Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hippocrates recognized hydrops fetalis as long ago as 400 BC, according to Ballantyne, who accepted Hippocrates’ account as the first reported cases of the syndrome. The first clear-cut description of hydrops fetalis, however, did not occur until 1641 when Felix Plater, the famed Renaissance physician, reported a case. Three more centuries would pass before physicians would understand the etiology of hemolytic disease of the fetus and newborn (HDFN).
In 1939, Levine and Stetson reported an atypical agglutinin in the blood of a woman who had delivered a macerated, hydropic, stillborn fetus. The patient subsequently suffered a transfusion reaction when transfused with apparently compatible blood. They postulated that the mother had become immunized to a paternal antigen, inherited by the fetus but lacking in the mother. In 1940 Landsteiner and Wiener discovered the Rh factor on the surface of red blood cells, and thereafter the study and management of HDFN could be conducted on a rational basis.
The first treatment for HDFN was introduced by Wallerstein in 1946 when he performed the first newborn exchange transfusion. In 1950, Allen and colleagues proved that hyperbilirubinemia led to kernicterus. They demonstrated the value of exchange transfusions in a series of 109 newborns, with only 1 developing kernicterus. Four years later, these authors reported the value of preterm delivery in decreasing the rate of stillbirth in fetuses with erythroblastosis fetalis. Subsequently the management of HDFN consisted mainly of timely delivery and treatment of neonatal anemia and hyperbilirubinemia by exchange transfusions. The timing of delivery, however, was rather imprecise, depending on history, physical examination, and Rh antibody titers. Then, in 1963, Liley performed the first successful intrauterine transfusion, saving a fetus too premature to survive early delivery. The intraperitoneal fetal transfusion was accomplished by injecting radiopaque dye into the amniotic fluid and subsequently into the fetal peritoneal cavity. Roentgenograms were then used to determine the proper placement of the needle. The early 1980s saw the introduction of real-time ultrasound for needle guidance. This was followed by the advent of the intravascular transfusion technique using direct access to the fetal intrahepatic umbilical vein or umbilical cord. Marked improvement in perinatal survival was noted, especially when the fetus was hydropic.
Prenatal diagnosis to detect the fetus affected by HDFN has undergone remarkable advances as well. In 1956, Bevis was the first to report the use of spectrophotometric analysis of amniotic fluid bilirubin pigments to detect elevations in severe erythroblastosis fetalis. In 1961, Liley reported his systematic graph for amniotic fluid optical density at a wavelength of 450 nm (ΔOD 450 ) in RhD-alloimmunized pregnancies between 28 weeks’ gestation and term to guide the timing of delivery. Further improvements in ΔOD 450 graphs followed. The Queenan four-zone graph provided values as early as 14 weeks’ gestation and stressed the importance of the trend of ΔOD 450 values. In a breakthrough study in 2000, Mari and coworkers presented a noninvasive method to detect fetal anemia using Doppler measurements of the peak systolic velocity in the middle cerebral artery. The method was widely embraced and has now eliminated the need for amniocentesis to detect fetal anemia. More recently, circulating cell-free fetal DNA has been shown to accurately determine the fetal RhD type as early as 12 weeks’ gestation. If the fetus is found to be RhD negative, the pregnancy can be managed in a routine fashion; if the fetus is found to be RhD positive, a referral for specialized care can be undertaken.
Throughout history, clinicians believed that the fetal and maternal circulations were totally separate. Then, in 1955, Chown published an article showing that significant fetal hemorrhage into the maternal circulation can result in anemic newborns. In the 1960s and 1970s, it was noted that most initial alloimmunizations were discovered postpartum; thus it was believed that fetomaternal hemorrhage usually occurred in the peripartum period. Further studies noted that fetal erythrocytes in the maternal circulation were less frequent in the first trimester, with an increase in both frequency and volume in the second and third trimesters. The largest fetomaternal hemorrhages were noted to occur during labor and delivery. Indeed, in a 1962 study instilling the maternal chromate-tagged red cells into a woman’s uterus following delivery of the placenta, a significant transfer of red cells in the maternal circulation was noted within minutes. This information led researchers in the 1960s to investigate the feasibility of postpartum RhD immunoprophylaxis.
Freda in the United States and Woodrow in the United Kingdom reported the successful prevention of maternal RhD alloimmunization by administering an anti-D antibody preparation following delivery. Postpartum RhD immune globulin (RhIG) received approval by the US Food and Drug Administration in 1968. The postpartum injection of RhIG was successful in decreasing the incidence of RhD alloimmunization in the United States by 90%. A decade later the initiation of antepartum RhIG at 28 weeks’ gestation further reduced the rate of Rh alloimmunization.
As successful as RhD immunoprophylaxis has been, there are shortcomings. It works by passive immunization; therefore it must be repeated for every potential immunizing event. Additionally, it is a blood product, and there are concerns over giving it unnecessarily. Currently in the United States this occurs in approximately 40% of pregnancies in which RhIG is administered antenatally and the fetus is determined to be RhD-negative at birth. The use of cell-free fetal DNA to triage the use of antenatal RhIG is now routinely practiced in some European countries. RhD immunoprophylaxis has been so successful that HDFN has become very infrequent in most parts of the United States. Finding centers with physicians skilled in fetal transfusions when they are needed has become a concern.
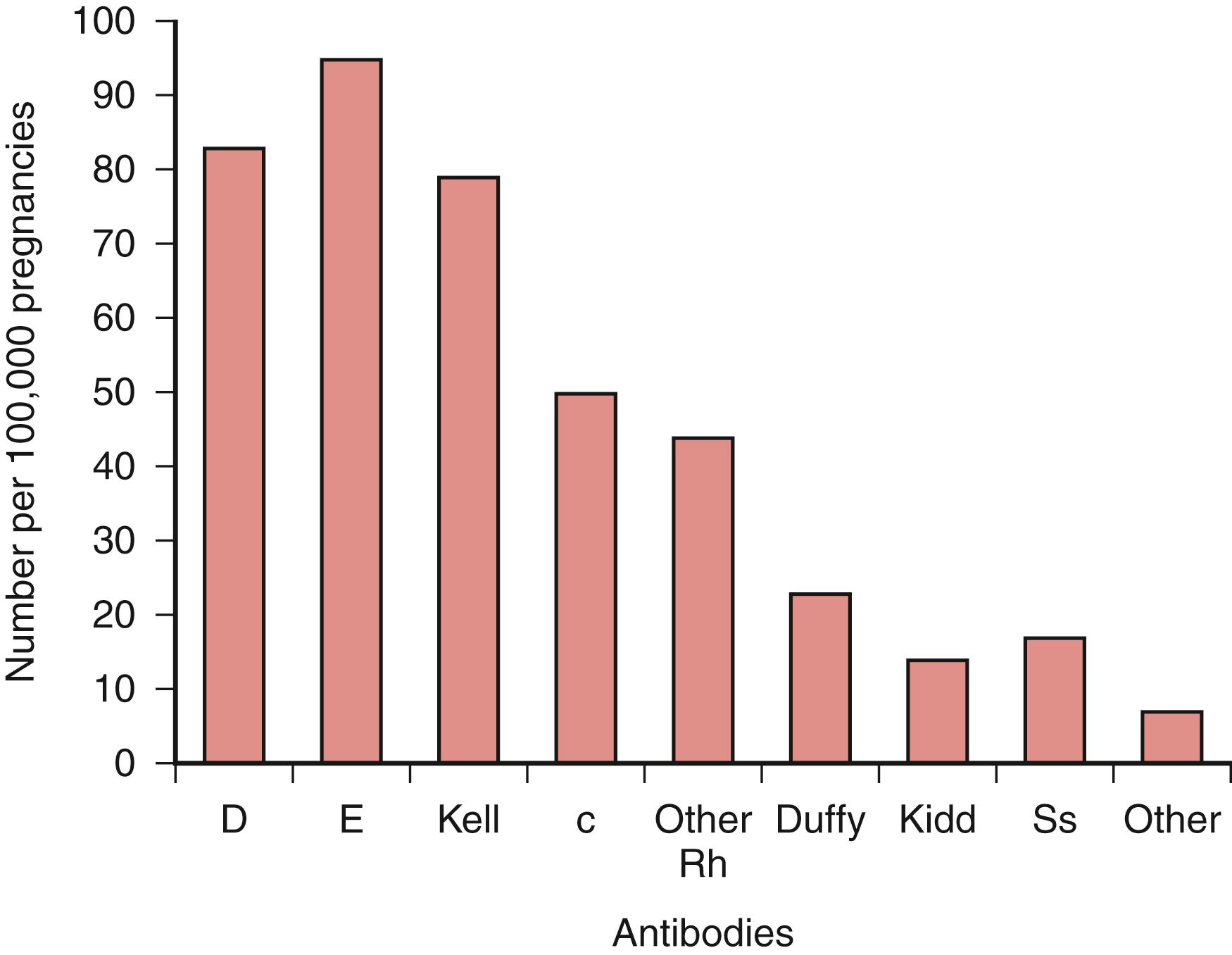
The widespread adoption of guidelines for the administration of antenatal and postpartum RhIG has resulted in a marked decline in the prevalence of red cell alloimmunization due to the RhD antigen. Cases continue to occur because of maternal sensitization in the first two trimesters of pregnancy, inadvertent omission of RhIG, and inadequate dosing after delivery when there has been a large fetomaternal hemorrhage (FMH). Immune globulins to prevent alloimmunization to other red cell antigens are unlikely to be developed by pharmacology companies, so red cell alloimmunization is likely to continue to complicate pregnancies for the foreseeable future. An analysis of 305,000 women in a national first-trimester screening program in the Netherlands revealed that anti-E was the most common red cell antibody associated with HDFN ( Fig. 35.1 ). Anti-D was the second most common antibody, followed by anti-Kell and anti-c.
The fetal-maternal interface is not an absolute barrier, and there is considerable trafficking of many types of cells between the fetus and the mother throughout gestation. In most cases, the antigenic load of a putative antigen on the fetal erythrocytes and erythrocytic precursors is insufficient to stimulate the maternal immune system. However, in the case of a large FMH before birth or at delivery, B lymphocyte clones that recognize the foreign red cell antigen are established. The initial maternal production of immunoglobulin M is short-lived and is followed by a rapid change to an immunoglobulin G (IgG) response. A human antiglobulin titer can usually be detected by 5 to 16 weeks after the sensitizing event.
After the initial antigenic exposure, memory B lymphocytes await the appearance of red cells containing the same antigen in a subsequent pregnancy. If stimulated by fetal erythrocytes, these B lymphocytes differentiate into plasma cells that can rapidly proliferate and produce IgG antibodies and an increase in the maternal titer. Maternal IgG crosses the placenta and attaches to fetal erythrocytes that have expressed the paternal antigen. These cells are then sequestered by macrophages in the fetal spleen, where they undergo extravascular hemolysis, producing fetal anemia. Fetal sex may play a significant role in the fetal response to maternal antibodies. Ulm and associates reported that the chance of hydrops fetalis was increased 13-fold in RhD-positive male fetuses compared with their female counterparts; the adjusted odds ratio for perinatal mortality was 3.38 in male fetuses.
Several important physiologic responses occur in fetuses as a result of this anemia. Enhanced bone marrow production of reticulocytes is noted when the fetal hemoglobin deficit, compared with norms for gestational age, exceeds 2 g/dL, and erythroblasts appear in the fetal liver at a hemoglobin deficit of 7 g/dL or greater. Cardiac output increases, and 2,3-diphosphoglycerate levels are enhanced.
Hydrops fetalis, a collection of fluid in at least two serous compartments, typically heralds end-stage disease, and it occurs with hemoglobin deficits of 7 g/dL or greater. This may be related to the gestational age when the anemia occurs. In one series of fetuses found to have severe anemia prior to 22 weeks’ gestation, more than 70% did not exhibit signs of hydrops on ultrasound. The initial ultrasound finding of hydrops is usually fetal ascites, followed later by pleural effusion and, finally, scalp edema. The exact pathophysiology of this condition is unknown. Reduced serum albumin levels have been reported, presumably resulting from depressed synthesis by the fetal liver, which shifts to an erythropoietic function. This results in a decrease in colloid osmotic pressure. However, congenital analbuminemia is not associated with hydrops fetalis, and experimental removal of fetal plasma proteins and replacement with saline in an animal model did not produce hydrops. , An alternative hypothesis suggests that tissue hypoxia resulting from anemia enhances capillary permeability. Iron overload secondary to ongoing hemolysis may contribute to free radical formation and endothelial cell dysfunction. Central venous pressures are elevated in the hydropic fetus with HDFN. This may cause a functional blockage of the lymphatic system at the level of the thoracic duct as it empties into the left brachiocephalic vein. This theory is supported by reports of poor absorption of donor red blood cells infused into the peritoneal cavity of hydropic fetuses. The widespread adoption of screening for fetal anemia with middle cerebral artery (MCA) Doppler may have contributed to a decreasing frequency of hydrops fetalis in HDFN cases. Zwiers and colleagues studied the incidence of hydrops in the time period 1987–92 as compared to 2011–16. The rate of all cases of hydrops declined from 41% to 6.4% while the rate of severe hydrops declined from 22% to 0.9%.
Although the RhD, C/c, and E/e antigens were once thought to be the product of three distinct genes, the presence of only two genes localized to the short arm of chromosome 1 has now been verified. Each of the two genes—an RHD gene and an RHCE gene—is 10 exons in length, and there is 96% homology between them ( Fig. 35.2 ). This has led some to conclude that these genes represent a duplication of a common ancestral gene. Production of two distinct proteins from the RHCE gene probably occurs as a result of alternative splicing of messenger RNA. One nucleotide difference, a change from cytosine to thymine in exon 2 of the RHCE gene, results in a single amino acid change of a serine to proline. This causes the expression of the C antigen as opposed to the c antigen. A single cytosine-to-guanine change in exon 5 of the RHCE gene produces a single amino acid change of proline to alanine, resulting in formation of the e antigen instead of the E antigen.
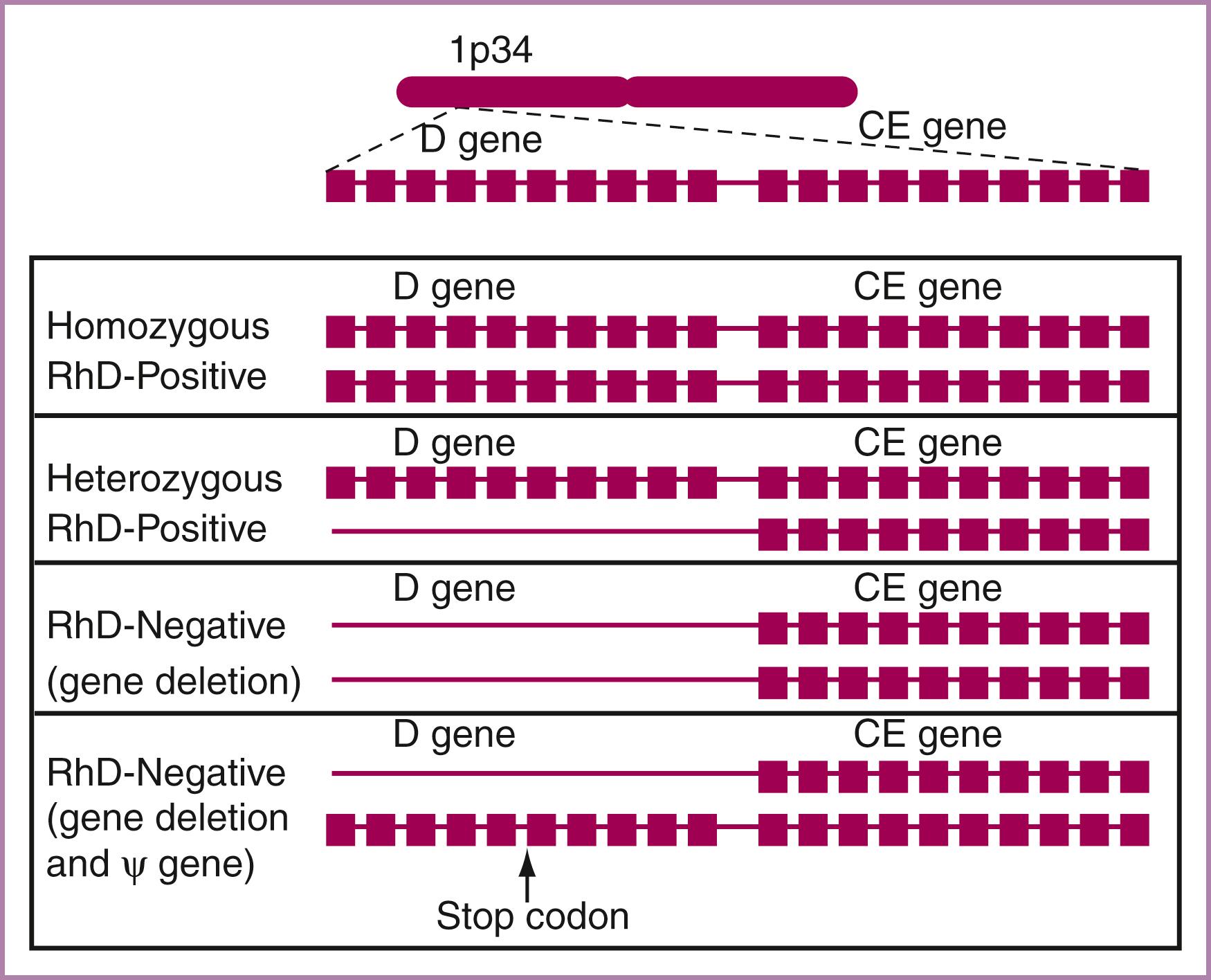
In most individuals, the RhD-negative serotype results from a gene deletion of both RHD genes. However, several unique gene arrangements can occur. An RHD pseudogene has been described in 69% of South African Blacks and 24% of African Americans. In this situation, all 10 exons of the RHD gene are present; however, translation of the gene into a messenger RNA product does not occur because of the presence of a stop codon in the intron between exons 3 and 4. Therefore no RhD protein is synthesized, and the patient is serologically RhD negative.
Finally, the red cells of 0.4% of non-Hispanic Caucasians, 0.57% of African Americans, and 0.8% of Hispanics exhibit a weak response when standard serologic RhD typing is undertaken. In the past, these individuals were reported as Du positive. The term was later changed in 1992 to weak D positive . Further genetic studies have indicated that these individuals belong to one of two populations. In the first group, a reduced number of intact RhD antigens are expressed on the patient’s red cells ( Fig. 35.3 ). One hundred and fifty subtypes of this group of weak D patients have been described. The second population of weak D patients is known as partial D. These patients exhibit amino acid substitutions in the active RhD epitopes of the D antigen on the surface of their red cells. These changes can result in “missing” portions of the epitopes, allowing the patient to form antibodies to these deleted portions if they are exposed to intact RhD-positive red cells. D variants continued to be discovered, with 118 partial D, 170 weak D, and 222 weakened D variants recently reported in an international database. Alloimmunization to partial D has been reported to result in severe HDFN and even fetal hydrops.
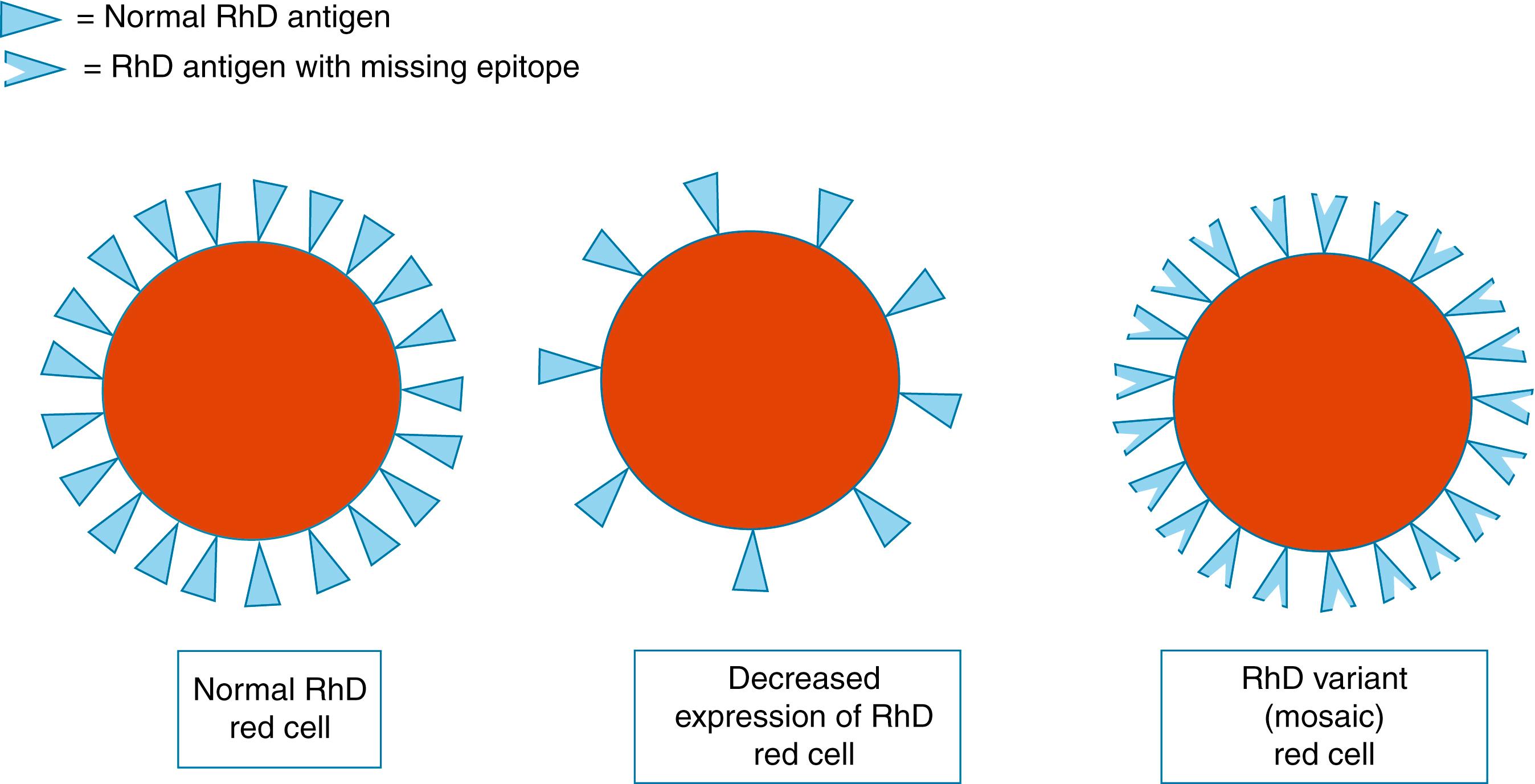
Monoclonal typing sera are now routinely used for RhD typing. Many of the weak D and D variants cannot be detected unless a Coombs phase is performed during the typing procedure. For prenatal testing, this is no longer recommended by the American Association of Blood Banks (AABB). Therefore some pregnant patients who exhibit reduced numbers of intact RhD antigens or partial RhD antigens on their red cells will be reported as RhD negative. Testing for weak D is indicated in blood donors and for all neonates born to RhD-negative women. This is to prevent alloimmunization in the case of a therapeutic red cell transfusion or an FMH. Confusion can therefore arise if the pregnant patient was previously a blood donor. The conflict of an RhD-positive result before pregnancy with an RhD-negative result during pregnancy can be easily explained to the patient, based on the different methods of red cell typing that have been employed.
Red cell alloimmunization is first detected when the maternal serum is mixed with a panel of indicator red cells containing known antigens. The antibody screen is called positive or negative. If an antibody is detected, it is first identified to determine its clinical significance; a titer is then obtained. The human antiglobulin titer (indirect Coombs test) is used to determine the degree of alloimmunization because it measures the maternal IgG response. By convention, titer values are reported as the reciprocal of the last dilution tube that showed a positive agglutination reaction; that is, a titer of 16 is the equivalent of a dilution of 1:16. Variation in results between laboratories is not uncommon, because many commercial laboratories use enzymatic treatment of red cells to prevent failure of detection of low-titer samples. However, in a single laboratory, the titer should not vary by more than one dilution if the two samples are run in tandem. This means that an initial titer of 8 that returns at 16 may not represent a true increase in the amount of antibody in the maternal circulation. In addition, the clinician should be aware that the newer gel microcolumn assays that are commonly used by laboratories today will result in higher titers than the older conventional tube testing. In one study, the mean titer was increased 3.4-fold with the gel technology. A critical titer is defined as the titer associated with a significant risk for fetal hydrops. If a critical titer is present, further fetal surveillance is required. This titer varies with the institution and the methodologies used; however, most centers use a critical value for anti-D antibody, and most other antibodies, of 16.
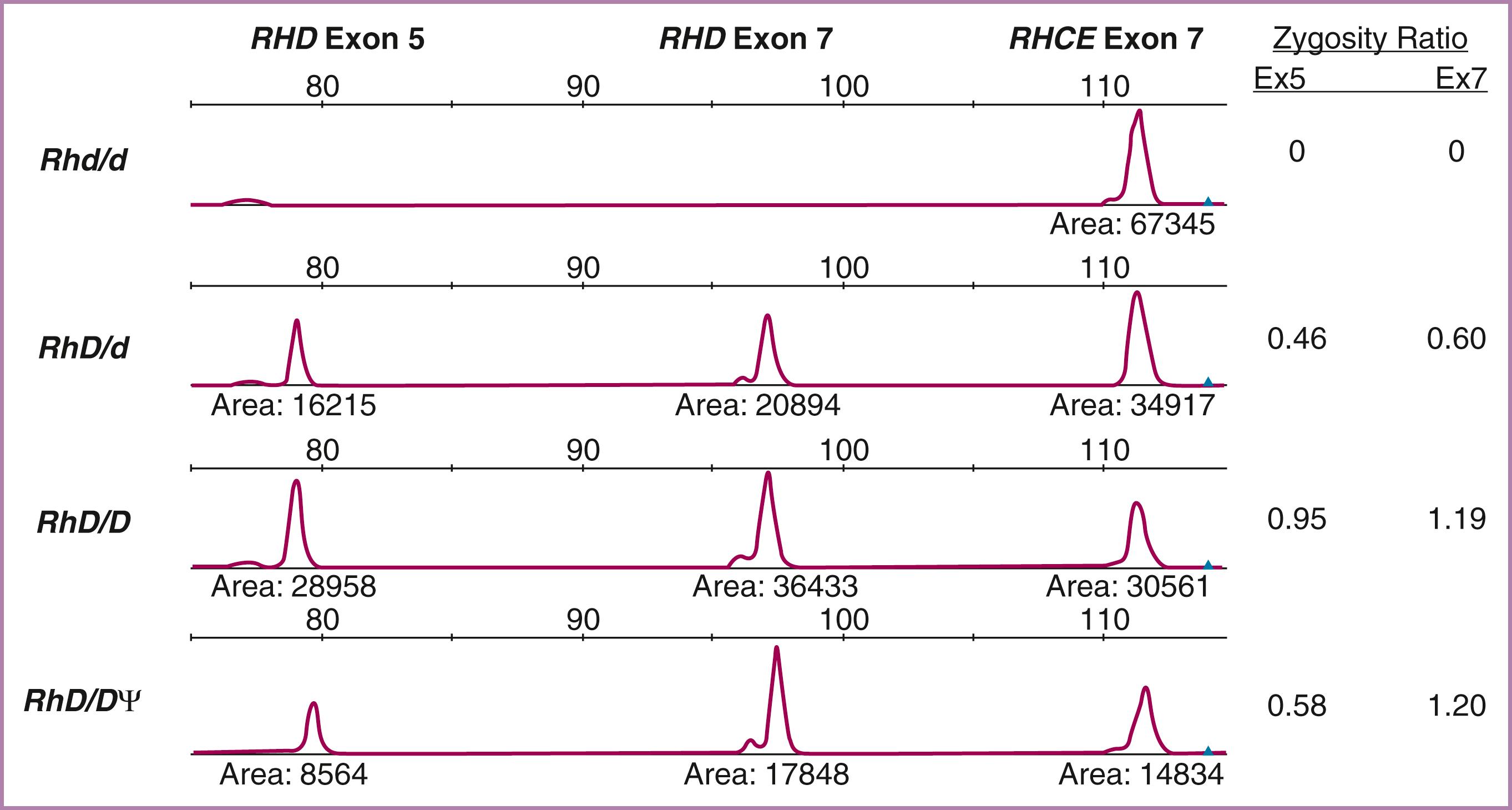
In cases of a heterozygous paternal genotype, 50% of the fetuses do not express the involved red cell antigen and therefore escape the hemolytic effects of the maternal anti–red cell antibody. In these cases, further maternal and fetal testing can be eliminated. Most red cell antigens are inherited through codominant alleles. As an example, for the Kell (K1) antigen, an individual can be homozygous K/K, homozygous k/k, or heterozygous K/k. Using anti-K and anti-k reagents, the Kell genotype of an individual can be easily determined by simply testing the red cells using serologic methods ( Table 35.1 ). This is not the case, however, for RhD, as there is no “d” antigen (and therefore no anti-d reagent) because it represents a gene deletion and a lack of translation to a protein. In the case of RhD, quantitative polymerase chain reaction can be used to test for the number of copies of the D gene by comparing the DNA peak to that of the RHCE gene, which is always present as two copies in all individuals ( Fig. 35.4 ). The assay has also proved effective in detecting the RHD pseudogene.
| Antigen | White Ethnicity | Black Ethnicity | ||
|---|---|---|---|---|
| Antigen + (%) | Heterozygous (%) | Antigen + (%) | Heterozygous (%) | |
| K (K1) | 9.0 | 97.8 | 2 | 100 |
| k (K2) | 99.8 | 8.8 | 100 | 2 |
| M | 78.0 | 64.0 | 70 | 63 |
| N | 77.0 | 65.0 | 74 | 60 |
| S | 55.0 | 80.0 | 31 | 90 |
| s | 89.0 | 50.0 | 97 | 29 |
| U | 100.0 | — | 99 | — |
| Fy a | 66.0 | 26.0 | 10 | 90 |
| Fy b | 83.0 | 41.0 | 23 | 96 |
| Jk a | 77.0 | 36.0 | 91 | 63 |
| Jk b | 72.0 | 32.0 | 43 | 21 |
In the past, a heterozygous paternal genotype for the RHD gene led to a recommendation for amniocentesis to determine the fetal RhD status using DNA from amniocytes. This allowed 50% of patients to receive routine obstetric care when the fetus was found to be RhD negative.
Based on a concept that cell-free tumor DNA could be found in the peripheral circulation of patients with cancer, Lo and colleagues first reported the presence of the RHD gene in the plasma of women pregnant with an RhD-positive fetus. Further studies have indicated that, near the end of the first trimester, circulating cell-free fetal DNA (cffDNA) comprises 10% of the total DNA in the maternal circulation. Between 10 and 21 weeks’ gestation, this increases by 0.1% per week, with a further increase of 1% per week between 20 weeks and term. Because apoptosis of placental trophoblasts is the source of this DNA, rapid clearance is noted after delivery. This makes cffDNA an attractive entity for prenatal diagnosis because there should not be any residual fetal DNA from previous gestations.
cffDNA testing to determine the fetal RHD genotype detection has evolved quickly in the past few years and is now standard practice in the management of the alloimmunized pregnancy in many European countries. Typically, a blood sample is obtained from the pregnant patient in the late first or early second trimester. Combinations of assays for the detection of exons 4, 5, 7, and 10 on the RHD gene are employed. The finding of these RHD exon products indicates that the fetus is RHD positive. In the case of an RHD-negative fetus, there is no amplification of the RHD exons. When the fetal RHD genotype is reported to be negative, like the mother’s, an RHD-negative result cannot be used to verify the presence of fetal DNA. In these cases, reflex fetal sex determination can be undertaken to confirm that fetal DNA is present. If gene products of the Y chromosome are detected, then the RHD -negative result can be considered valid (male, RHD -negative fetus). However, in the case of an RHD -negative female fetus, the presence of fetal DNA cannot be confirmed. Single nucleotide polymorphisms that differ between the maternal white cells in the buffy coat of the sample and the plasma indicate the presence of paternally derived DNA, assuring that fetal DNA is present. Another method of confirming fetal origin of the cffDNA is to identify the hypermethylated RASSF1A promoter unique to the fetus.
Two meta-analyses have been undertaken to assess the accuracy of cffDNA in determining the fetal RHD status. The first included 41 studies of 11,129 patients and found a sensitivity of 99% and a specificity of 98%. In the more recent meta-analysis, 30 studies including 10,290 patients revealed a false-positive rate of 0.53% and a false-negative rate of 0.54%. Moise and colleagues prospectively collected maternal samples in the first, second, and third trimesters in 520 patients. Only one false-negative cffDNA RHD result was noted in the first trimester; this was later determined to be due to a labeling error.
cffDNA testing to determine the fetal antigen status is available in Europe for the C, c, E, and Kell (K1) antigens but is not yet available in the United States. In the case of alloimmunization to a non-RhD antibody, amniocentesis can be used in the case of a heterozygous paternal genotype to test fetal DNA to determine the fetal antigen status for the majority of the red cell antigens associated with HDFN.
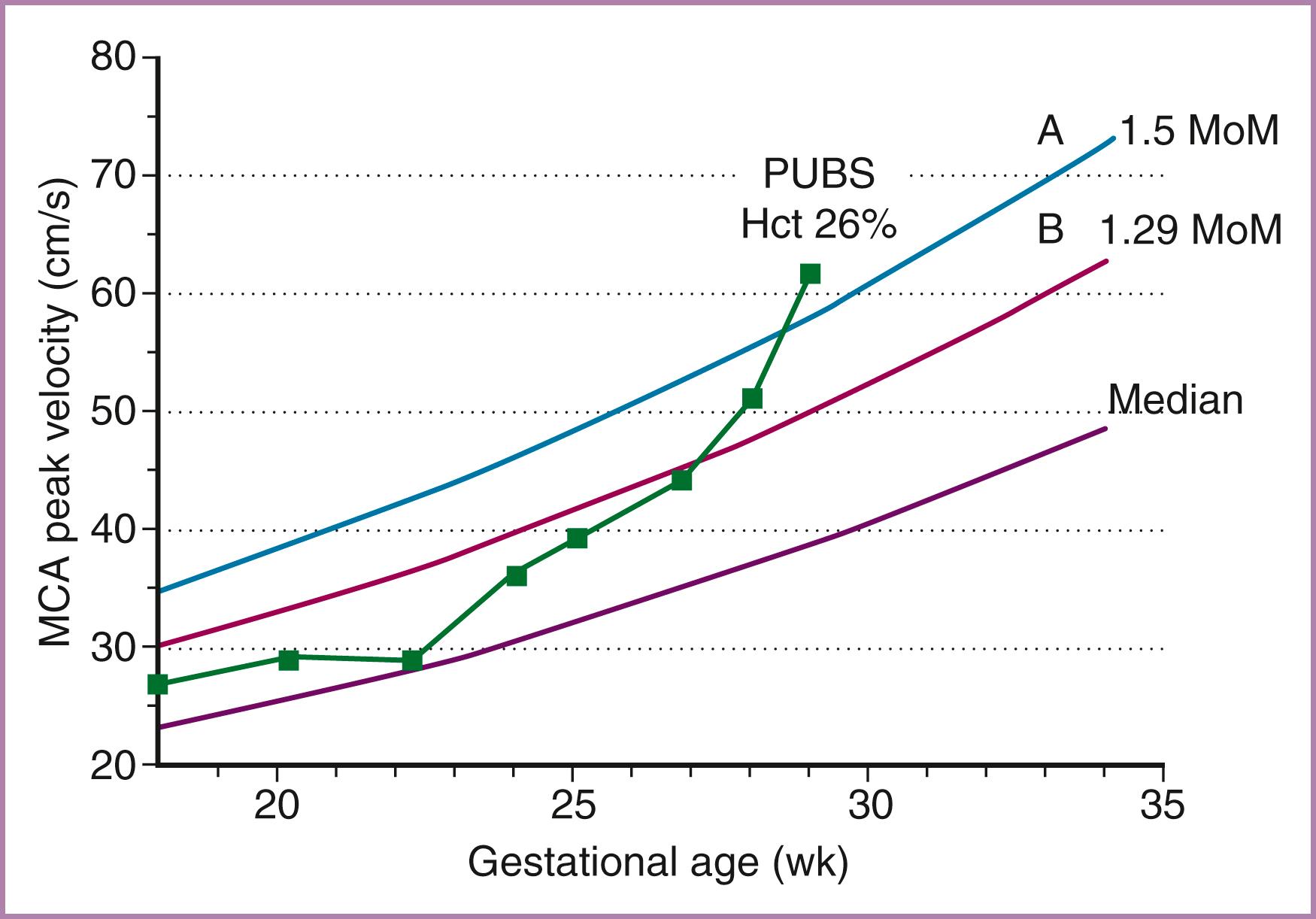
Measurement of the peak systolic velocity (PSV) in the fetal MCA has been shown to be an accurate noninvasive method for detecting fetal anemia. In the initial report by Mari and coworkers, normative data for gestational age were established. Using receiver operating characteristic curve analysis, a threshold value of 1.5 multiples of the median (MoM) was used to predict moderate to severe anemia (<0.65 MoM for fetal hemoglobin). The results suggested that more than 70% of invasive tests could be avoided if this test were used to monitor all alloimmunized pregnancies. A meta-analysis of noninvasive monitoring of alloimmunized pregnancies reported that the MCA PSV had a positive likelihood ratio of 8.45 (95% confidence interval [CI], 4.7 to 15.6) for the detection of fetal anemia; the negative likelihood ratio was 0.02 (95% CI, 0.001 to 0.25). In a prospective series, Oepkes and associates found that the peak MCA Doppler velocity was superior to measurements of optical density at a wavelength of 450 nm using both the Queenan and Liley amniotic fluid bilirubin curves in the detection of severe fetal anemia. The accuracy of MCA Doppler assessment was 85%, compared with 76% for the Liley curve and 81% for the Queenan curve. These data have led to the abandonment of serial amniocenteses in favor of the MCA PSV as the primary surveillance tool for detecting fetal anemia in the red cell–alloimmunized pregnancy.
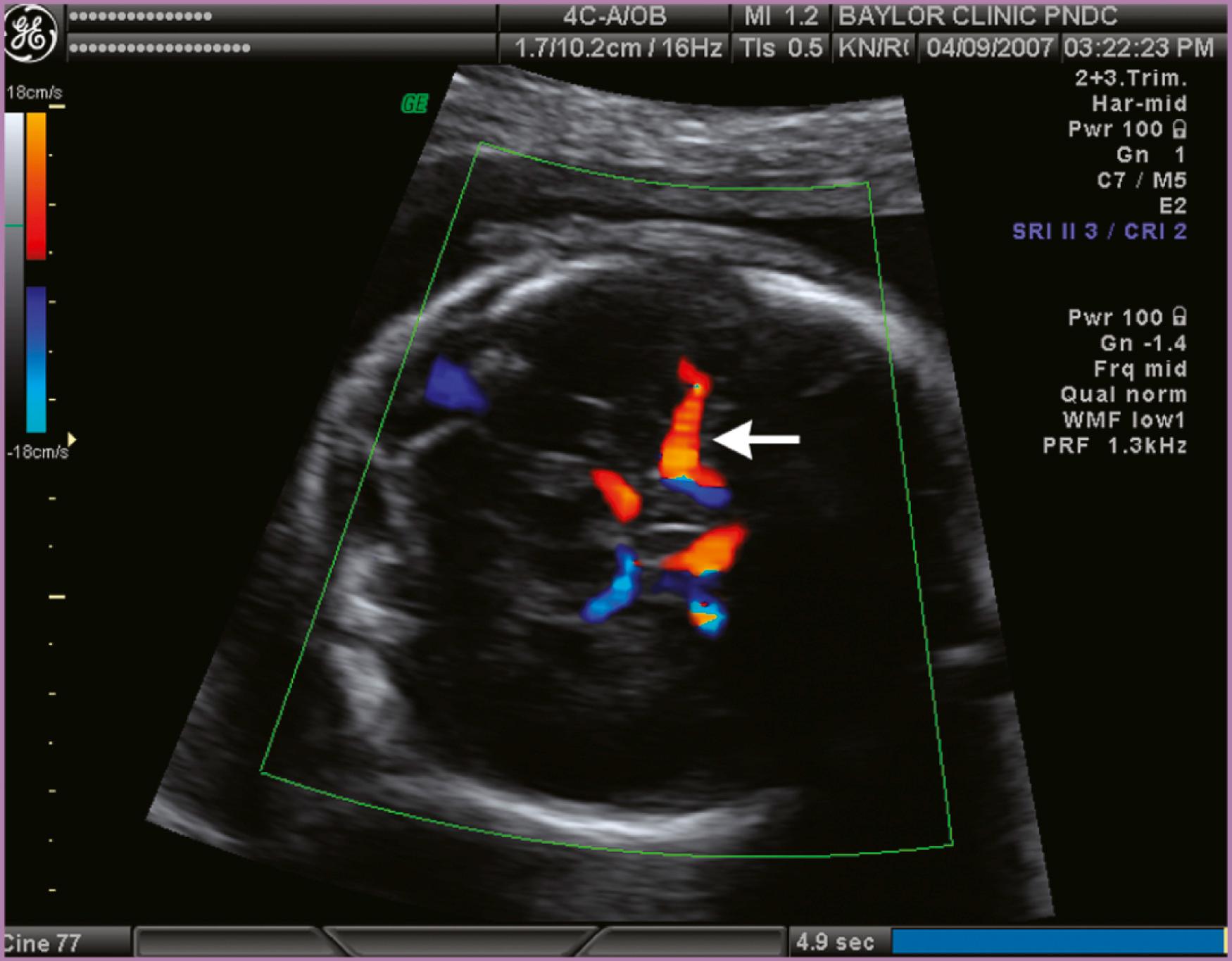
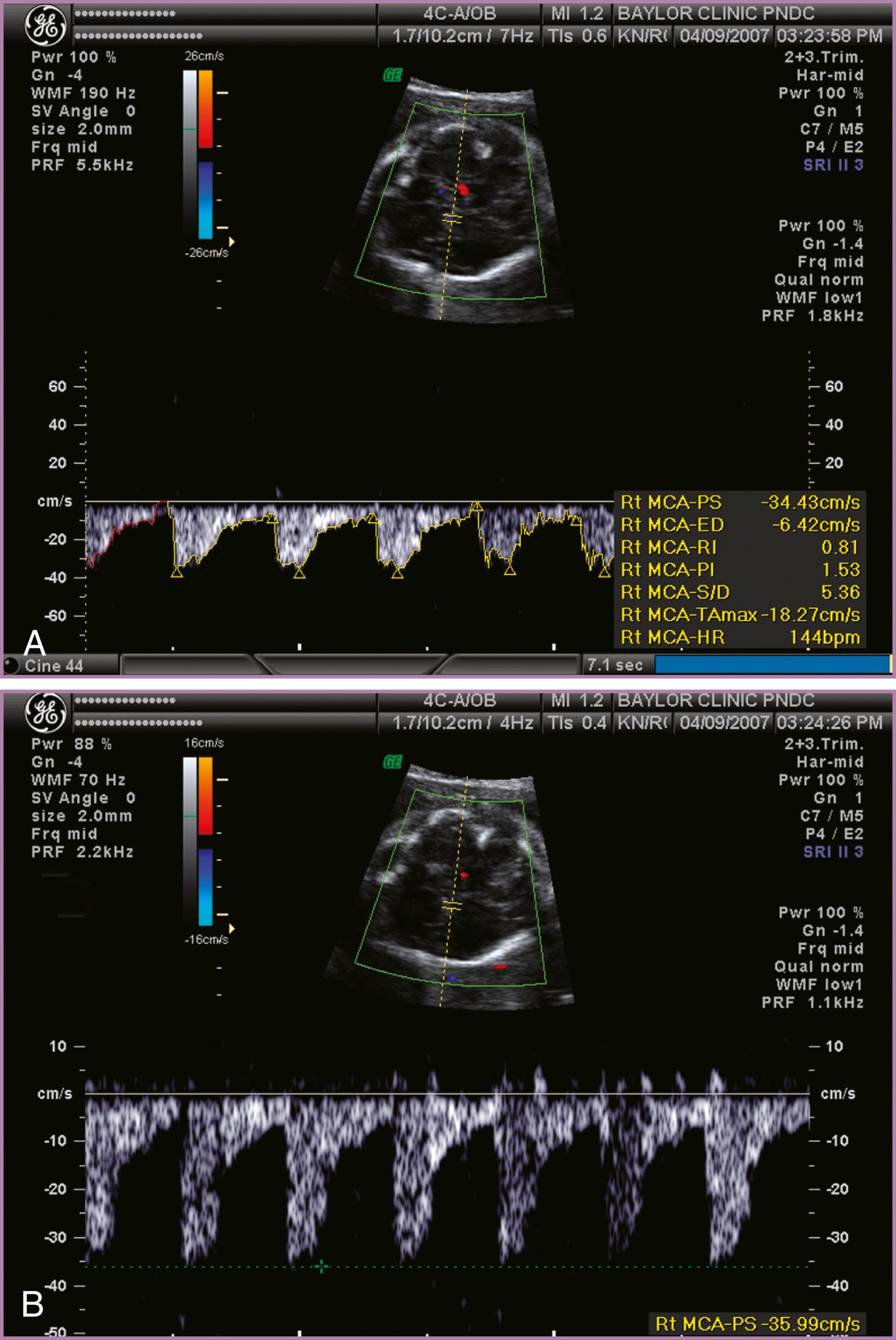
To accurately measure the MCA PSV, the anterior wing of the sphenoid bone at the base of the skull is located. Color or power Doppler is then used to locate the MCA vessel closest to the transducer, although the more distant MCA will give similar results ( Fig. 35.5 ). The Doppler gate is then placed in the proximal MCA as the vessel arises from the carotid siphon, because measurements in the more distal aspect of the vessel will be inaccurate because of reduced peak velocities. In the original description by Mari and coworkers, the angle of insonation between the Doppler beam and the vessel was maintained near zero, to more closely estimate the true velocity. Ruma and colleagues demonstrated that angle correction software can be used to correct an insonation angle of up to 30 degrees, with good correlation to the true velocity. An additional consideration is that the pulse repetition frequency should be optimized to the approximate peak velocity ( Fig. 35.6 ). These adjustments optimize the appearance of the waveform and make the true MCA PSV more discernible. Peak systolic measurements should be made with electronic calipers; automated software to trace the waveform may overestimate or underestimate the true peak velocity. Finally, the fetus should be in a quiescent state during the Doppler examination because accelerations of the fetal heart rate can result in a false depression in the MCA PSV, especially late in the third trimester. Measurements can be initiated as early as 15 weeks’ gestation; we start with weekly checks for 3 weeks and then check biweekly if the values are reassuring ( Fig. 35.7 ). Values should be reported in MoM to account for changes in gestational age. Internet-based calculators are available (see http://perinatology.com/calculators/MCA.htm ). MCA PSV values in pregnancies prior to 16 weeks’ gestation have been reported in a population at risk for fetal anemia secondary to α-thalassemia ( Table 35.2 ).
| GA (wk) | MCA PSV (cm/s) | 1.5 MoM | ||||
|---|---|---|---|---|---|---|
| 5th | 10th | 50th | 90th | 95th | ||
| 11 | 3.2 | 5.2 | 10.7 | 15.2 | 19.5 | 16.1 |
| 12 | 4.5 | 6.5 | 12.1 | 16.9 | 21.2 | 18.2 |
| 13 | 5.8 | 7.8 | 13.6 | 18.5 | 22.8 | 20.3 |
| 14 | 7.1 | 9.0 | 15.0 | 20.2 | 24.5 | 22.4 |
| 15 | 8.4 | 10.3 | 16.4 | 21.8 | 26.1 | 24.5 |
| 16 | 9.8 | 11.5 | 17.8 | 23.4 | 27.7 | 26.7 |
| 17 | 11.1 | 12.8 | 19.2 | 25.1 | 29.4 | 28.8 |
| 18 | 12.4 | 14.1 | 20.6 | 26.7 | 31.0 | 30.9 |
| 19 | 13.7 | 15.3 | 22.0 | 28.3 | 32.6 | 33.0 |
| 20 | 15.0 | 16.6 | 23.4 | 30.0 | 34.3 | 35.1 |
| 21 | 16.4 | 17.8 | 24.8 | 31.6 | 35.9 | 37.2 |
| 22 | 17.7 | 19.1 | 26.2 | 33.3 | 37.6 | 39.3 |
Severe HDFN is usually not present in the patient’s first alloimmunized pregnancy; subsequent gestations are generally associated with a worsening degree of fetal anemia.
Once an antibody reported to cause HDFN has been detected in an initial maternal antibody screen, a titer should be obtained to assess the amount of antibody present ( Fig. 35.8 ). If the value remains below a critical value (16 for anti-D antibody and most other antibodies; 4 for anti-Kell, see Anti-K [K1], later), we obtain titers every month until approximately 24 weeks’ gestation and then every 2 weeks thereafter. Paternal blood is drawn to determine if the patient’s partner is positive for the involved red cell antigen and to investigate his zygosity. DNA techniques are employed in the case of RhD; for other antigens, serology is used to determine the paternal genotype. In cases of a heterozygous paternal genotype for RHD, cffDNA can be used to determine the fetal RHD status. In the case of other red cell antigens, amniocentesis may be performed after 15 weeks’ gestation to determine the fetal antigen status. If the paternal blood typing is negative for the involved red cell antigen, further maternal and fetal monitoring is unnecessary as long as paternity is ensured. When the patient’s partner is not available for testing or paternity is in question, cffDNA allows accurate assessment of the fetal RHD status. A homozygous paternal phenotype or a genotype that indicates an antigen-positive fetus obviates the need for cffDNA or amniocentesis for fetal typing.
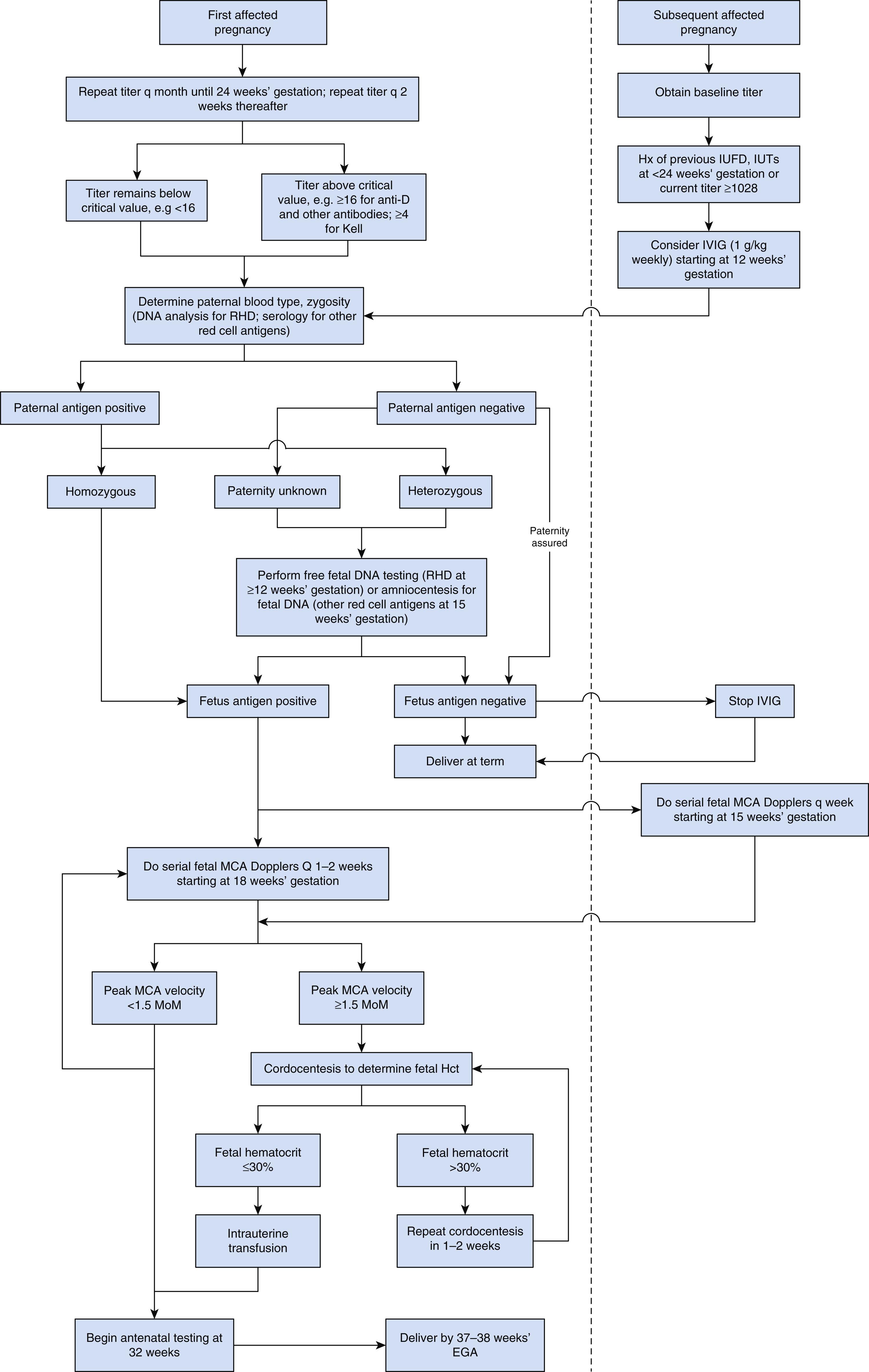
If there is evidence of an antigen-positive fetus, Doppler assessment of the MCA is performed serially at 1- to 2-week intervals starting at around 18 weeks’ gestation. An MCA PSV greater than 1.5 MoM is an indication for cordocentesis for fetal hematocrit determination and intrauterine transfusion (IUT) as needed. Antenatal testing (nonstress test or biophysical profile) should be initiated after 32 weeks’ gestation. Induction by 38 weeks’ gestation should be considered.
If the patient has a new partner and there is a history of a previous perinatal loss related to HDFN, a previous need for IUT, or a previous need for neonatal exchange transfusion, referral to a perinatal center with experience in the management of severely alloimmunized pregnancies should be considered. In general, maternal titers should not be used for deciding when to initiate fetal surveillance. However, the detection of an extremely elevated maternal titer at the start of pregnancy may warrant consideration for immunomodulation (see Immunomodulation, later). Paternal red cell typing and zygosity testing should be undertaken. In the case of a partner who tests negative for the involved red cell antigen with assured paternity, no further testing is necessary. In the case of a homozygous partner or an antigen-positive fetus as determined by cffDNA or amniocentesis, serial MCA PSV testing is usually initiated as early as 15 weeks’ gestation and repeated every 1 to 2 weeks. In the rare case in which IUTs are not required, the remaining management should be identical to that used in a first affected pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here