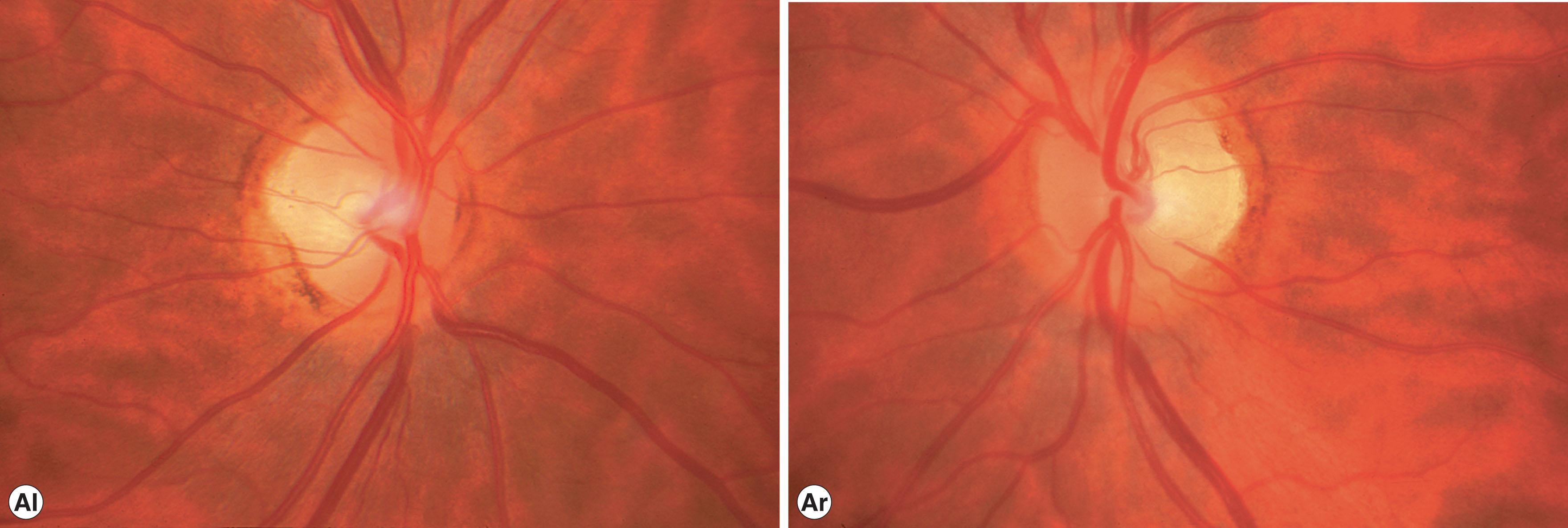
Dominant optic atrophy is believed to be a primary degeneration of the retinal ganglion cells. The majority of cases of DOA (50%−60%) are the result of mutations in the OPA1 gene, located on the long arm of chromosome 3 (3q28–q29). This nuclear gene is widely expressed in mitochondria and encodes a dynamin-related protein anchored to the inner membrane of mitochondrial cristae, abundantly represented in the retina. More than 300 pathogenic mutations in the OPA1 gene have been identified, including missense, nonsense, deletion/insertion, and splicing mutations. However, the monosymptomatic DOA phenotype is genetically heterogeneous, and other genetic loci have been implicated. In a single family of German descent with DOA, the causative OPA4 locus was mapped to chromosome 18 (18q12.2−12.3), and in two unrelated French families the OPA5 locus was mapped to chromosome 22 (22q12.1−13.1). Interestingly, linkage analysis of patients with normal-tension glaucoma has shown an association with polymorphisms of the OPA1 gene.
Classically, DOA presents with monosymptomatic optic atrophy and no other neurologic or systemic manifestations. However, it has long been known that some pedigrees with individuals manifesting classic DOA have also included affected family members with other clinical features such as sensorineural hearing loss and even chronic progressive external ophthalmoplegia, ataxia, myopathy, peripheral neuropathy, and, rarely, phenotypes suggestive of hereditary spastic paraplegia. Most of these families are not genetically distinct from pedigrees with monosymptomatic DOA, and, indeed, OPA1 gene mutations have been confirmed as the causative genetic defects in many of these syndromic forms of DOA, so-called “DOA plus.” Some patients with monosymptomatic DOA have been found to have subclinical features of DOA plus. In these patients, a severely dysfunctional OPA1 protein may result in aberrant replication of mtDNA and multiple large-scale deletions of the mtDNA, and hence a more dramatic phenotype suggestive of mitochondrial disease. While these new observations emphasize the common final pathway of mitochondrial dysfunction in the hereditary optic neuropathies, they also blur the classic classification distinctions of the non-syndromic from the syndromic forms of hereditary optic neuropathy.
This form of optic atrophy is present at birth or develops at an early age and is usually discovered before the patient is 3 or 4 years old. It is presumed to have autosomal recessive transmission and there is often consanguinity between parents. The visual acuity is so severely affected that the patient may be blind, with sensory nystagmus. The optic discs are completely atrophic and often deeply cupped. By funduscopy alone, it is difficult to differentiate this entity from infantile retinal degeneration, making electroretinography essential. In one consanguineous family of French origin, isolated autosomal recessive optic atrophy was linked to chromosome 8 (8q21−q22), and the gene was designated OPA6 . In a number of consanguineous North African families with autosomal recessive optic atrophy, both with and without mild (often asymptomatic) sensorineural hearing loss, defects were found in a gene designated OPA7 on chromosome 11 (11q14.1−q21), which codes for transmembrane mitochondrial protein TMEM126A. A mutation in the gene ACO2 has been described in syndromic and non-syndromic optic atrophy patients and has been called OPA9 . Several individuals with both consanguineous and unrelated parents with optic atrophy with or without encephalopathy, mental retardation, and seizures have been described as having OPA10 due to mutations in RTN4IP1 .
Pedigrees with optic atrophy inherited in a documented sex-linked fashion are extremely rare, especially with the primary manifestation of only monosymptomatic optic atrophy. In two families, linkage to the X chromosome at the same locus (Xp11.4−Xp11.2) has been established and the gene designated OPA2 . Other families with presumed X-linked optic atrophy more consistently have other neurologic and systemic signs.
Several pedigrees with DOA and hearing loss have been described. In many of these pedigrees there are no other systemic or neurologic abnormalities. Some, but not all, of these pedigrees have now been shown to harbor OPA1 mutations that in other pedigrees cause only optic neuropathy. In one Italian family, a new locus on chromosome 16 (16q21−q22) was designated OPA8 , and preliminary studies suggest its pathogenesis may also be via mitochondrial dysfunction.
In a Dutch pedigree with DOA and deafness, OPA1 mutations were excluded, but a novel missense mutation was found in the WFS1 gene on chromosome 4 (4p16.1), a common locus for mutations typically causing the autosomal recessive DIDMOAD or Wolfram syndrome (see below). Similarly, in another DOA pedigree with hearing impairment and impaired glucose regulation, mutation analysis excluded mutations within the OPA1 , OPA3 , OPA4 , and OPA5 genes, but identified a novel missense mutation in the WFS1 (Wolfram) gene.
In other pedigrees with DOA and deafness, there may be associated ataxia, limb weakness, or polyneuropathy. The hearing loss in these pedigrees may be severe at birth with poor speech development or may be only moderate and slowly progressive. “DOA plus” with OPA1 mutations may ultimately account for many of these more complicated syndromic pedigrees, but clearly the syndromic combination of DOA and hearing loss is genetically heterogeneous. The syndrome CAPOS (cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural deafness) has been identified as due to specific pathogenic mutations in ATP1A3 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here