Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The porphyrias and the sideroblastic anemias are metabolic disorders that involve defects in heme biosynthesis. Most forms of porphyria are inherited in a Mendelian autosomal dominant pattern, but some types are recessive, and others are acquired through exposure to porphyrinogenic drugs and chemicals. A linked group of diseases, the porphyrinurias, are not porphyrias but have in common alterations of heme biosynthesis following exposure to various toxins. Porphyrins are tetrapyrroles, which are ubiquitous in nature and exhibit characteristic red fluorescence on exposure to ultraviolet light. The iron-porphyrin complex, called heme , is central to all biologic oxidation reactions. In plants, the porphyrin molecule is combined with magnesium to form chlorophyll. Porphyrin biosynthesis is one of the most essential biochemical processes in most life forms.
In humans, loss-of-function mutations affecting the first enzyme of the heme biosynthetic pathway, 5-aminolevulinate synthase (ALAS), produce sideroblastic anemia. Gain-of-function mutation of ALAS, and inborn errors that occur at subsequent sites in this pathway, usually result in metabolic disorders known as the porphyrias ( Fig. 39.1 ). It has been hypothesized that Vincent van Gogh suffered from porphyria, most likely acute intermittent porphyria, based on the intermittent nature of gastrointestinal complaints, neurological disturbances, and exacerbations caused by inadequate nutrition and absinthe abuse.
Heme biosynthesis is an essential pathway and occurs in all metabolically active cells that contain mitochondria. It is most active in erythropoietic tissue, where it is required for hemoglobin synthesis, and in hepatic tissue, where the heme forms the basis of various heme-containing enzymes such as the cytochromes P450, catalase, cytochrome oxidase, and tryptophan pyrrolase. The synthetic pathway starts with the condensation of glycine and succinyl CoA to form 5-aminolevulinate (ALA) under the control of the mitochondrial enzyme ALAS. This enzyme requires pyridoxal phosphate as a cofactor. A series of enzymes then controls the conversion of ALA first to the monopyrrole porphobilinogen (PBG) and then to the various porphyrins. Iron is inserted into protoporphyrin by the enzyme ferrochelatase (FECH) to form heme (see Fig. 39.1 ). During the past 50 years, complementary deoxyribonucleic acid (cDNA) clones have been obtained for all the enzymes of heme biosynthesis, the structures of the corresponding genes have been determined, and molecular analyses have revealed numerous heterogeneous defects in all the porphyrias. These advances are certain to improve understanding of the pathogenesis of the porphyrias and methods for identification of carriers. The enzymes of the biosynthetic pathway have all been mapped to specific chromosomes ( Table 39.1 ). Heme synthesis and its disorders have been the subject of reviews, and advances in our knowledge of mitochondrial iron trafficking and metabolism have recently been reviewed.
| Porphyria (Synonym) | Acute Attack, Skin and Organ Involvement | Enzyme of Heme Biosynthesis Affected (Gene) | Chromosome Location |
|---|---|---|---|
| — | — | Hepatic, 5-aminolevulinate synthase, nonspecific, mitochondrial (ALAS1) | 3p21.2 |
| X-linked sideroblastic anemia | Bone marrow | 5-Aminolevulinate synthase, erythroid-specific, mitochondrial (ALAS2; low function mutation) | Xp11.21 |
| X-linked dominant protoporphyria | Skin, red cells, liver | 5-Aminolevulinate synthase, erythroid-specific, mitochondrial (ALAS2; high function mutation) | Xp11.21 |
| ALA dehydratase deficiency porphyria (plumboporphyria) | Acute liver | ALA dehydratase (ALAD; porphobilinogen synthase) | 9q32 |
| Acute intermittent porphyria | Acute liver | Porphobilinogen deaminase (hydroxymethylbilane synthase; HMBS) | 11q23.3 |
| Congenital erythropoietic porphyria (Günther disease) | Skin, red cells, bone marrow | Uroporphyrinogen III synthase (UROS) | 10q26.2 |
| Porphyria cutanea tarda (symptomatic porphyria, cutaneous hepatic porphyria) | Skin, liver | Uroporphyrinogen decarboxylase (UROD) | 1p34.1 |
| Hereditary coproporphyria | Acute skin, liver | Coproporphyrinogen oxidase (CPOX) | 3q11.2 |
| Variegate porphyria (porphyria variegata) | Acute skin, liver | Protoporphyrinogen oxidase (PPOX) | 1q23.3 |
| Erythropoietic protoporphyria (erythrohepatic protoporphyria) | Skin, red cells, liver | Ferrochelatase (heme synthase; FECH) | 18q21.3 |
The overproduction of porphyrins and their precursors in the different porphyrias is mainly hepatic or erythropoietic in origin. In the acute porphyrias and in porphyria cutanea tarda (PCT), the liver is the main source of overproduction; in congenital erythropoietic porphyria (CEP), the marrow is the main source; and in erythropoietic protoporphyria (EPP), porphyrins are overproduced by the liver and marrow.
Control of hepatic heme biosynthesis is regulated by the rate of the initial enzymatic step, ALAS1, a housekeeping form of ALAS that is expressed in all cell types, including erythroid cells, whereas ALAS2 encodes a red cell-specific form. ALAS1 is under negative-feedback control by heme, which occurs by more than one mechanism. Heme represses transcription of the ALAS1 gene and increases the rate of degradation of the messenger ribonucleic acid (mRNA) ( Fig. 39.2A ). At the posttranslational level, heme blocks the translocation of pre-ALAS1 into the mitochondrion. In the mitochondrion, the molecular mass of ALAS1 is smaller than that of the cytosolic pre-ALAS1 because of the removal of the mitochondrial targeting sequence. Finally, heme regulates levels of mature ALAS1 by activation of a mitochondrial proteolysis system.
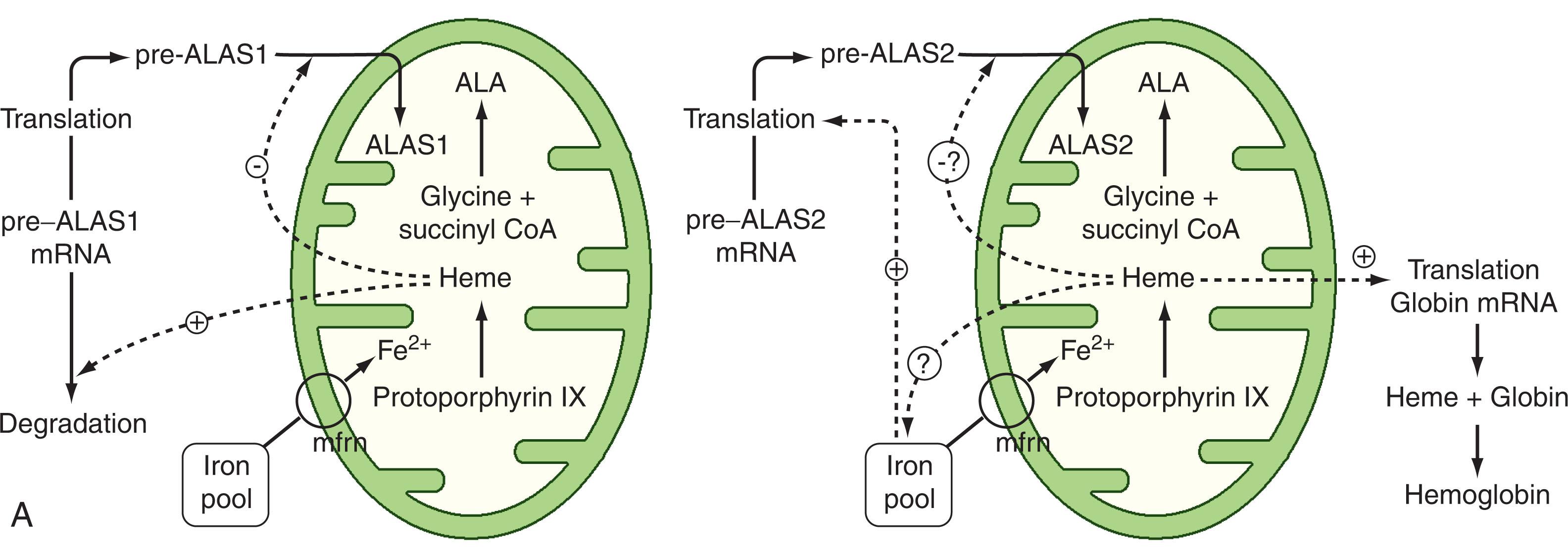
The erythroid bone marrow is the major heme-forming tissue in the body, producing 85% of the daily heme requirement. Heme synthesis in erythroid cells varies from that in hepatocytes; it is linked to tissue differentiation, and the half-life of the same end product of the two is quite different. Heme complexed with globin is preserved in circulating red blood cells for approximately 120 days, whereas heme produced in liver for cytochromes and enzymes, such as catalase, is subject to much more rapid turnover, measurable in hours. Regulation in the liver is exquisitely sensitive to fluctuations in intracellular heme levels and responds rapidly to the requirements for synthesis, as described in Fig. 39.2A . However, heme synthesis in the bone marrow shows a more leisurely response. This fundamental difference is explained by the finding of two different tissue-specific isoenzymes and two different cDNAs for human liver or “housekeeping” ALAS1 and erythroid specific ALAS2, which is expressed exclusively in erythroid cells. The gene for ALAS2 has been mapped to the X chromosome and that for the hepatic enzyme to chromosome 3. The ALAS2 gene has 12 exons; exons 4 to 11 encode the catalytic domain of the enzyme and include a lysine residue that forms a Schiff base with the pyridoxal phosphate cofactor. Exon 1 contributes to the 5′ untranslated region (UTR) whose structure allows iron to regulate ALAS2 mRNA translation, whereas exons 1 and 2 contribute the sequence that targets the enzyme to the mitochondria and is cleaved after import. Succinyl CoA synthetase associates specifically with ALAS2 within the mitochondrion, which helps promote the first step of heme synthesis.
Enzyme levels of ubiquitous and erythroid isoenzymes of ALAS are controlled by different mechanisms. Ubiquitous ALAS1 levels in the liver are regulated by negative feedback by heme that inhibits gene transcription and import of pre-ALAS1 (see Fig. 39.2A ). More recently ALAS1 has been shown to be upregulated by peroxisome proliferator-activated receptor-gamma, coactivator 1, alpha (PPAR-γ coactivator 1-α), which regulates mitochondrial biogenesis and oxidative metabolism. Transcription of PPAR-γ coactivator 1-α is controlled by glucose availability. PPAR-γ coactivator 1-α production increases when glucose levels are low, leading to increased levels of ALAS1 and heme. These conditions are conducive to an acute attack of porphyria. However, the relative contribution of heme and PPAR-γ coactivator 1-α in regulating ALAS1 expression remains to be resolved. In contrast, heme does not affect transcription of the ALAS2 gene, which is under the control of erythroid-specific promoters such as GATA1, a globin transcription factor. Whether heme inhibits import of pre-ALAS2 into the mitochondrial matrix remains to be unequivocally established, although this is supported by in vitro experiments. Heme also regulates the transcription of ferroportin, and H- and L-ferritin during erythroid differentiation, which may ensure adequate iron supply to mitochondria for heme synthesis (see Fig. 39.2B ). In addition to transport of iron from plasma to the cytosol by the transferrin receptor, a second transport step is required for mitochondrial uptake of iron. The passage of iron across the outer mitochondrial membrane is ill-defined but is due largely to the presence of voltage-dependent anion channels. Iron import across the inner mitochondrial membrane is fulfilled by mitoferrin, a member of the solute carrier 25 family of proteins located in the inner mitochondrial membrane, which, to import iron into the mitochondrion, must interact both with FECH and with the adenosine triphosphate (ATP)-binding cassette transporter ABCB10. Levels of intracellular iron regulate the translation of ALAS2 mRNA. Cellular iron homeostasis is maintained through a posttranscriptional regulatory mechanism, which is mediated by iron regulatory proteins that bind to iron-responsive elements (IRE) in mRNA of target genes to either increase or decrease translation. The RNA binding activity of iron-responsive proteins (IRP) is regulated by mitochondrial iron-sulfur cluster synthesis and cytosolic iron levels. When iron is available for heme synthesis, translation of ALAS2 is allowed to proceed as a result of decreased IRP binding to the 5′ UTR iron-responsive elements of ALAS2 mRNA. In contrast, under iron-depleted conditions, increased IRP binding to ALAS2 mRNA blocks translation and ensures that ALAS2 and protoporphyrin levels are not produced in excess of available iron. Furthermore, to prevent the cell from becoming iron deficient, increased translation of mRNA from genes that increase cellular iron, such as the transferrin 1 gene, results from stabilization of mRNA by binding of IRPs to mRNA (see Chapter 36 ). This effect ensures that protoporphyrin synthesis is coupled to iron availability.
A second rate-limiting step in the overall heme synthetic pathway lies at the level of porphobilinogen deaminase (PBGD), which has a low endogenous activity and is inhibited by protoporphyrinogen and coproporphyrinogen. There are also two forms of PBGD. The PBGD gene (hydroxymethylbilane synthase; HMBS) encodes two enzymes, which arise from alternative splicing of PBGD mRNA. One isoform is expressed in all cells, whereas a second is restricted to red cells. Erythroid PBGD is stimulated by erythropoiesis in vitro and may play a regulatory role in heme biosynthesis during differentiation.
HMBS, the human PBGD gene, has attracted extensive investigation because of the practical importance of detecting carriers of the gene for acute intermittent porphyria (AIP). Studies of the genetic locus of PBGD on chromosome 11 show great molecular heterogeneity, with over 380 mutations associated with AIP in the Human Gene Mutation Database (HGMD Professional 2019, www.hgmd.org ). Most human mutations have been described in exons 10 and 12, which is consistent with alteration of the binding sites for the dipyrromethane cofactor for the enzyme. The three-dimensional structure of PBGD has been defined by X-ray crystallography, which has allowed study of the structural and functional implications of mutations.
The porphyrias are classified as acute or nonacute (cutaneous) according to their clinical and biochemical features ( Table 39.2 ).
| Classification | Disease | Biochemistry | Clinical Features |
|---|---|---|---|
| Acute porphyria | Acute intermittent porphyria | Increased ALA and PBG | Acute attack |
| Variegate porphyria | Increased ALA and PBG; increased porphyrin | Acute attack; photosensitivity | |
| Hereditary coproporphyria | Increased ALA and PBG; increased porphyrin | Acute attack; photosensitivity | |
| ALA dehydratase deficiency porphyria | Increased ALA; increased porphyrin | Acute and chronic neuropathy | |
| Nonacute porphyria | Porphyria cutanea tarda | Increased porphyrin | Photosensitivity |
| Erythropoietic protoporphyria | Increased porphyrin | Photosensitivity | |
| Congenital erythropoietic porphyria | Increased porphyrin | Photosensitivity | |
| X-linked dominant protoporphyria | Increased porphyrin | Photosensitivity | |
| Porphyrinurias | Lead, alcohol, iron deficiency anemia, liver disease | Various biochemical manifestations | Various clinical presentations |
Each of the different types of porphyria is linked to a reduced activity or deficiency of a specific enzyme in the heme biosynthetic pathway, with the exception of X-linked dominant erythropoietic protoporphyria, which results from inheritance of a gain-of-function mutation in the ALAS2 gene (see Fig. 39.1 ). When porphyria is caused by a loss-of-function mutation, the resulting enzyme deficiency impairs the production of the end-product heme, and there is overproduction and increased excretion of the heme precursors formed by the steps before the enzyme defect. There is also a compensatory increase in activity of the initial and rate-controlling enzyme ALAS. In the acute porphyrias, there is overproduction of all the porphyrins and porphyrin precursors (e.g., ALA, PBG) formed proximal to the enzyme defect. The increased excretion of porphyrin precursors in the acute porphyrias is caused by decreased activity of PBGD in these conditions. The decrease can be caused by genetic mutation of the enzyme (in AIP) or by inhibition of PBGD by protoporphyrinogen and coproporphyrinogen in variegate porphyria and hereditary coproporphyria, respectively.
In the nonacute porphyrias, there is overproduction of all porphyrins formed before the enzyme defect but no overproduction of porphyrin precursors. The cause of this lack of overproduction of porphyrin precursors in the nonacute porphyrias is unclear, but it may result from a compensatory increase in the activity of the enzyme PBGD in addition to increased activity of ALAS and site-specific heme synthesis. The pattern of overproduction and excretion of porphyrins and porphyrin precursors in the various porphyrias is shown in Table 39.3 . A consequence is that each of the different porphyrias is characterized by a different excretion pattern. Quantitative studies of the different porphyrins and precursors in the urine and feces usually identify the particular type of porphyria. The porphyrin precursors ALA and PBG and the more water-soluble porphyrins (with multiple carboxyl groups) are excreted mainly in the urine. Other porphyrins are mainly excreted in the feces by way of bile (see box on Measurement of Porphyrins and Precursors ).
PBG quantitation in a random urine sample collected during symptoms is recommended as the initial screening test for the acute porphyrias, whereas plasma fluorescent spectroscopy is the best initial test for diagnosis of cutaneous porphyrias. Diverse techniques such as high-pressure liquid chromatography, quantitative extraction, and various forms of fluorometry are used to measure porphyrins and precursors. The International Federation of Clinical Chemistry and Laboratory Medicine presents diagnostic information on its website ( www.ifcc.org ).
| Porphyrias and Other Conditions | ALA | PBG | Urine Uroporphyrin | Urine Coproporphyrin | Feces Coproporphyrin | Feces Protoporphyrin | Erythrocyte Protoporphyrin |
|---|---|---|---|---|---|---|---|
| Acute Porphyrias | |||||||
| Acute intermittent porphyria | Raised, very high in attack | Raised, very high in attack | Usually raised a | Sometimes raised | Sometimes raised | Sometimes raised | Normal |
| Variegate porphyria | Raised in attack | Raised in attack | Usually raised in attack | Usually raised in attack | Raised | Raised | Normal |
| Hereditary coproporphyria | Raised in attack | Raised in attack | Sometimes raised in attack | Usually raised, always in attack | Raised | Usually normal | Normal |
| ALA dehydratase–deficiency porphyria | Raised in attack | Normal | Normal | Usually raised in attack | Normal | Normal | Occasionally raised |
| Nonacute Porphyrias | |||||||
| Porphyria cutanea tarda | Normal | Normal | Raised (7-/8- carboxylate porphyrin levels very high in attack) | Slightly raised | Isocoproporphyrin raised in remission | Raised in remission | Normal |
| Erythropoietic protoporphyria | Normal | Normal | Normal | Normal | Normal | Usually raised | Raised, usually very high |
| Congenital erythropoietic porphyria | Usually normal | Usually normal | Raised, isomer I | Raised, isomer I | Normal | Usually raised | Usually raised |
| X-linked dominant protoporphyria | Normal | Normal | Normal | Normal | Normal | Usually raised | Raised, usually very high |
| Other Conditions | |||||||
| Hereditary sideroblastic anemia | Normal | Normal | Normal | Normal | Normal | Normal | Occasionally raised |
| Lead poisoning | Raised | Normal | Normal | Sometimes raised | Normal | Normal | Raised when blood lead level >2 μM |
| Hereditary tyrosinemia | Raised | Normal | Normal | Normal | Normal | Normal | Normal |
| Iron deficiency anemia | Normal | Normal | Normal | Normal | Normal | Normal | Raised |
The clinical manifestations of an acute attack of porphyria can be explained by dysfunction of the central, peripheral, and autonomic nervous systems. The mechanism by which altered heme synthesis results in dysfunction is unknown, although porphyrin-induced protein aggregation may be a mechanism for both external and internal tissue damage in porphyrias that involve fluorescent porphyrin accumulation.
Perhaps the most likely hypothesis is that the neurologic and muscular manifestations of acute porphyria arise as a result of heme deficiency within the nerve cells, which causes dysfunction of the energy-dependent Na + /K + ATPase. The proposal that axonal dysfunction results from impaired energy metabolism is supported by the findings of axonal membrane depolarization during acute attacks of porphyric neuropathy and reduction in inward rectification between episodes. However, this does not exclude the possibility that ALA may also act as a pharmacologic agent in these diseases, compounding the effects of heme deficiency. ALA has a pro-oxidant effect on rat brain tissues and generates free radical species during its auto-oxidation, and this oxidant stress has been proposed to directly damage myelination by Schwann cells. The concept of auto-oxidation or oxidative stress is supported by the hypothesis that manganese excess could contribute to induction of superoxide dismutase and increased indicators of such stress in lead exposure. There is evidence that ALA enters cells by a pathway common to it and γ-aminobutyric acid (GABA).
The enzymatic links and genetic loci in each of the hereditary porphyrias are shown in Table 39.1 . Nearly all are inherited as autosomal dominant traits. The Chester porphyria family pedigree ( Fig. 39.3 ) shows autosomal dominant inheritance of acute porphyria with attacks of neurovisceral dysfunction without cutaneous hypersensitivity. This was originally reported as a dual porphyria ; however, identification of a heterozygous truncating mutation in the HMBS gene and no mutations in other heme biosynthesis enzymes in affected individuals has confirmed that Chester porphyria is a variant of classic AIP. HMBS is transcribed from two promoters to produce ubiquitous and erythroid specific isoforms. In classic AIP both isoforms are deficient; however, in the rare, nonerythroid variant, only the ubiquitous HMBS variant is defective. The rare CEP shows autosomal recessive inheritance. The mutations producing each of the acute porphyrias are heterogeneous at the molecular level and include complete or partial gene deletions, alterations of splicing or stability of mRNA, and missense mutations. In South Africa, the founder effect ensures a predominance of the Arg59Tryp mutation in protoporphyrinogen oxidase, although over 175 other mutations have been reported worldwide. Homozygotic or compound heterozygotic inheritance has been found in a number of the porphyrias, as has concurrent inheritance of more than one defect. This may present as two types of porphyria in one family or as two types in one patient. Dual porphyrias most commonly arise from a combined deficiency of uroporphyrinogen decarboxylase with PBGD, coproporphyrinogen oxidase, or protoporphyrinogen oxidase.
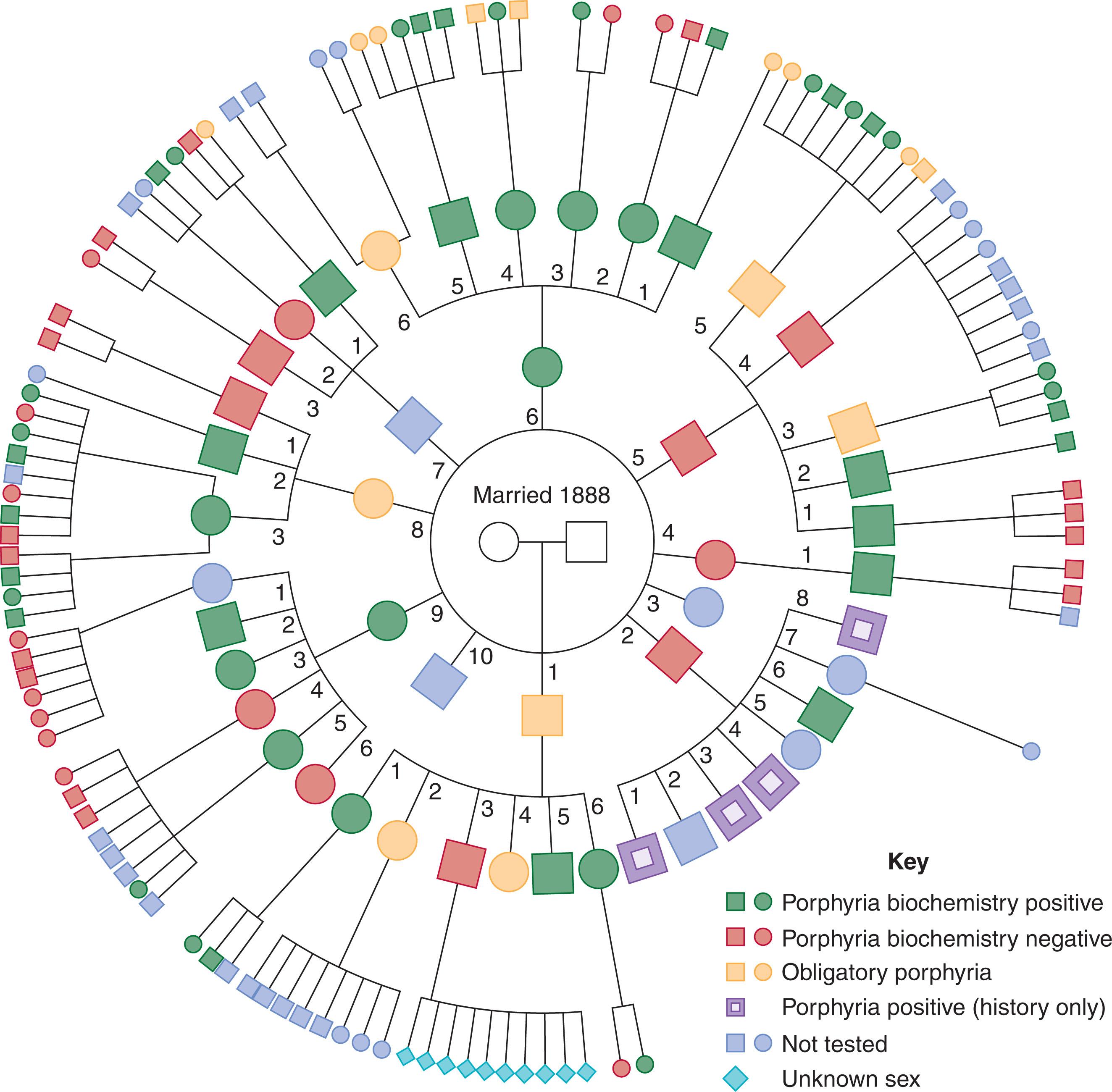
The prevalence of the different forms varies widely. For example, in northern Europe and North America, approximately 1 of 10,000 individuals carries the gene for AIP, although only about 10% of the affected persons will present with clinical features. It has been suggested that spontaneous mutation accounts for 3% of AIP cases. Variegate porphyria occurs in 1 of 400 white South Africans. There is a reduction in gene frequency in variegate porphyria from generation to generation that suggests that the allele associated with it is selectively deleterious. The same is probably true of the other porphyrias.
Acute intermittent porphyria is the most severe of the acute porphyrias. During an attack, patients display abdominal and neuropsychiatric or neurovisceral disturbances. Onset occurs in puberty; female patients exhibit a fourfold greater incidence of attacks than males. Attacks occur mainly in young adults and become less frequent after menopause. It is uncommon to see attacks in children. Crises may vary in duration from several days to months. They are most commonly followed by complete remission, although deaths are still reported, especially with AIP (see box on Precipitating Factors in Acute Porphyria ). Late complications of AIP include renal impairment, hypertension, and hepatocellular carcinoma, and patients should be monitored for these complications.
Most patients who have inherited acute porphyria enjoy normal health and go through life without any knowledge of their disorder or ever experiencing an acute attack. All porphyric patients, however, are at risk for developing an attack if exposed to various precipitating factors. Drugs are the most common precipitating agents. Other factors that may trigger attacks include alcohol ingestion, reduced caloric intake (from fasting or dieting), and infection. Smoking can cause more frequent attacks.
Hormonal status is also important. Attacks are more common in females, and they rarely occur before puberty or after menopause. Although generally uncomplicated, pregnancy in addition to oral contraceptives may precipitate attacks. Some women experience regular attacks, commencing in the week before the onset of menstruation. These may require luteinizing hormone–releasing hormone (LH-RH) antagonists for control ( Table 39.4 ).
| Drugs | Other Stimuli |
|---|---|
| Alcohol | Fasting or dieting |
| Barbiturates | Hormones, stress |
| Angiotensin-converting enzyme (ACE) inhibitors | Smoking |
| Anticonvulsants | |
| Antidepressants | |
| Calcium channel blockers | |
| Cephalosporins | |
| Ergot derivatives | |
| Erythromycin | |
| Steroids or anabolic steroids | |
| Contraceptives, hormone replacement therapy | |
| Sulfonamides | |
| Sulfonylureas |
Before prescribing any medication to a porphyric patient, advice must be sought from an appropriate specialist. Full drug lists are available on the Internet at https://porphyriafoundation.org/ (in the United States) and www.porphyria-europe.com (in European Union countries, South Africa, and Canada), and a guide for drug prescription at www.drugs-porphyria.org . It should be borne in mind that such lists are far from encyclopedic, that new drugs are constantly being introduced to the pharmacopeia, and that any form of combined preparation must be viewed with suspicion, because little is known about metabolic interactions in these diseases. More details of such use and side effects of drugs can be sought from the literature.
Gastrointestinal symptoms occur in 95% of cases; most patients present with acute colicky central abdominal pain. Examination reveals tenderness but little rigidity, and patients may also experience limb pain or generalized muscular aches. Severe vomiting may occur, and constipation is usual. Hyponatremia occurs in severe attacks.
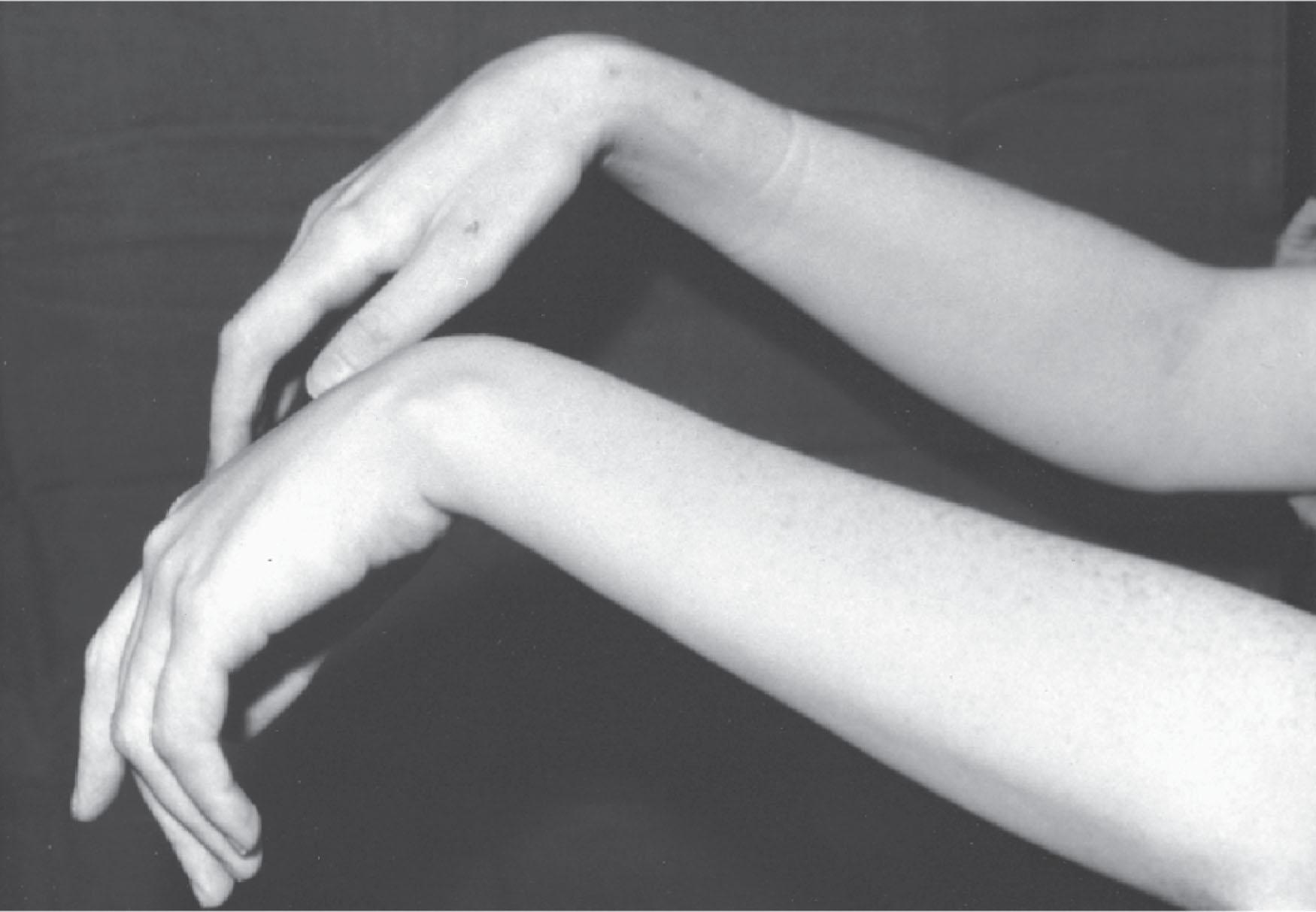
Motor neuropathy complicates two-thirds of porphyric attacks and may be the presenting feature. Motor involvement is most common, but paresthesias may also occur. Paralysis usually starts peripherally and then spreads proximally; however, in some patients, shoulder girdle involvement may be the first manifestation. The neuropathy may progress rapidly, resulting in respiratory insufficiency. Weakness, usually symmetric, involves proximal and distal limb muscles more often than those of the trunk. Upper limbs and proximal muscles are often affected. Involvement of the wrists, ankles, and small muscles of the hand may lead to a permanent deformity ( Fig. 39.4 ), and trunk muscle weakness can lead to respiratory embarrassment. Death is usually caused by respiratory paralysis. Progressive weakening of the voice may suggest this; treatment requires tracheotomy and intermittent positive pressure ventilation. Paresthesias, numbness, and objective evidence of sensory impairment may occur with loss of pinprick sensation, which is most marked around the shoulder and hip areas; generalized tonic-clonic seizures occasionally occur.
Severe anxiety, depression, and frank psychosis are the main psychiatric manifestations of porphyric attacks. These psychiatric manifestations may result in a patient being misdiagnosed as suffering from a primary psychiatric disorder. Agitation, mania, depression, hallucinations, and schizophrenic-like behavior may occur. Psychiatric manifestations may persist between attacks. Quality of life is severely affected in those suffering from repeated attacks of acute porphyria.
The cardiovascular system is involved in approximately 70% of attacks. Sinus tachycardia (to 160 beats/min) and hypertension can occur; these elevations usually revert to normal after an attack. There is evidence that hypertension may occasionally be permanent, even in latent cases of AIP (see box on Differential Diagnosis of Acute Intermittent Porphyria ).
Attacks of acute porphyria must be distinguished from other causes of acute abdominal pain or peripheral neuropathy sometimes associated with psychosis. Heavy metal poisoning (i.e., lead or arsenic) and Guillain-Barré syndrome must be considered, as well as paroxysmal nocturnal hemoglobinuria with its characteristic early morning hemoglobinuria and abdominal pain.
During an attack, all patients excrete a massive excess of the porphyrin precursors, 5-aminolevulinate (ALA) and porphobilinogen (PBG), in their urine. Urine, when first voided, is clear and darkens on exposure to light as the hexahydroporphyrins, the porphyrinogens, are oxidized to porphyrins.
A rapid screening test during an acute attack is to mix equal volumes of urine and Ehrlich aldehyde reagent and observe for the pink color of porphobilinogen; alternatively, the urine can be left standing in sunlight to observe darkening in color. The differential diagnosis of porphyrias uses qualitative and quantitative measurement of porphyrins and precursors, with subsequent use of enzymatic assay and identification of familial genetic alterations.
Hereditary coproporphyria combines the clinical features of acute porphyria with photosensitive skin manifestations. It results from various mutations in the gene encoding coproporphyrinogen oxidase, the activity of which is decreased, leading to overproduction of coproporphyrin. A clinically distinct variant of hereditary coproporphyria, harderoporphyria, is associated with severe jaundice and hemolysis and is caused by specific mutations that lead to accumulation of harderoporphyrins.
The porphyrin precursors ALA and PBG and the more water-soluble porphyrins (with multiple carboxyl groups) are excreted mainly in the urine. Other porphyrins are mainly excreted in the feces by way of bile.
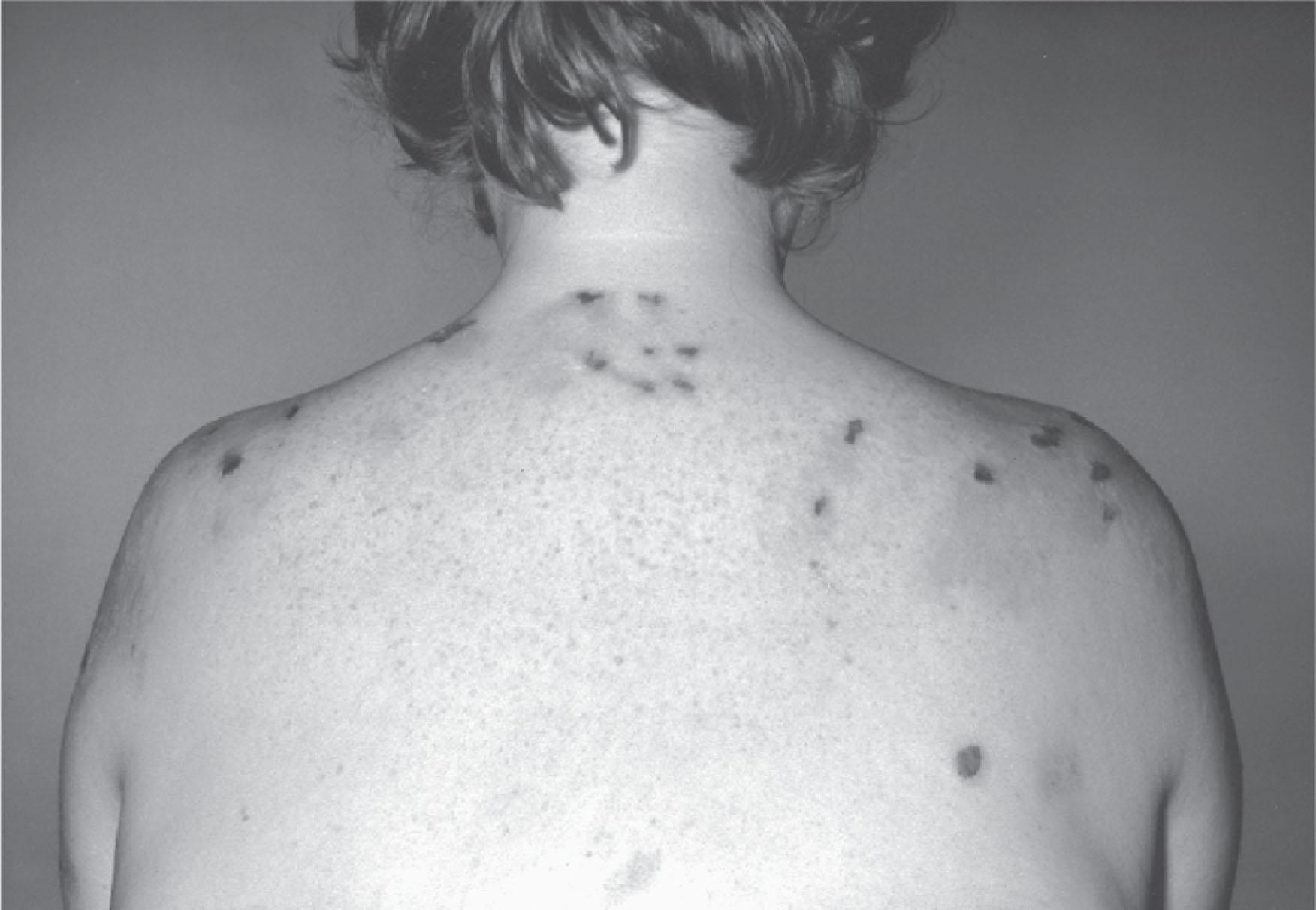
Variegate porphyria is similar to hereditary coproporphyria, except that there are more severe skin lesions, sometimes with scarring ( Fig. 39.5 ). Protoporphyrinogen oxidase is the affected enzyme, and protoporphyrin is the major circulating porphyrin. Conventionally, variegate porphyria is most readily diagnosed by measurement of fecal porphyrin concentrations. However, it has been reported that biliary porphyrin levels may provide a better discriminator from normal patients in the asymptomatic phase. As in erythropoietic protoporphyria, there is a tendency toward cholelithiasis. The mechanism by which gallstones form is not certain, but some studies have suggested that porphyrins are cholestatic. In hereditary coproporphyria and variegate porphyria, the pathway intermediates produced in excess, coproporphyrinogen and protoporphyrinogen, respectively, are inhibitors of the secondary rate-controlling enzyme PBGD. Numerous mutations in the protoporphyrinogen oxidase gene have been described leading to 50% reduction in enzyme activity. The functional consequences of many of these mutations have been predicted based on the crystal structure of the enzyme. In a few cases, homozygosity or compound heterozygosity has been described.
In this porphyria (also known as acute hepatic porphyria or plumboporphyria), the ALA dehydratase activity is depressed, such as seen with a low-function variant of the enzyme in a subject exposed to lead. The clinical picture resembles AIP, but very few cases have been described, and the disease only manifests in homozygous cases when there is a precipitating factor.
The concurrent porphyrias are a rare group of conditions in which there is concurrent inheritance of two different defects within the heme biosynthetic pathway, although this requires confirmation from molecular evidence. Previous descriptions have shown the presence of concurrent porphyria within a family, and toxicologically there is good evidence that exposure to poisons such as lead can induce multiple changes within the pathway. The first reported example of concurrent porphyria in a family combined the clinical features of acute and cutaneous porphyria, and biochemical analysis confirmed the segregation of variegate porphyria and PCT as independent inherited traits. In another patient, dual genetic defects involving ALA dehydratase and coproporphyrinogen oxidase have been described (see box on Management of Acute Porphyria ).
A carbohydrate intake of 1500–2000 kcal/24 h should be maintained throughout the attack to reduce porphyrin synthesis; give this orally or, for more severe attacks, through a fine-bore Teflon nasogastric tube. If this cannot be tolerated, intravenous dextrose (e.g., 20% solution, 2 L/day) should be given. If early in the attack (2–4 days from onset), give intravenous hematin as heme arginate (Normosang, Recordati Rare Diseases, Lebanon, NJ) at a recommended daily dose of 3 mg/kg once daily for four days to further reduce the overproduction of porphyrin and precursors. Hematin (Panhematin, Recordati Rare Diseases) in similar doses may also be used, although it should be reconstituted in human albumin solution to avoid phlebitis and mild transient prolongation of coagulation times. No renal complications have occurred with the standard recommended dosages, and even patients with renal insufficiency tolerate hematin well, although the dosage should be reduced slightly. Severe hepatic toxicity has been associated with rapid infusion of higher doses of heme arginate. The action of heme therapy may be extended by heme oxygenase blockers such as tin protoporphyrin. Liver transplantation may be an effective treatment for life-threatening acute intermittent porphyria that fails to respond to medical therapy.
Many drugs are contraindicated, and the patient must be warned to avoid precipitating factors. Alcohol should be restricted and smoking discouraged. Dieting (<800 kcal/day) must be avoided. Pregnancy should also be avoided if the disease is active. If a patient requires an anesthetic, nitrous oxide, ether, and cyclopropane are safe, and suxamethonium appears to be a safe muscle relaxant. The opiates and belladonna derivatives can be used for premedication and propofol for maintenance of anesthesia. Infection can precipitate an attack and should therefore be sought and treated. Blood relatives of patients should be screened to see if they carry the gene.
Luteinizing hormone-releasing hormone (LH-RH) antagonists that suppress ovulation are a valuable form of prophylaxis in menstrually related attacks. Estrogens and progestogens such as those in the contraceptive pill must be avoided in acute porphyria. The same applies to most steroids and receptor antagonists such as mifepristone.
In all cutaneous porphyrias, porphyrins (which are photosensitizing) are deposited in the upper layers of the skin, and they are responsible for the characteristic skin lesions. In the development of these lesions, reactive oxygen species and other radicals are formed and probably induce oxidative membrane damage, particularly to mast cells, which enables complement activation as one part of the inflammatory reaction (see box on Management of Nonacute Porphyria .
Patients should avoid exposure to sunlight and use sunblock (to filter Soret band light) and physical barriers such as cotton gloves.
The clinical features are reversed by removing any precipitating agent such as alcohol, halogenated hydrocarbons, and drugs. The patient should be screened for hepatic neoplasm and hepatitis C infection. The mainstay of treatment is to remove liver iron by venesection of 500 mL of blood weekly until clinical remission occurs or until the hemoglobin level falls below 12 g/dL. In patients with PCT where HCV is the primary risk factor, antiviral treatment with direct-acting antiviral agents such as simeprevir and sofosbuvir, is effective as a single-agent therapy. Chloroquine, at low doses of 125 mg twice per week for several months, has also been helpful because it enhances urinary clearance of porphyrin. When this is not possible, oral cimetidine has been used. When venesection is difficult, parenteral and orally active iron chelators can be used to reduce liver iron stores. It is of value to screen the patient and first-degree relatives for hereditary hemochromatosis.
Oral β-carotene offers effective protection in erythropoietic protoporphyria (EPP) against solar sensitivity. It does so by quenching the radical formation that is a feature of the skin damage. Yellowing of the skin (i.e., carotenemia) is one side effect. Afamelanotide, an alpha-melanocyte-stimulating hormone that is now approved by the US Food and Drug Administration, is effective for decreasing photosensitivity in EPP by increasing melanin production, and has shown efficacy and tolerability for the long-term treatment of EPP. Interruption of the enterohepatic protoporphyrin circulation by bile salt–sequestering agents, such as cholestyramine, reduces plasma protoporphyrin levels and may retard the development of the liver disease. Liver transplantation has been reported to be an effective measure in preventing the progression of this disease.
The severity of hematologic manifestations are predictors of a poor prognosis, and patients with hemolytic anemia or thrombocytopenia should be considered for allogeneic hematopoietic stem cell transplantation (HSCT). The majority of patients treated with allogeneic HSCT have achieved long-term symptomatic cures. Erythropoiesis should be reduced by means of erythrocyte hypertransfusion and by hematin or heme arginate infusion. However, care should be taken when prescribing heme arginate, as overdosing may cause acute hepatic failure. Splenectomy and chloroquine therapy (125 mg twice weekly) have an ameliorating effect, as does hypertransfusion, but life expectancy is usually severely shortened. Iron chelation with deferasirox to inhibit ALAS2 mRNA translation in a CEP patient has resulted in decreased porphyrin accumulation and reduced hemolysis and photosensitivity. Therapeutic potential has been shown with the use of clinically approved proteasome inhibitors, such as bortezomib, which prevent enzymatically active uroporphyrinogen III synthase (UROS) mutants from early degradation in a murine model, and gene therapy using induced pluripotent stem cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here