Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hemophilia is the most common, severe inherited bleeding disorder recognized in humans. The X-linked mode of inheritance has been appreciated since biblical times, and the previous occurrence of the disease in the European royal family has added further interest in this condition.
Hemophilia is subdivided into hemophilia A and B; the former being a deficiency or absence of factor (F) VIII while the latter is a deficiency or absence of FIX. Both are single-gene disorders. Ultimately both disorders result in a similar bleeding pattern whose severity is generally proportional to the amount of active factor VIII (FVIII) or FIX that the person produces. As both types of hemophilia result in deficiency of a coagulation protein, for the past more than half-century treatment has involved replacement of the missing protein (either FVIII or FIX) either episodically (on demand) to treat bleeds or prophylactically to prevent them. In the last 5 to 10 years, the management of both hemophilia A and B has transformed with the availability of coagulation protein replacement products with longer half-lives and with the introduction of emicizumab, a FVIII mimetic.
Additionally, clinical trials on novel therapies that aim at rebalancing coagulation in patients with hemophilia by targeting natural anticoagulants are ongoing and progress in gene therapy over the last decade is poised to provide a cure for hemophilic patients with imminent licensing of gene therapy products.
Therefore, the management of hemophilia is rapidly evolving.
The prevalence of hemophilia is expected to be the same in all populations globally, apart from rare clusters of mild-moderate disease in areas where founder variants have been propagated (e.g., mild hemophilia A in Twillingate, Newfoundland; mild/moderate hemophilia B in Old Order Amish, United States). In many parts of the developing world, the true prevalence of the condition is unknown because of inadequate diagnostic facilities, but from experience in the developed world, it can be assumed that the incidence of hemophilia A and hemophilia B in males at birth is approximately one in 4000 (slightly higher than the previous estimate of one in 5000) and one in 20,000, respectively.
Due to the X-linked recessive mode of inheritance, most affected subjects are male. However, for every male with hemophilia there are likely two carrier females (including, in most cases, the mother of the affected male as well as potentially some sisters of the male and his daughters). In addition to mothers and sisters of boys with hemophilia there are likely many other women who are carriers but are never diagnosed because they are not related to any male with hemophilia. Hence the hemophilia carrier state is almost certainly much more common than hemophilia in males. Most carriers without affected sons/brothers will likely never be investigated, as most (particularly women with non-O blood type) will not experience significant bleeding because their FVIII or FIX levels are in the normal range or, if their FVIII levels are low, will be misdiagnosed as having von Willebrand disease (VWD) rather than being carriers of hemophilia. For further discussion on hemophilia carrier detection see the box on Hemophilia Carrier Detection and Prenatal Diagnosis . Even though most carrier females have clotting factor levels in a normal range, some hemophilia carrier women have levels low enough to cause mild, moderate, or even severe disease due to lyonization (X-linked inactivation). A new nomenclature reclassifies these women as having mild, moderate, or severe hemophilia in a similar manner to hemophilic males.
The X-linked recessive nature of hemophilia results in most affected subjects being males. Females, however, carry and transmit the hemophilic trait and can sometimes also have clinical manifestations of the disease. Female carriers of hemophilia rarely have factor levels low enough to cause moderate or severe hemophilia; however, this can occur under the genetic circumstances listed in Table 134.1 .
|
There are four ways in which hemophilia carriers can be identified. The first three apply to women with a high a priori likelihood of being a carrier based on a positive family history of hemophilia while the last applies to those without a family history of hemophilia.
First, considering the X-linked transmission of the disease, pedigree analysis will determine the carrier status of some women. Thus, all daughters of hemophilic fathers and all women with an affected son and another first-degree male relative (another son, brother, uncle, cousin) are obligate carriers.
The second mode of carrier detection involves laboratory testing. In a minority (≈20%–30%) of carriers, the PTT may be prolonged and plasma levels of FVIII, or FIX may be reduced. These findings, together with a family history of hemophilia, are predictive of a carrier state. The etiology of low factor levels in carrier females is multifactorial and includes a woman’s blood type and VWF levels as well as the ratio of inactivation of the hemophilic and normal X chromosomes; a random process that occurs early in embryonic development. If there is markedly skewed inactivation of the normal X chromosome, the plasma level of FVIII or FIX may be correspondingly reduced, and the carrier female may have a bleeding disorder. It is important to determine the FVIII or FIX levels in all carrier females to inform the risk of bleeding with surgical procedures or childbirth. However, carriers rarely have a prolonged PTT or low plasma levels of FVIII or FIX. Therefore, a normal factor level does not rule out the carrier state. With hemophilia A, a low FVIII to VWF ratio is a good predictor of the carrier state.
The third and most definitive approach to carrier diagnosis is to use molecular genetics to identify the causative FVIII or FIX mutation. The results of such testing can be available within a few days if the information is needed urgently. Genetic carrier determination enhances family counseling for hemophilic kindreds and enables optimal preparation for delivery and future planning.
Very rarely a hemophilia carrier may be identified in a family in which there is no history of hemophilia. This would likely occur if the woman had abnormal bleeding associated with a low FVIII or FIX level. Although estimates of the percentage of hemophilia carriers with excessive bleeding (most often heavy menstrual bleeding) vary, it is likely in the 20%–30% range. Therefore, most de novo carriers will never be diagnosed or may only be diagnosed if they have affected sons or grandsons with hemophilia. Without a family history of hemophilia, it is difficult to confirm a diagnosis of hemophilia carriership even in a woman with excessive bleeding and a low FVIII or FIX level. Often carriers of hemophilia A are incorrectly labeled as having type 1 VWD particularly if they have an O blood type and, as a result low VWF levels. In this setting, a FVIII level that is much lower than the VWF level may be helpful.
Ideally, carrier detection should be undertaken before the woman contemplates starting a family. In many countries, testing for carrier status of a genetic disease is prohibited before adolescence to ensure that the subject can meaningfully participate in discussions of testing options.
Prenatal diagnosis of hemophilia begins with determination of the fetal sex, which can usually be determined by ultrasound examination. As levels of fetal DNA in maternal blood increase late in pregnancy examination for the presence of Y chromosome sequences in maternal blood enables definitive sex determination. If the fetus is female, no additional studies are required. If the fetus is male, fetal DNA for molecular genetic analysis can be isolated from chorionic villus samples obtained after 10 weeks of gestation or from amniocytes obtained by amniocentesis from 12 to 34 weeks. The risk of miscarriage with either procedure is approximately 1%. If the studies are being used to inform decisions about therapeutic abortion, they should be performed as early as possible. Determination of the hemophilic status of the fetus will also help planning for delivery, although consensus about optimal obstetric management of an affected baby is lacking.
Over the past decade, international studies indicate that the number of reported persons with hemophilia has increased by approximately 2% each year. The likely reasons for this trend include increasing awareness and improved diagnosis of hemophilia, increasing longevity of persons with hemophilia due to improved treatment and decreased mortality from human immunodeficiency virus (HIV) and hepatitis C. This also leads to greater propagation of hemophilia mutations as affected males are more likely to reach adulthood and “pass their genes” to the next generation. Potentially there may be other reasons to explain the increase in hemophilia.
The clinical signs and symptoms and inheritance patterns for hemophilia A and B are identical, and it was not until the early 1950s that the two forms of hemophilia were differentiated. Subsequently, in the early 1980s, the two genes encoding FVIII, and FIX were cloned, and the specific variants responsible for hemophilia began to be determined.
The FVIII gene (F8) encoding the FVIII protein is located at Xq28, the most distal band of the long arm of the X chromosome ( Fig. 134.1 ). It is a large and complex structure, 186 kb in length, consisting of 9 kb of exonic DNA arranged into 26 exons and 177 kb of intronic DNA in 25 introns. Most exons are small, ranging in size from 69 base pairs (exon 5; the smallest) to 3106 base pairs (exon 14; the largest–encoding the central B domain).
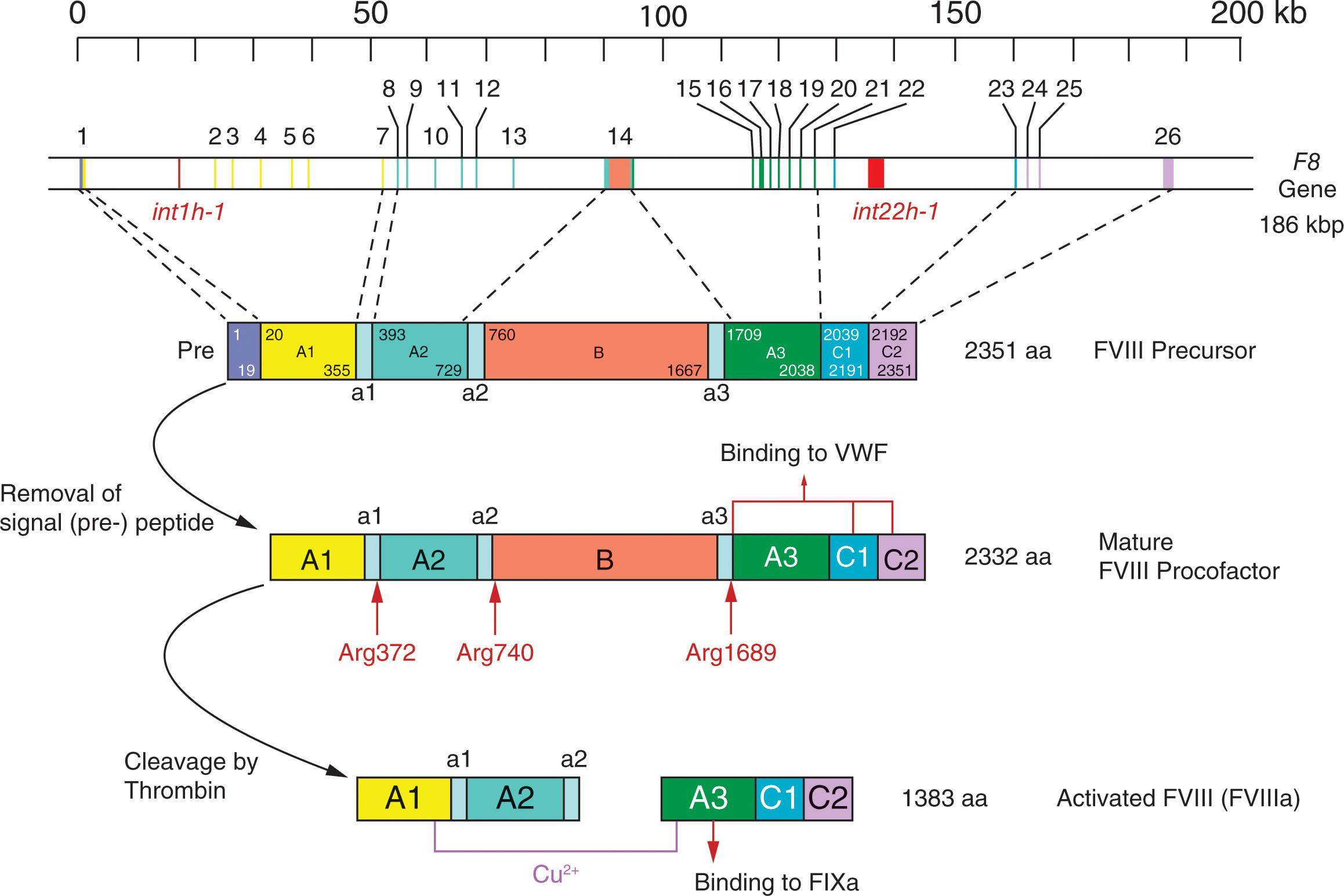
Within intron 22 of the F8 gene there are two additional coding elements, F8A and F8B . Although its function is unclear, the F8A sequence within intron 22 is frequently involved in intrachromosomal recombination that is described later. The F8B transcript is expressed in the same orientation as the native F8 mRNA, and this transcript comprises an initial intron 22 sequence that is spliced onto exons 23 to 26 of F8 . As with F8A, the F8B transcript is also ubiquitously expressed. Although the function of the protein is unknown because it contains the phospholipid binding region of FVIII it may play a role in membrane binding.
Transcription of the F8 gene is regulated by a promoter that contains binding sites for both tissue-specific and ubiquitous transcription factors. The mRNA transcribed from F8 is 9 kb in length and contains 7053 nucleotides of coding (i.e., translated) sequence and short 5′ and long 3′ untranslated sequences. Two reports using different conditional knockout approaches in mice have shown that the vascular endothelium is the predominant site of FVIII expression in this animal model. There is strong evidence that the liver sinusoidal endothelium is the source of FVIII production. This fits with the recognition that hemophilia can be cured by orthotopic liver transplantation. Other endothelial cell vascular beds, such as those found in lymph nodes, and spleen, may also contribute to FVIII production.
The transcription factors that regulate constitutive production of FVIII are not well defined but may include hepatocyte nuclear factor 1 (HNF1) and members of the CCAAT-enhancer-binding protein (C/EBP) and HNF4 families. FVIII also is an acute phase reactant, and studies have shown that FVIII expression can be induced through nuclear factor kappa-B (NFκB) and C/EBP. Working through these transcription factors, interleukin 6 can elevate FVIII expression as part of an acute systemic inflammatory response. FVIII levels are strongly influenced by circulating levels of its carrier protein, von Willebrand Factor (VWF). Both proteins are lower in blood group O individuals due to higher endogenous clearance of VWF and of the FVIII attached to it. This impacts on the pharmacokinetics of FVIII administration—lasts longer in non-O blood type individuals than in O blood type individuals.
FVIII biosynthesis has been extensively investigated in vitro using cell types that do not normally express this protein, such as Baby Hamster Kidney (BHK), Chinese Hamster Ovarian (CHO), and Human Embryonic Kidney (HEK 293) cells, among others. Little work has been performed on sinusoidal endothelial cells, the predominant cell type responsible for endogenous FVIII synthesis. More recently, transgene-produced FVIII in hepatocytes following gene therapy results in in-vivo expression of subnormal to normal circulating levels in this heterologous cell type. The biosynthesis of transgenic FVIII has also not been well characterized.
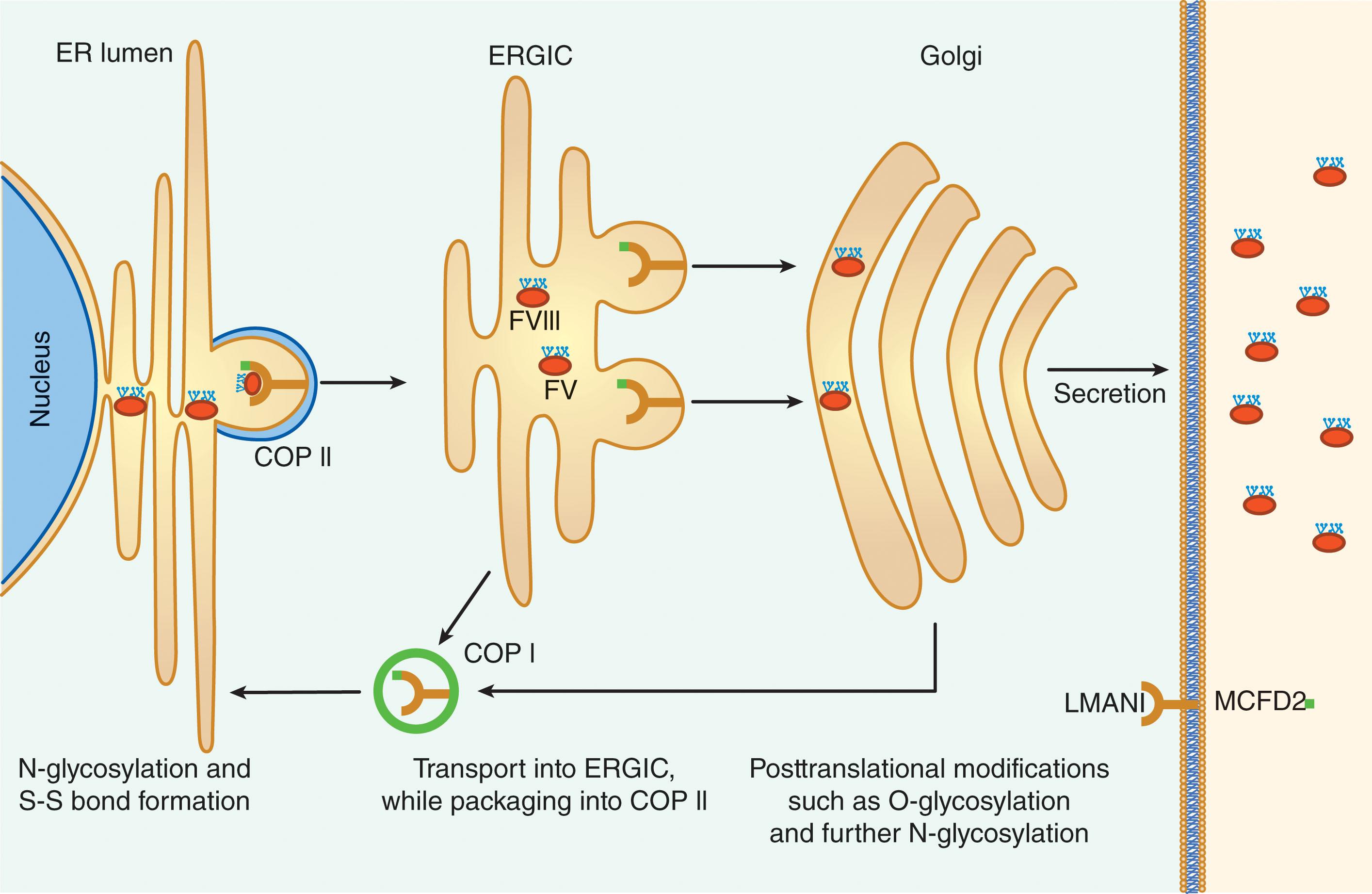
After production of the primary polypeptide chain and cleavage of the 19 amino acid signal peptide, the protein undergoes a series of posttranslational modifications, including N- and O-linked glycosylation mainly in the B domain and sulfation of tyrosine residues (see Fig. 134.1 ). Furthermore, when the nascent protein transits the endoplasmic reticulum (ER), it interacts with ER chaperones, including calreticulin and immunoglobulin binding protein (BiP). Although these interactions limit the transport of misfolded or aggregated forms of the protein in heterologous cells with high levels of FVIII expression, their relevance in native FVIII-producing cells is unknown. Indeed, under conditions of high-level FVIII expression, cells can activate a classical unfolded protein response (UPR) and can succumb to apoptotic cell death. The relatively unique challenge of processing FVIII has implications for gene therapy where high expression of B-domain-deleted FVIII in a small number of heterologous hepatocytes may trigger an UPR.
Efficient transit through the ER–Golgi boundary requires an interaction with glycans within the B domain of the protein. Absence of these glycans or lack of a specific cargo receptor complex that transports proteins (e.g., LMAN also known as ERGIC-53, multiple coagulation factor deficiency 2 [MCFD2]) markedly reduces levels of both secreted FVIII and FV, the latter resulting in the autosomal recessive disorder of combined deficiency of FVIII and FV ( Fig. 134.2 ).
The DNA sequence of the F8 gene predicts a translated single chain polypeptide with a molecular weight of 260 kDa consisting of 2351 amino acid residues, including a 19-residue signal peptide. Upon translocation to the ER, the signal peptide is cleaved. The remaining polypeptide is 2332 residues long.
The FVIII protein consists of three types of domains: the three A domains, which have a 35% to 40% amino acid sequence homology to ceruloplasmin and to FV; a central B domain with no known homologues; and two C-terminal domains that are also 35% to 40% homologous to ceruloplasmin and to FV (see Fig. 134.1 ). There are also three small “a” linker regions (each between 20 and 40 amino acids) consisting of predominantly acidic residues. The sequence of these domains within the protein is NH2-A1-a1-A2-a2-B-a3-A3-C1-C2-COOH. Tyrosine residues in the a2 and a3 domains, when sulfated, contribute to the cofactor function of FVIII and, with residues in the C1 domain, enable its interaction with VWF. Given the structural similarities between FVIII and FV it is not surprising that both function as cofactors for serine protease enzymes in the coagulation cascade: FVIII in the intrinsic tenase complex to generate FXa, and FV in the prothrombinase complex to generate thrombin. Of note, the FVIII B domain is not required for cofactor function; its principal role is to facilitate trafficking and secretion of the nascent polypeptide, and B-domain-deleted FVIII is fully active.
Subsequent proteolysis of the FVIII polypeptide chain in the Golgi generates a heavy chain of A1-A2-B domains with a mass of 90 kDa and a light chain consisting of the A3-C1-C2 domains with a mass of 80 kDa (see Fig. 134.1 ).
Circulating FVIII exists as a heterodimer of the NH 2 -terminus heavy chain and the COOH-terminus light chain. The heavy and light chains are bound to one another at the A1 and A3 domains by noncovalent bonds that are divalent metal ion dependent. The involved ion is likely copper, which has been found in association with the FVIII A3 domain.
After translation and ER/Golgi processing, FVIII is secreted into the circulation, where it forms a tight noncovalent complex with its multimeric partner VWF. Whereas the plasma concentration of FVIII is 100 to 200 ng/mL (≈1 nM), the concentration of VWF is approximately 10 μg/mL (50 nM); thus, the molar ratio of FVIII to VWF in the FVIII-VWF complex is about 1 to 50. The majority of VWF in plasma is synthesized and secreted by vascular endothelial cells. VWF binds strongest to the A3 and C1 regions of FVIII through sequences in the D’ region (D’D3) of the mature VWF monomer. The site at which FVIII and VWF first interact has been debated. However, confirmation that FVIII is expressed by endothelial cells suggest that at least some of the circulating FVIII may interact with VWF before secretion and may be co-stored with VWF in Weibel-Palade bodies.
Circulating levels of FVIII vary three-fold in the normal population (~50% to 150%), likely due to a combination of variability in synthesis and probably more importantly, variability in the clearance of FVIII mediated by VWF clearance. This in turn is related to ABO blood type as well as to other mechanisms leading to proteolysis and clearance of circulating FVIII/VWF. In plasma, VWF protects FVIII from proteolysis by lipid-binding proteases including FXa. Without this interaction—for example, in cases of type 3 VWD (in which VWF is absent) or type 2N VWD (in which mutations occur in the FVIII binding region of VWF—D’ region)—the plasma half-life of FVIII is markedly reduced and, consequently, plasma levels of FVIII may be very low.
VWF also protects FVIII from intracellular proteolysis by blocking binding of FVIII to cell surface lectin domain and scavenger receptors which target it for lysosomal degradation. VWF, with or without FVIII, is degraded via binding to lectin and scavenger receptors on the surfaces of Kupffer cells, hepatocytes, and endothelial cells. As the vast majority of FVIII circulates bound to VWF, FVIII much like VWF is cleared by VWF-specific catabolic pathways. However, VWF-free circulating FVIII, representing 2% to 5% of the total circulating FVIII, has accelerated clearance (6- to 8-fold greater), thus representing a physiological VWF-independent clearance pathway. Thus, most of the clearance and half-life of FVIII is regulated by the clearance of VWF. This places a ceiling on the potential gain in half-life achieved by bioengineering FVIII molecules via Fc fusion or PEGylation technologies. Knowing this, one extended half-life (EHL) FVIII (efanesoctocog alpha; Sanofi) still in development has overcome this ceiling effect on FVIII half-life extension by engineering FVIII to no longer bind to endogenous VWF.
VWF may also protect FVIII from recognition by the immune system in persons with hemophilia A. Endocytic uptake into antigen-presenting cells for presentation to CD4 + T cells occurs largely through the C1–C2 domains, which are blocked when FVIII is bound to VWF. All of this is in keeping with some clinical evidence suggesting a lower rate of neutralizing antibody development (inhibitors) with plasma-derived FVIII containing VWF than with recombinant FVIII.
FVIII plays a critical role in the propagation (amplification) phase of coagulation. The physiological activator of FVIII is thrombin, which proteolytically cleaves FVIII at three sites: Arg372 at the NH 2 -terminus of the A2 domain, Arg740 at the NH 2 -terminus of the B domain, and Arg1689 at the NH 2 -terminus of the A3 domain (see Fig. 134.1 ). These cleavages release FVIII from VWF and result in the formation of a noncovalently associated A1-A2-A3/C1/C2 heterotrimeric activated FVIII (FVIIIa) molecule. FVIII can also be activated by FXa and FIXa, although the physiological contribution of activation by these proteases is less clear.
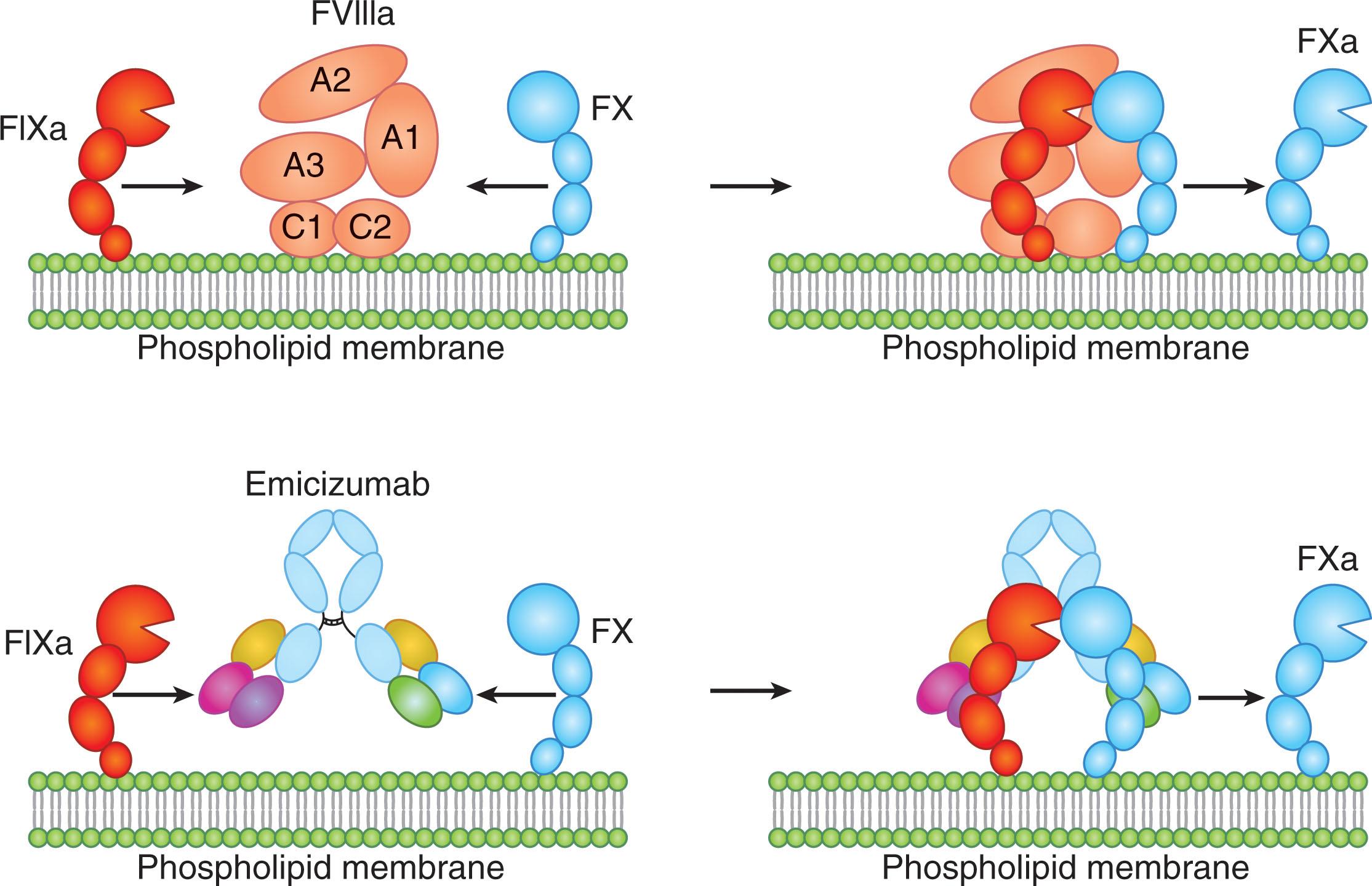
In its activated form, FVIIIa provides essential cofactor activity in the intrinsic tenase complex where FIXa is the serine protease and FX is the substrate ( Fig. 134.3 ). This reaction takes place on a procoagulant phospholipid surface, which in normal hemostasis is likely the activated platelet. Participation of FVIIIa in the complex enhances the catalytic efficiency of this reaction about 200,000-fold, and thus severe FVIII deficiency profoundly reduces the rate of FXa generation and renders this reaction biologically futile. The cofactor role of FVIIIa is assumed to be based on it serving as a “scaffold” protein that optimally aligns the enzymatic and substrate components of the complex (FIXa-FVIIIa-FX) on the phospholipid surface. The cofactor binds to the phospholipid surface through hydrophobic residues in the FVIII C1 domain. Emicizumab was developed by capitalizing on the scaffolding effect of FVIIIa. Serving as a FVIII mimetic, emicizumab is a bispecific monoclonal antibody that binds to both FIXa and FX and bridges them together thus substituting for the cofactor role of FVIIIa.
FVIIIa is inactivated via two processes (see Chapter 120, Chapter 124 ). The predominant mechanism is through spontaneous dissociation of the A2 domain. The second is via activated protein C–mediated proteolysis at Arg336 and Arg562 in the FVIIIa heavy chain (see Fig. 134.1 ).
Hemophilia A is a disorder characterized by congenital deficiency of FVIII. Almost all patients with hemophilia A have F8 gene mutations. Because the F8 gene is in the most distal band (Xq28) on the X chromosome, hemophilia A follows an X-linked inheritance pattern. As a result, most affected individuals are male. Severe and moderately severe cases of hemophilia A are unusual in females but can result from the genetic mechanisms listed in Table 134.1 .
Approximately 50% of cases of hemophilia A are caused by sporadic mutations and occur without a family history of the disorder. The sporadic mutation most commonly occurs in a female making her a carrier. However, because most carriers do not have excessive bleeding, the “new carrier” is unlikely to ever be diagnosed, or if diagnosed, not until later in life if she has an affected son or grandson.
The molecular genetic basis of hemophilia A has been extensively characterized over the past 35 years. A comprehensive Internet database of hemophilia A mutations is maintained and updated at https://f8-db.eahad.org/ .
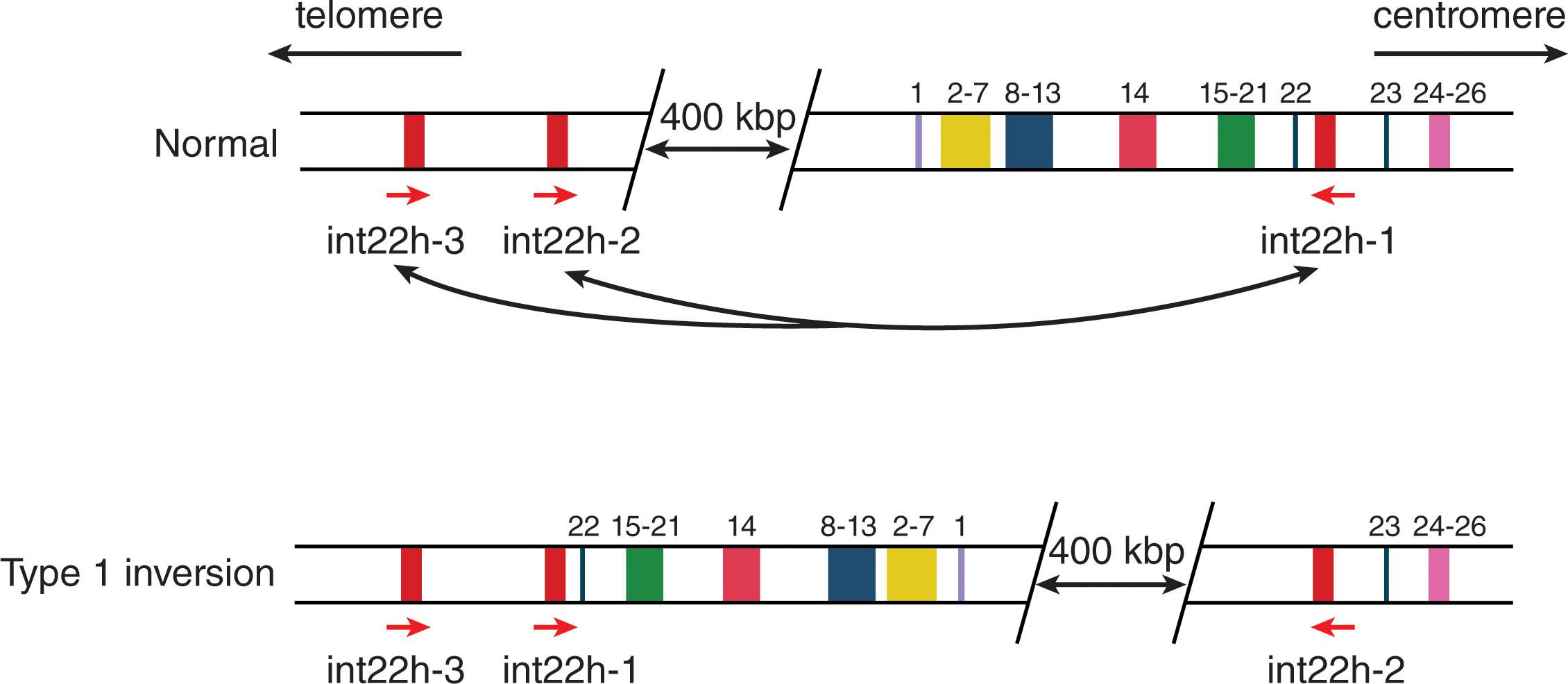
Approximately 45% of cases of severe hemophilia A are the result of a recurrent mutation, an inversion mutation involving sequences in intron 22 of the gene ( Fig. 134.4 ). The mechanism responsible for this mutation involves an intrachromosomal recombination event in which there is an exchange between the F8A gene in intron 22 and one of two extragenic copies of this sequence that are located 5′ and telomeric to F8 . The consequence of this event is inversion of the F8 gene between exons 1 and 22 and although two distinct F8 transcripts are expressed from separate promoters (exons 1 to 22 and exons 23 to 26), a contiguous FVIII protein sequence is not synthesized. The origin of this recurrent mutation is almost exclusively in the male germline (i.e., in sperm cells) where the single X chromosome has no partner to pair with, thereby potentially facilitating the F8A-mediated intrachromosomal recombination. The origin of this mutation in male germline explains why hemophilia often arises in daughters of older men whose sperm cells carry a greater number of mutations.
A second recurrent inversion mutation involving intron 1 of the F8 gene is responsible for approximately 2% of severe hemophilia A cases. Other mutations responsible for hemophilia A include more than 3000 DNA variants with an array of missense, nonsense, frameshift, insertion or deletion, duplication, and splicing mutations. In addition, transcriptional mutations have been identified in the F8 promoter region. All regions of the gene are potential mutation targets but certain sequences, such as the CpG dinucleotide in arginine codons, are more prone to mutation because of spontaneous methylation of the 5′ cytosine and spontaneous deamination to thymine. Indeed, the recent identification of mutations deep within introns of the gene, which may alter F8 mRNA splicing, suggests that almost all hemophilia A mutations are likely to be found within or adjacent to the F8 gene.
Extensive genotype-phenotype studies in hemophilia A reveal a good correlation between null mutations (mutations that result in no production of factor) and severe disease and between non-null (largely missense) mutations and moderate or mild phenotypes. The phenotype of the disease remains generally consistent with specific mutations. One exception may be mutations within the B domain, which is dispensable for FVIII activity but is important for secretion of the protein after it undergoes post-translational modifications. Proteins synthesized with putative hemophilia causative B domain variants have normal activity, suggesting that many may be polymorphisms.
In addition to its utility for family counseling, the F8 genotype is also a strong predictor for the development of anti-FVIII antibodies ( Table 134.2 ). Multidomain deletions are associated with the highest risk (≈75%) of inhibitor development, while most missense and splicing mutations are associated with the lowest risk (<10%). The largest group is those with an average risk of 20% to 30%; this includes patients with inversion, nonsense, and insertion/deletion mutations. Even within these categories there is significant variability in the risk of inhibitor development; for example, some missense mutations in the C2 and C3 domains are associated with much higher risk of inhibitor development than missense mutations in other domains.
| Large insertion/deletions | ≈75% |
| Light chain nonsense mutations | 30%–40% |
| Inversion mutations (I-22 and I-1) | 20%–25% |
| Small insertion/deletions | 15%–25% |
| Small non-A run insertions/deletions | 15%–20% |
| Heavy chain nonsense mutations | 10%–20% |
| Factor VIII missense mutations | <10% |
| Small A run insertions/deletions | <5% |
| Splicing mutations | <5% |
The gene that encodes FIX ( F9) was cloned and characterized by two groups in the early 1980s. The gene is located on the X chromosome at cytogenetic band Xq27 and spans 34 kb of genomic sequence ( Fig. 134.5 ). The eight exons (previously designated as exons A to H and now as exons 1 to 8) of the F9 gene encode an mRNA transcript of 2.8 kb that is exclusively expressed in hepatocytes.
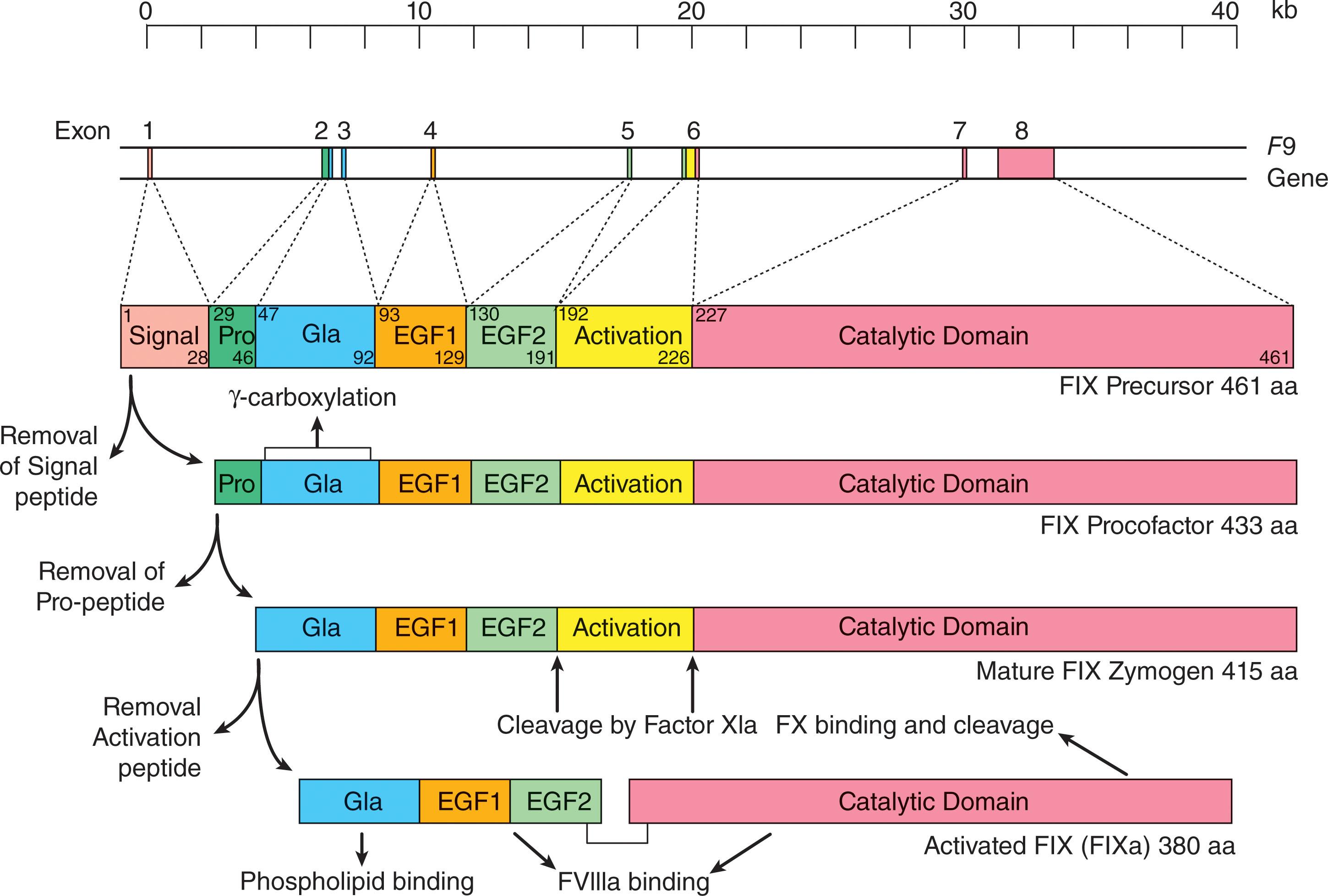
FIX circulates in plasma at a concentration of approximately 5 μg/mL (90 nM). The mature circulating protein consists of 415 amino acids and has a molecular weight of 57 kDa (see Fig. 134.5 ). The protein is initially synthesized as a pre-pro-polypeptide with a short signal sequence, which facilitates entry into the ER, and a pro-polypeptide sequence, which interacts with γ-glutamylcarboxylase in the ER. The interaction with this microsomal enzyme catalyzes the post-translational conversion of the 12 amino-terminal glutamic acid residues of mature FIX into γ-carboxyglutamyl residues (the GLA domain). This post-translational modification enables FIX to form calcium-dependent interactions with procoagulant phospholipid membranes (see Chapter 125 ). A second post-translational modification at residue 64 in the first epidermal growth factor (EGF)–like domain of the mature polypeptide involves β-hydroxylation of asparagine. This modification also facilitates interactions with calcium and phospholipid membranes.
The molecular arrangement of FIX consists of an amino-terminal GLA domain followed by two EGF-like domains, an activation peptide, and the carboxyl-terminus catalytic domain, which is similar to the organization of procoagulant FVII and FX and the anticoagulant protein C (see Chapter 125 ). The characteristic feature of all serine proteases is the catalytic triad which in FIX is comprised of His267-Asp315-Ser411. These three residues form the active site of the enzyme which is responsible for cleavage of the Arg234-Ile235 scissile bond in FX that is required for its activation.
The FIX zymogen is activated by two processes; proteolytic cleavage mediated by FXIa and by a similar process involving the FVIIa–tissue factor complex. The activation of FIX involves sequential proteolysis of two peptide bonds at Arg191-Ala192 and Arg226-Val227 (see Fig. 134.5 ). These cleavages release the FIX activation peptide and result in the formation of the disulfide-linked FIXa enzyme with an NH 2 -terminus light chain and a COOH-terminus heavy chain. Whereas the light chain binds to procoagulant phospholipid surfaces through a calcium-mediated process involving the GLA domain, the C-terminus catalytic domain cleaves and activates the substrate FX. This reaction involves the so-called intrinsic tenase complex in which FVIIIa participates as an essential cofactor. Absence of FVIIIa in the tenase complex reduces the catalytic efficiency of the reaction by approximately 200,000-fold.
FIXa is inhibited through an interaction with the natural anticoagulant protein antithrombin (AT). The fate of FIXa–AT complexes is incompletely understood, but specific AT–protease receptors on the surface of hepatocytes participate in this process.
To date, all variants responsible for hemophilia B have involved the F9 gene. Genetic analyses of large numbers of patients with hemophilia B have detected variants in the F9 gene in more than 98% of cases, and it may well be that the remaining variants are located within intronic sequences that are not readily accessible using standard techniques ( Fig. 134.6 ).
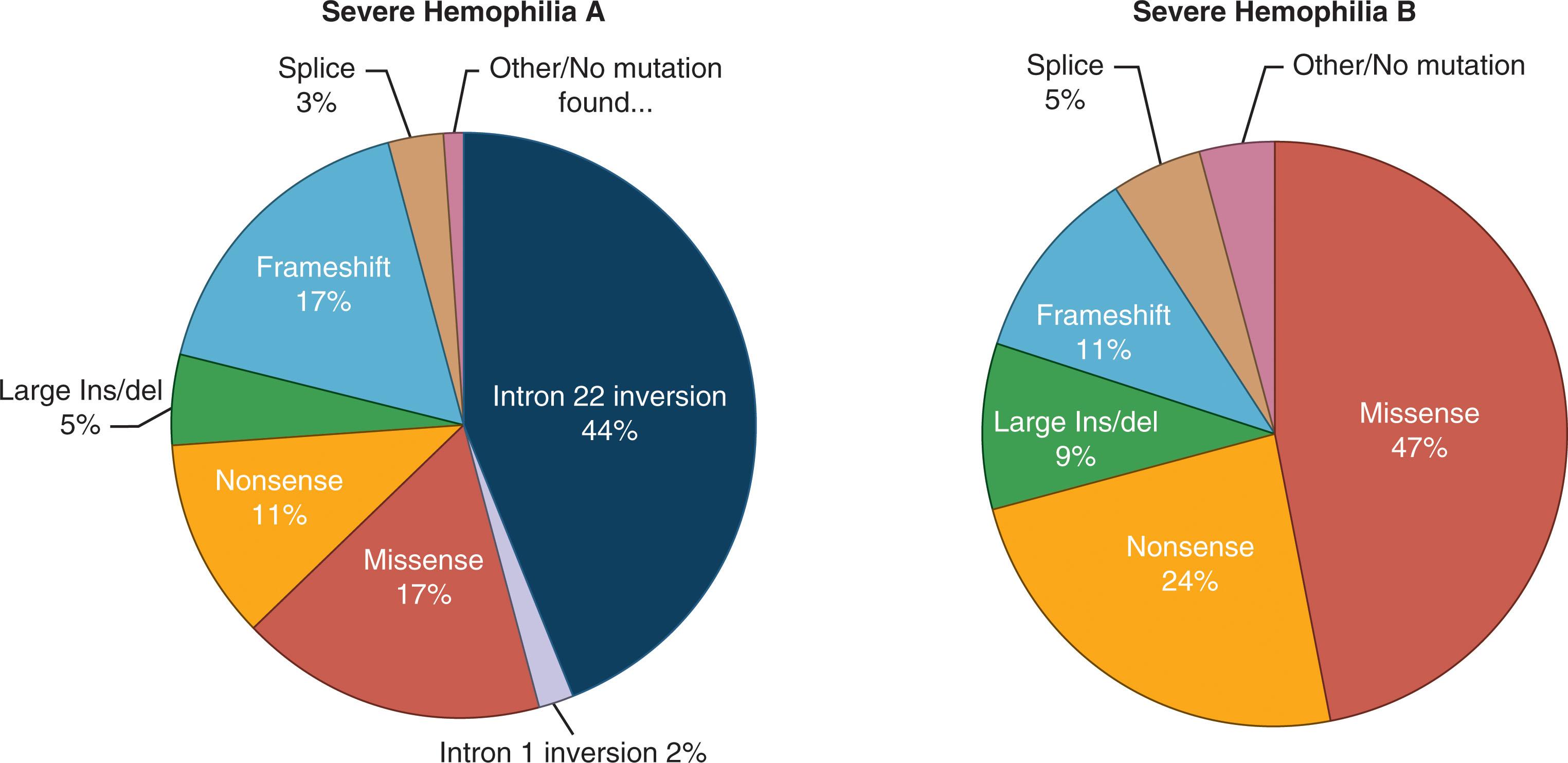
In marked contrast to hemophilia A, in which approximately 50% of variants resulting in severe disease are caused by two recurrent gene inversion events, approximately 75% of variants in hemophilia B are missense substitutions. There are no common recurrent F9 variants, although mild forms of hemophilia B may show a founder effect in some populations.
Despite the predominance of missense variants, multiple other variants can produce hemophilia B ranging from large gene deletions to a mix of nonsense, insertion or deletion, and splicing changes. The full array of F9 variants responsible for hemophilia B can be viewed in an internet database at http://f9-db.eahad.org/ .
The most biologically remarkable hemophilia B variant was discovered in Leyden and is eponymously named after that city. FIX deficiency with this variant results from disruption of a 40-bp region of the F9 promoter around the hepatic transcription factor binding sites which are responsible for gene expression during early childhood. If the variant spares an androgen responsive element in the promoter, gene expression normalizes with increasing androgen (testosterone) levels in puberty and beyond. In this form of hemophilia B, FIX levels gradually increase from less than 5% at birth to greater than 30% by early adulthood. Because of the low FIX levels early in life, boys with this disorder often have bleeding episodes consistent with moderate hemophilia B. After puberty, FIX levels and bleeding phenotype improve or even resolve to the extent that the label of hemophilia is often removed from these patients.
Since the cloning of the F9 gene in the 1980s, the association of F9 gene deletions with FIX antibody formation has been well recognized. While most missense mutations causing hemophilia B are associated with a low risk of inhibitor development, large deletion mutations are associated with a higher risk (30% to 50% risk). In addition to neutralizing FIX enzymatic function, these antibodies can also be associated with anaphylactic or anaphylactoid reactions to FIX infusion. Antibodies usually develop in young children (1 to 2 years old) after 10 to 20 exposures to FIX concentrate. In such patients, FIX concentrate use is generally no longer of benefit and attempts at inducing immunologic tolerance to FIX may be complicated by the development of nephrotic syndrome. Further discussion about inhibitors in hemophilia B is found later.
Hemophilia B is the bleeding disorder which in the past was associated with many of the royal families of Europe. Although the clinical picture in these individuals was consistent with severe hemophilia, the precise diagnosis was not reported until 2009. The “Royal” F9 variant is a splicing abnormality occurring at the 3′ end of intron 3 that creates a new splice acceptor sequence that results in a novel out-of-frame transcript with 11 new amino acids followed by a premature stop codon. This variant is predicted to result in nonsense-mediated decay of the aberrant FIX mRNA, which would inevitably result in a severe phenotype.
Missense variants elsewhere in the F9 gene have been informative with regard to our detailed understanding of protein structure and function. Variants at both activation peptide cleavage sites have been described, and as with many genes, recurrent “hotspot” variants have been documented at arginine codons with CpG dinucleotide sequences.
The initial diagnosis of hemophilia depends on the family context. In families in which hemophilia has previously been identified, family counseling can determine the risk of hemophilia transmission and usually results in a diagnosis being made in utero or early in neonatal life. In contrast, when there is no family history of the disease, the diagnosis will not be made until there are signs of bleeding, and the timing of this depends on the extent of the factor deficiency (i.e., disease severity). With severe deficiency, the diagnosis is usually made in the first 1 to 2 years of life, but with moderate disease, the diagnosis may be made later, and with mild hemophilia, the diagnosis may be delayed until well into adulthood when bleeding occurs after a surgical intervention.
The diagnosis of hemophilia involves measurement of FVIII or FIX coagulant levels in platelet-poor plasma using a functional clotting assay. Although most clinical hemostasis laboratories use a one-stage PTT (i.e., clot-based) test to quantify FVIII, some laboratories use either a two-stage or a chromogenic assay that measures the generation of FXa in a purified system (see Chapter 127 ). In these latter assays, the incubation time is often longer, which may influence test results. For example, some missense mutations causing mild hemophilia A result in the synthesis of an unstable FVIII with enhanced dissociation of the A2 domain from the remainder of the protein ( Fig. 134.7 ). These unstable mutants can occasionally be missed when a one-stage FVIII assay is used but are readily apparent with two-stage or chromogenic assays that use a prolonged incubation time. Consequently, when a diagnosis of mild/moderate hemophilia A is made using a one-stage assay, it is advisable to also perform a chromogenic assay to better determine the extent of the deficiency.
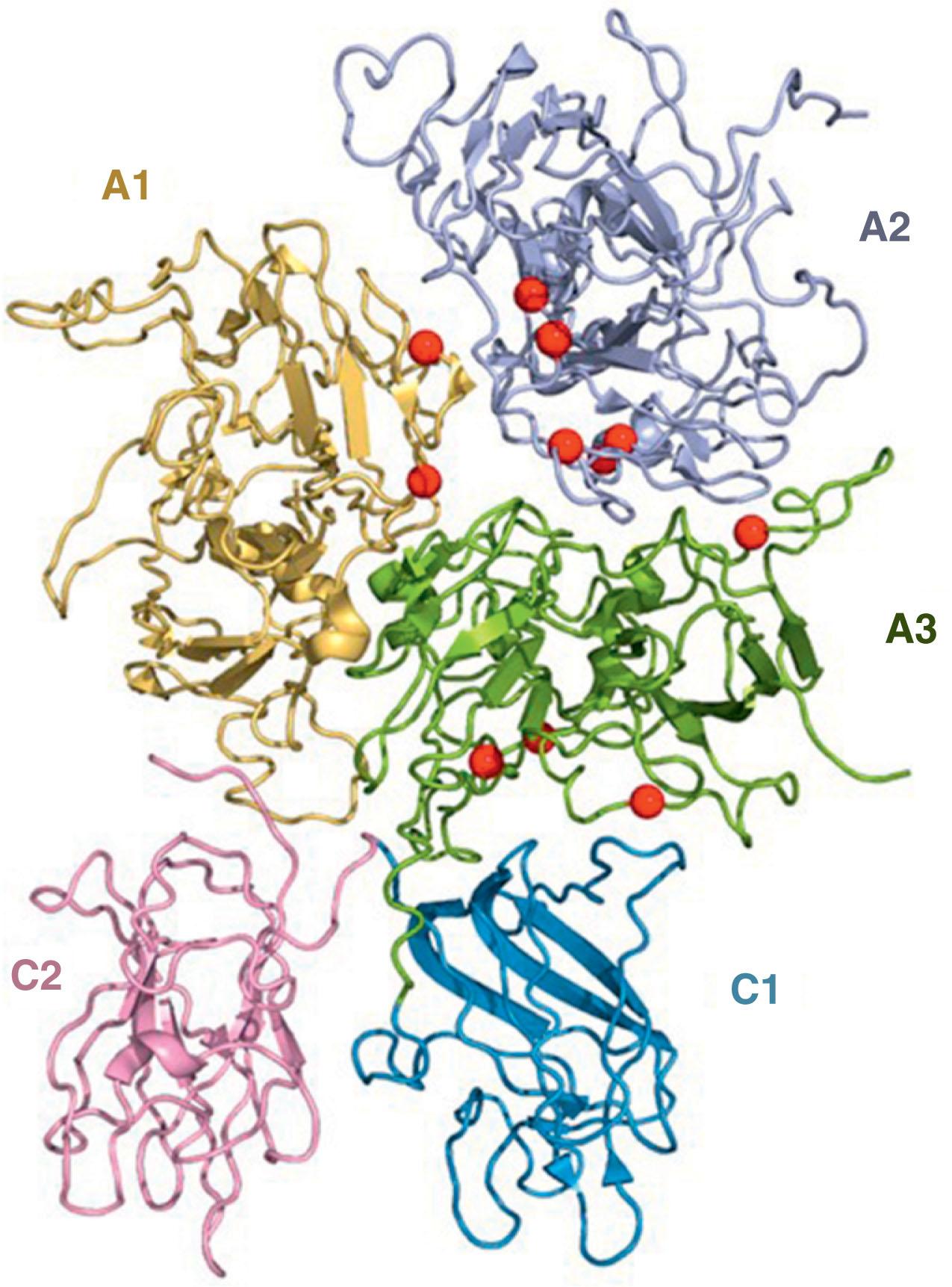
The severity of hemophilia is based on the clotting factor level (severe, <1% [<0.01 IU/mL]; moderate, 1% to 5% [0.01 to 0.05 IU/mL]; and mild, >5% to 40% [>0.05 to 0.40 IU/mL]). When making a diagnosis of hemophilia B, caution must be taken in neonates because levels of FIX can be low in newborns because of their immature glutamylcarboxylase system. FIX levels double in the first few years of life and continue to rise slowly until puberty. Consequently, a child initially labeled as having moderate hemophilia may be reclassified as having mild hemophilia later in life and a child initially diagnosed as having mild hemophilia B may later have normal FIX levels. In general, a FIX level greater than 20% in a neonate is considered normal as is a FIX level greater than 30% at 12 months of age.
The other diagnostic strategy that can be used for the diagnosis of hemophilia involves DNA analysis. Since the cloning of the FVIII and FIX genes in the early 1980s, molecular genetic approaches to hemophilia diagnosis have advanced dramatically. There are now greater than 3000 different F8 mutations associated with hemophilia A and greater than 1000 F9 mutations documented in hemophilia B.
The strategy for genetic analysis depends on the type of hemophilia and the severity of the phenotype. For example, all patients with severe hemophilia A should be screened initially for the recurrent F8 intron 22 and intron 1 inversion mutations. In contrast, most patients with hemophilia B require full-sequence analysis of the entire FIX promoter, coding region, and splice sites.
If a familial mutation has already been identified in the FVIII or FIX gene, prenatal diagnosis can be accomplished with chorionic villus sampling or by amniocentesis. Outside of the prenatal context, mutation testing in hemophilia can be used for carrier detection for family planning purposes and as one component of the risk analysis for inhibitor development (see box on Hemophilia Carrier Detection and Prenatal Diagnosis ). Determination of the hemophilic genotype is now regarded as standard of care.
The initial clinical suspicion of hemophilia usually arises from signs and symptoms of excessive bleeding, a family history of a bleeding problem, or abnormal coagulation test results.
The initial laboratory screening test suggestive of hemophilia is a prolonged PTT. If the PTT normalizes after mixing with control plasma, factor levels are then measured to pinpoint the cause of the prolonged PTT. Although FVIII or FIX deficiency are associated with a prolonged PTT, deficiencies of FXI or FXII or combined deficiencies of various coagulation factors are other possibilities (see Chapter 135 ).
A low plasma FVIII level is not synonymous with a diagnosis of hemophilia A. Several disorders are associated with low FVIII levels ( Table 134.3 ). Levels of FVIII below 10% are most often the result of inherited or acquired hemophilia A (in adults) or a severe form of inherited or acquired VWD (severe type 1 or type 3 VWD). Determination of VWF antigen and VWF activity levels is essential to differentiate these conditions. Levels of FVIII below 10% can also occur with type 2N VWD (see Chapter 133 ). This diagnosis can be confirmed with FVIII binding studies or by genotypic analysis of the FVIII binding codons of VWF (exons 17 to 25, which encode the D′D3 regions of the VWF protein). Levels of FVIII between 10% and 50% are likely the result of either mild hemophilia A or VWD (types 1 or one of the type 2 variants: 2A, 2B, 2M, or 2N). Assessment of VWF levels (antigen and activity) must be undertaken in these cases. Rarely, mild or moderate FVIII deficiency can be co-inherited with FV deficiency. Combined inherited deficiency of FVIII and FV (levels are usually between 5% and 20%) has a prevalence of approximately 1 per million. This rare inherited trait is caused by recessive mutations in one of two genes involved in protein transport across the ER-Golgi interface: lectin mannose binding protein type 1 (LMAN1) or MCFD2.
|
|
|
|
Isolated low plasma levels of FIX are almost always caused by congenital hemophilia B. In contrast to acquired hemophilia A, autoantibody development against FIX (i.e., acquired hemophilia B) is rarely encountered. Other situations in which FIX deficiency is found usually involve concomitant reductions in the other vitamin K–dependent clotting factors, such as occurs with vitamin K antagonists, vitamin K deficiency, or severe liver disease (see Chapter 126 ). A much less common cause of a mild deficiency of all the vitamin K–dependent proteins (FII, VII, IX, and X) is hereditary combined vitamin K-dependent clotting factors deficiency (VKCFD), an autosomal recessively inherited defect in the γ-glutamylcarboxylase ( GGCX ) gene, coding for the γ-carboxylase enzyme required for the posttranslational modification of these proteins.
The symptoms and signs of hemophilia A and B are identical and involve a propensity for prolonged and excessive bleeding (see Chapter 126 ). The bleeding tendency in hemophilia is determined in large part by the baseline activity level of the deficient or defective clotting factor. Thus, in severe hemophilia (A and B), in which the baseline clotting factor activity level is below 1% (0.01 IU/mL), pathological bleeding can occur in the neonatal period, especially intracranial bleeding from the delivery process. However, most frequently, severe disease manifests with easy bruising or soft tissue or joint bleeding beginning at around 6 to 12 months of age when children become more mobile. Excessive bleeding after circumcision can prompt diagnosis in the perinatal period. Without clotting factor prophylaxis, the annual incidence of bleeding increases in the first 5 to 6 years of life and plateaus at an average of 20 to 40 bleeds per patient per year.
Most patients with mild hemophilia (factor levels of >5% [>0.05 IU/mL]) only experience bleeding with trauma or surgery and the disease may go undiagnosed until later in life.
The bleeding phenotype in patients with moderate hemophilia (FVIII or FIX levels between 1% and 5% [0.01 and 0.05 IU/mL]) can resemble that of patients with severe or mild hemophilia. Thus, the severity and frequency of bleeding may correlate poorly with the factor activity level and even patients with FVIII or FIX levels less than 1% or with the same mutation may have variable phenotypes for the reasons listed in Table 134.4 . All these reasons for phenotypic variability may explain why some patients bleed much less than would be expected based on their factor levels while others bleed much more. These differences may also explain why some patients with joint bleeds develop arthropathy and others do not.
| 1. | Differences in factor levels |
| 2. | Differences in mutations causing hemophilia: null vs. non-null mutations |
| 3. |
|
| 4. |
|
| 5. | Differences in the pharmacokinetic handling of factor. In the case of hemophilia A this includes the effect of ABO blood group and endogenous levels of von Willebrand factor (for patients on factor prophylaxis) |
| 6. | Differences in the pharmacokinetics and pharmacodynamics of emicizumab (for those patients on emicizumab) |
| 7. | Differences in levels of physical activity |
| 8. | Differences in the structural integrity of joints, making patients more/less susceptible to joint bleeds and joint damage |
Hemophilia A and B are characterized by similar types of bleeds. Although not conclusively proven, several studies suggest that with similar factor levels, the bleeding phenotype in hemophilia A is less severe than that in hemophilia B as evidenced by a younger median age for the first joint bleed and for start of prophylaxis, as well as increased use of prophylaxis and greater need for joint arthroplasty in patients with severe hemophilia A than in those with severe hemophilia B. Likewise, in children with moderate disease, a higher proportion with hemophilia A than with hemophilia B require prophylaxis. Although the reasons for these differences are incompletely understood, the genetics of the disorders are a likely cause; most patients with severe hemophilia A have null mutations and produce no FVIII, whereas most of those with hemophilia B have non-null mutations and synthesize small amounts of FIX. In addition, in patients with hemophilia B, the stress-induced release of FVIII with bleeds may augment the hemostatic potential of the residual FIX as FVIII is a cofactor for FIX.
Although bleeding is the hallmark of hemophilia, the types of bleeds and issues facing patients vary depending on age. This is particularly true in newborns where issues related to the birthing process can occur, which are not encountered later in life. Also, the problems in infancy, which is the highest risk period for development of inhibitors and the time when prophylaxis protocols are established, are different from those encountered later in life.
The hazards of hemophilia are greatest in the neonatal period, particularly for those with moderate or severe disease. Newborns with hemophilia can be born to mothers who are known or suspected hemophilia carriers or can be born to mothers who are not known to be carriers as there is no family history of hemophilia. The latter scenario, which is encountered in about 50% of cases, is associated with the highest risk of bleeding because no precautions are taken. The optimal method for delivery of children with known or suspected severe hemophilia is uncertain. Most experts recommend atraumatic vaginal delivery to avoid the maternal risks associated with cesarean section and because delivery by cesarean section does not eliminate the risk of intracranial hemorrhage (ICH) ( Fig. 134.8 ). Vacuum extraction or forceps delivery should be avoided because they increase the risk of extracranial bleeding (e.g., subgaleal hemorrhage and cephalohematoma) and ICH. Fetal blood sampling is not a significant risk factor for ICH.
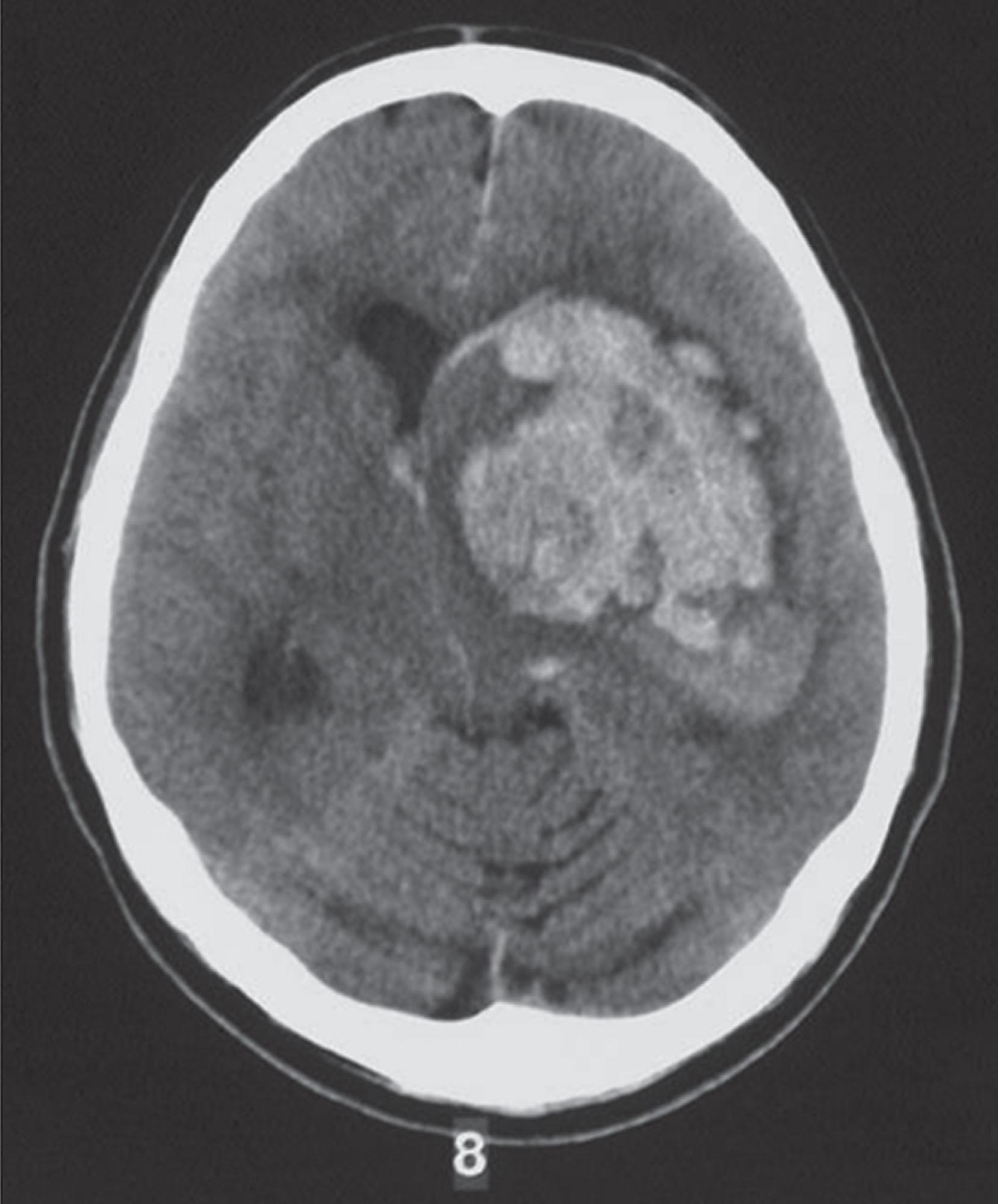
The incidence of ICH in newborns with severe hemophilia is approximately 3% to 4%, which is 40- to 80-fold higher than the risk in the non-hemophilic population. If a newborn male suspected of having hemophilia has any sign of ICH (e.g., unequal pupils, seizures, vomiting, or lethargy), the appropriate factor concentrate should be promptly administered.
If signs of ICH occur in newborn boys without a family history of hemophilia, the PTT should be determined along with central nervous system imaging. If the PTT is prolonged and imaging reveals ICH, the levels of FVIII, FIX, and VWF should be urgently quantified. While waiting for the test results, fresh plasma (10 mL/kg) can be given. Alternatively, because FVIII deficiency is more common than FIX deficiency, a FVIII concentrate can be administered, and the PTT determined 10 to 15 minutes later. If the patient has hemophilia A or type 3 VWD, the PTT will correct; with type 3 VWD, the correction will be transient because of the short half-life of FVIII in this disorder (see Chapter 133 ). If the PTT fails to correct, the child likely has FIX deficiency, and a FIX concentrate can then be given. If FVIII concentrate is given, fresh plasma can be administered while waiting for the PTT results to cover the possibility of hemophilia B.
Surprisingly, excessive bleeding after circumcision only occurs in about half of boys with severe hemophilia. Consequently, the lack of excessive bleeding after circumcision does not exclude the diagnosis of hemophilia. In children suspected of having hemophilia, the diagnosis should be confirmed before circumcision. If hemophilia is confirmed and circumcision is desired, the appropriate factor concentrate should be administered before the procedure. A single dose prior to circumcision is usually sufficient although some clinicians give more after the procedure.
With no or with inadequate treatment, incomplete resorption of blood after repeated hemorrhage into muscles can result in the formation of pseudotumors—walled-off cystic structures surrounded by a fibrous membrane that can become multivacuolated and calcified over time. These cystic lesions can expand and destroy adjacent structures leading to bone fractures and deformities. Another rarer type of pseudotumor seen mostly in adults arises within the bone secondary to repeated subperiosteal bleeding. This type of pseudotumor usually occurs in the long bones of the lower extremities and in the pelvis. Distal pseudotumors in the hand are more common in children and are associated with pain from rapid growth or nerve compression.
Pseudotumors are usually diagnosed with ultrasonography or MRI and can be misdiagnosed as a neoplasm (e.g., Ewing sarcoma or osteosarcoma) or an infection (e.g., osteomyelitis or tuberculous abscess). Biopsy is contraindicated because of the potential for bleeding or infection. Small pseudotumors, particularly distal ones or pseudotumors in patients with inhibitors, can be treated conservatively with aggressive prophylactic therapy with clotting factor replacement (there are no data on long-term prophylaxis with emicizumab in this setting) along with immobilization of the affected limb.
If prophylactic therapy alone is insufficient, surgical excision is needed. This carries potential morbidity and even mortality and should only be undertaken by skilled surgeons in conjunction with appropriate hemophilia specialists. Embolization and radiation have been successful for treatment of small pseudotumors of the hand.
The bleeding pattern in severe hemophilia is distinct and is rarely seen with other bleeding disorders. Although all types of bleeds can occur in hemophilia, musculoskeletal bleeds (hemarthrosis, muscle bleeds, and hematomas) are the most common and are responsible for most of the long-term complications. Other common sites for bleeding include the oral cavity, gastrointestinal (GI) and genitourinary tracts, and intracranial bleeding. Additionally, insufficiently treated muscle or soft tissue bleeds may result in pseudotumor formation (see box on Hemophilic Pseudotumor ) or compartment syndrome (see box on Compartment Syndrome ). Finally, bleeds can occur with surgery, dental procedures, or trauma.
A compartment syndrome arises when a bleed (usually trauma- induced) occurs into a closed (encapsulated) space, such as the forearm or calf. The capsule restricts exit of blood, thereby raising the pressure within the compartment with resultant vascular compression, obstruction of blood flow, tissue ischemia, and the potential for nerve and muscle damage. Compartment syndrome is characterized by severe pain and swelling, limb pallor, paresthesia, and reduced limb movement. This condition requires urgent factor replacement and surgical decompression (fasciotomy) may be required. Without treatment, compartment syndrome may lead to permanent neuropathy, tissue necrosis, and even loss of the limb.
Joint bleeds account for about 75% of all bleeding events in hemophilia and are a hallmark of the disorder. Although bleeds can occur in any joint, the most commonly involved are the ankles, knees, and elbows, the so-called index joints . Less commonly involved joints are the shoulders, hips, and wrists. The least involved are the joints in the hands and feet.
The first onset of joint bleeding is highly variable. Although the median age for the first joint bleed is 1.8 years, some children experience their first bleed earlier as they begin to ambulate and others have it later in the fourth, fifth, or sixth years of life. Without prophylaxis, hemarthroses, which can be spontaneous or related to trauma, become more common with age. The trauma may be imperceptible, particularly in infants who are unable to vocalize the occurrence of trauma. Even minimal trauma during sleep can trigger awakening with joint pain and swelling.
In patients with severe/moderate hemophilia, bleeding into joints continues until hemostatic therapy is given or until the extravascular pressure within the joint increases to the point where bleeding vessels are occluded. With the latter scenario, the joint pain may be excruciating. Although bleeding may stop spontaneously in patients with mild hemophilia, there still can be substantial accumulation of blood in the joint. Therefore, joint bleeds should be treated promptly with factor concentrates to reduce the risk of long-term complications. Joint aspiration to remove blood may be of benefit with bleeds into large joints such as the hips (see box on Hip Pain in Hemophilia ).
Pain in the hip joint region can be caused by several conditions, for example, hip joint bleed, iliopsoas muscle bleed, bleed into surrounding muscles, retroperitoneal bleed, or appendicitis. Bleeds into the hip joint and iliopsoas muscle are serious and difficult to differentiate. Both cause pain and restricted movement of the hip joint with a tendency to maintain the leg in a partially flexed position, the position of lowest pressure. Consequently, ultrasonography or MRI is essential for accurate diagnosis; plain radiographs are insufficient.
Iliopsoas muscle bleeds can rapidly expand because there is no surrounding connective tissue to restrict their growth. This can lead to a significant decrease in the hemoglobin level and a need for blood transfusion. Furthermore, pressure on the femoral nerve can cause paresthesia, hyperesthesia, or weakness of the quadriceps muscle.
Treatment of iliopsoas muscle bleeds or bleeding into the hip joint involves prolonged administration of factor concentrates and immobilization and subsequent physical therapy, often necessitating hospitalization.
The management of an acute hip joint bleed is somewhat different from management of bleeds in other joints because of the vascular anatomy of the hip joint, which renders the head of the femur vulnerable to ischemia. Persistent pain despite appropriate factor replacement may indicate ischemia in the femoral head and ultrasound-guided joint aspiration by an experienced interventional radiologist or surgeon should be considered. Graded physiotherapy should be instituted when there is symptomatic improvement. Follow-up imaging studies (MRI, bone scan, or both) should be considered for assessment of avascular necrosis.
With repeated episodes of joint bleeding, most older children, adolescents, and adults with hemophilia can identify early intraarticular bleeding before the classical signs appear through a feeling of warmth and tingling in the affected joint.
Joint damage occurs through at least three mechanisms: iron toxicity, inflammation, and mechanical distension of the joint. Repeated joint bleeding causes inflammation and hyperplasia of the synovial tissue ( synovitis ) which is the first step in the development of hemophilic arthropathy.
Repeated bleeding into the same joint three times over a 6-month period qualifies that joint as a target joint. Such joints are susceptible to further bleeding unless prophylaxis is given resulting in a vicious cycle that ultimately leads to destructive joint damage.
Early stage hemophilic arthropathy is characterized by synovial hyperplasia, extensive destruction of articular cartilage, progressive loss of joint space, cystic changes within the subchondral bone, osteoporosis, and atrophy of surrounding muscles. The final stages of hemophilic arthropathy are associated with deformed and dysfunctional joints with wasting of the muscles in the affected limb ( Fig. 134.9 ). At this late stage, joint bleeds are less frequent because synovial hypertrophy is less prominent.

Over the past 10 to 20 years, clinical and radiographic scoring systems have been developed to objectively evaluate the status of the joints over time ( Table 134.5 ). Clinical scores are more readily available, do not require expensive radiological equipment, and better reflect the extent of joint disability. Plain radiographs are relatively inexpensive and widely available but are insensitive to early soft tissue changes and involve radiation exposure. Magnetic resonance imaging (MRI) is the most sensitive test for detection of early soft tissue changes and is not associated with radiation exposure but is more expensive than plain x-rays and less available. In addition, young children may require sedation to avoid movement during image acquisition. Ongoing studies are evaluating the utility of point-of-care ultrasonography for evaluation of hemophilic arthropathy. Ultrasonography has the advantages of being inexpensive, not requiring sedation and being able to differentiate between synovitis and hemosiderin deposition. However, it is operator dependent, interpretation of its findings can be subjective, and it fails to identify joint structures such as cartilage. Some of these problems may be overcome with automated ultrasonography but more work is needed.
| Scores | Reference | |
|---|---|---|
| Clinical | WFH Joint score (Gilbert score); this has largely been supplanted by the Hemophilia Joint Health Score (HJHS) | |
| HJHS | ||
| Plain radiography | Pettersson | |
| Ultrasound | Head-US (Hemophilia Early Arthropathy Detection with Ultrasound) | |
| MRI | Single compatible IPSG MRI scoring system |
Patients with hemophilia are prone to excessive and prolonged soft tissue and muscle bleeding. Superficial hematomas (bruises) may resolve spontaneously without the need for treatment, and as such, bruising is not an indication for treatment. However, in moderate and severe hemophilia, soft tissue hematomas often undergo progressive enlargement and may need to be treated. Furthermore, some soft tissue bleeds (e.g., retroperitoneal bleeds or hematomas of the neck) can cause extensive blood loss and be life or organ threatening because of their propensity to expand, thus causing compression of adjacent organs, blood vessels, and nerves and the airway in the case of a neck hematoma.
The muscles most often involved are, in descending order of frequency, the calf, thigh, buttocks, and forearm. Bleeds into these locations can lead to compartment syndrome, which is an emergency (see box on Compartment Syndrome ).
While epistaxis is uncommon in hemophilia, oral bleeding frequently occurs. Bleeding from the frenulum after falling forward is often one of the first manifestations of hemophilia as is bleeding in the tongue. Such bleeds can result in significant blood loss and swelling to the point of airway obstruction. Excessive bleeding with loss of deciduous teeth and eruption of secondary dentition can occur but is uncommon. Fortunately, most oral bleeds can be managed with antifibrinolytic agents such as tranexamic acid or epsilon aminocaproic acid although factor replacement may be needed for more serious cases. For dental surgery, particularly if a nerve block is required, factor replacement or DDAVP (desmopressin) must be given to raise the factor level to at least 30% of normal.
Hematuria is usually spontaneous, episodic, and painless but may also occur with trauma. Rarely, hematuria can be caused by renal calculi, which are thought to be more common in males with hemophilia than in those without hemophilia. Consensus on the optimal management of hematuria in patients with hemophilia is lacking, but increased fluid intake and bed rest are recommended. If bleeding continues or is particularly severe, prophylactic therapy (e.g., daily factor replacement) should be given. The value of steroids is controversial and use of antifibrinolytic agents is contraindicated because of the risk of ureteral obstruction by clots. Fortunately, hematuria in patients with hemophilia is not associated with progressive loss of renal function, and as such, its natural history is probably benign.
GI bleeding can occur in adults with hemophilia, particularly in association with chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) for arthropathy. Life-threatening upper GI bleeding can occur because of ruptured esophageal varices in patients with portal hypertension secondary to long-standing hepatitis C. Bleeds are usually treated with high doses of factor concentrate for several days together with antifibrinolytic agents.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here