Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hematopoietic stem cells (HSCs) are characterized by their unique ability to self-renew and give rise to the entirety of the blood and immune system throughout the lifetime of an individual. HSCs are very rare cells, representing approximately one in 100,000 bone marrow (BM) cells in the adult. The concept of the existence of an HSC that is capable of reconstituting hematopoiesis in vivo was first introduced more than 70 years ago, when Jacobson et al. demonstrated that lead shielding of the spleen protected mice from otherwise lethal γ-irradiation. Subsequently, Jacobson and colleagues reported that similar radioprotection of mice could be achieved via shielding of one femur. Shortly thereafter, it was demonstrated that intravenous injection of BM cells also provided radioprotection of lethally irradiated mice. Interestingly, investigators initially hypothesized that the radioprotected spleen or BM provided soluble factors that mediated radiation protection. However, subsequent experiments by Nowell et al. and Ford et al. critically demonstrated that transplanted BM cells provided radioprotection directly via cellular reconstitution of the blood system. The historical significance of these studies cannot be overestimated because they provided the basis for not only the ultimate isolation and characterization of HSCs but also for the field of hematopoietic stem cell transplantation.
Subsequent landmark studies by Till and McCulloch demonstrated that transplantation of limiting doses of BM cells gave rise to myeloid and erythroid colonies in the spleens of irradiated recipient mice. Importantly, Till and McCulloch showed that the numbers of colonies detected in recipient mice was proportional to the numbers of BM cells injected into the irradiated mice, suggesting that a particular population of hematopoietic cells was capable of reconstituting hematopoiesis in vivo. The clonogenic nature of a subset of BM cells was definitively shown when these investigators irradiated BM cells and then transplanted the cells into lethally irradiated mice. Persistent chromosomal aberrations were demonstrated in spleen colonies in recipient mice. It was subsequently shown that cells within the spleen colonies were radioprotective of lethally irradiated mice and contained myeloid, erythroid, and lymphoid cells. Taken together, these data strongly suggested the presence of hematopoietic stem or progenitor cells that were capable of in vivo engraftment and generation of multilineage progeny from a small number of parent cells.
Mammalian hematopoiesis occurs in several waves, which are separated temporally and spatially and produce different cell types: a transient first “primitive” is followed by a “prodefinitive” and then a “definitive” wave, which persists through life. While most of the evidence is derived from the mouse, data from humans, albeit limited, point to a very comparable hematopoietic program.
During embryogenesis, the hematopoietic cells of the first, primitive wave are formed when cells from the epiblast that constitute the prospective mesoderm ingress and migrate through the primitive streak between the endoderm and ectoderm, both in the embryo proper and in the extraembryonic yolk sac (YS). In the latter, mesodermal cells aggregate to form blood islands surrounded by visceral endodermal cells on mouse embryonic day (E) 7 to 7.5. The close proximity of erythroid cells and vascular endothelium in YS blood islands, their origin from mesoderm, and their simultaneous differentiation led to the proposal of a common precursor, the hemangioblast, over a century ago. In support of this hypothesis, a spontaneous zebrafish mutant, cloche (named for its bell-shaped heart because of the loss of endothelium), lacks both vasculature and hematopoietic cells but no other mesodermal lineages such as cardiac progenitors. The gene mutated in cloche was recently cloned and encodes a PAS (PER-ARNT-SIM) domain–containing basic helix-loop-helix (bHLH) transcription factor ( npas4l ), which belongs to the same class that also includes the aryl hydrocarbon receptor and hypoxia-induced factor (HIF)-1α. Also, mice lacking FLK1 (VEGFR2, a receptor for vascular endothelial growth factor), expressed on endothelial (progenitor) cells, fail to develop both vascular endothelium and blood islands during embryogenesis. Indeed, gene tracing studies in mouse and human embryonic stem cell cultures have identified a progenitor with both hematopoietic and endothelial potential.
Primitive hematopoiesis encompasses the generation of primarily large erythroid cells and primitive macrophages. Following this initial wave, beginning at mouse E8.25, erythromyeloid progenitors (EMPs) are generated as prodefinitive progenitors. Both waves arise transiently in the YS during a time comparable to the first trimester in humans, but the cells lack the capacity for self-renewal and multilineage differentiation present in definitive HSCs. The emergence of EMPs during development is presaged by ckit+ EMP precursors that are characterized by the expression of toll-like receptor 2 (TLR2) at E7.5. Three lineage-tracing studies recently demonstrated that embryonic erythropoiesis is sustained completely by YS EMPs, rather than HSC-derived progenitors. Remarkably, Hoxa neg/low Kit+CD41+CD16/32+ HSC-independent EMPs from the YS have also been shown to give rise to NK cells with cytotoxic capability, and YS EMPs produce osteoclast precursor cells that create space for post-natal BM hematopoiesis. Interestingly, epidermal γδ T cells, also known as dendritic epidermal T cells, function in the adult epidermis, and are also derived from YS progenitors. Utilizing single cell transcriptomic analysis and single cell cultures of YS-derived myeloid progenitors, Bian et al. recently provided a comprehensive characterization of the spatiotemporal dynamics of early macrophage development from YS progenitors during embryogenesis. This study provides unprecedented insight into the distinct biology of tissue resident macrophages that are derived from YS progenitors. Importantly, Stremmel et al. showed that CX3DR1+ YS-derived pre-macrophages migrate between embryonic day 8.5 through day 12.5 via the bloodstream into embryonic tissues where they take up long-term residence.
Definitive HSCs capable of long-term (LT), multilineage reconstitution of irradiated adult recipient mice appear at E10.5 in the intraembryonic region encompassing the aorta, gonads, and mesonephros (AGM), in particular in hematopoietic intra-aortic clusters in the ventral wall of the dorsal aorta. Then, within a remarkably short period of 1.5 days during embryonic development, virtually all HSCs are generated that will replenish the hematopoietic system throughout fetal and adult life. Studies by Ganuza et al. utilizing cultured 2 to 7 somite pairs (sp) murine embryonic explants and 2 to 7 sp YS explants confirmed that the embryo, not the YS, is the source of definitive HSCs. Several complementary studies using lineage tracing experiments in both mice and zebrafish have demonstrated that within the dorsal aorta, hemogenic endothelial cells (ECs) are the direct precursors of definitive HSCs. In a process known as endothelial-to-hematopoietic transition (EHT), HSCs bud off the hemogenic endothelium to form intra-aortic hematopoietic clusters from which they are released into circulation. Interestingly, while the AGM gives rise to HSCs, it is not the site of hematopoietic differentiation. Rather, HSCs colonize the fetal liver where they expand and then differentiate (Ref. and references therein).
Recent advances in single cell labeling and single cell transcriptomic analysis has allowed the isolation and characterization of a population of “pre-HSCs” during murine development. CD45+ pre-HSCs in the AGM were shown to have a unique molecular signature and activation of mechanistic target of rapamycin (mTOR) was reported to be indispensable for the emergence of HSCs. Separate studies which evaluated ex vivo maturation of HSCs from pre-HSCs showed that ex vivo matured HSCs and fetal liver HSCs express programmed death ligand 1 (PDL1), among other immune response genes, although PDL1 expression was not required for engraftment of embryonic HSCs.
Evidence from studies in mice suggests that some definitive hematopoiesis also occurs at sites other than the AGM. By E12 the fetal liver contains more HSCs than can be accounted for by HSCs generated in the AGM alone. Quantitative analysis of HSC distribution showed that both YS and placenta generate definitive HSCs that migrate to the liver and other hematopoietic sites. Lastly, a c-Myb- and thus HSC-independent cell lineage that emerges between E8.5 and E9.5 in the YS has recently been shown to give rise to YS macrophages and later on to tissue macrophages in brain (microglia), liver (Kupffer cells), and skin (Langerhans cells).
Human induced pluripotent stem cell models and studies of zebrafish have suggested that a population of immune precursors may originate directly from hemogenic endothelium rather than HSCs. Utilizing a RAG1:GFP human reporter system, Motazedian et al. showed that early RAG1+ cells could differentiate into CD4+CD8+ T cells, also possessed B-cell, myeloid, and erythroid potential, while also expressing endothelial markers, and resided within CD31+ endothelial structures. The authors concluded that a wave of T-cell development might originate directly from hemogenic endothelium via a RAG1+ intermediate population. Utilizing a novel technique called “ScarTrace,” a single cell sequencing strategy to quantify the clonal history and cell type of thousands of cells in different organs of the developing zebrafish, Alemany et al. identified a novel population of immune cells in the zebrafish fin that had a distinct clonal origin from other hematopoietic cells. In keeping with these studies, Tian et al. utilized temporal-spatial fate mapping analysis and time-lapse imaging to show that a wave of T lymphopoiesis could be detected in the developing zebrafish, arising from ventral endothelium in the AGM and posterior blood islands. This generation of CD4+ T cells is transient and occurs only in the early larval stage, and is later replaced by HSC-dependent T cells of all subtypes. Taken together, these studies provide the impetus for further exploration of evidence for HSC-independent immune cell generation during mammalian development.
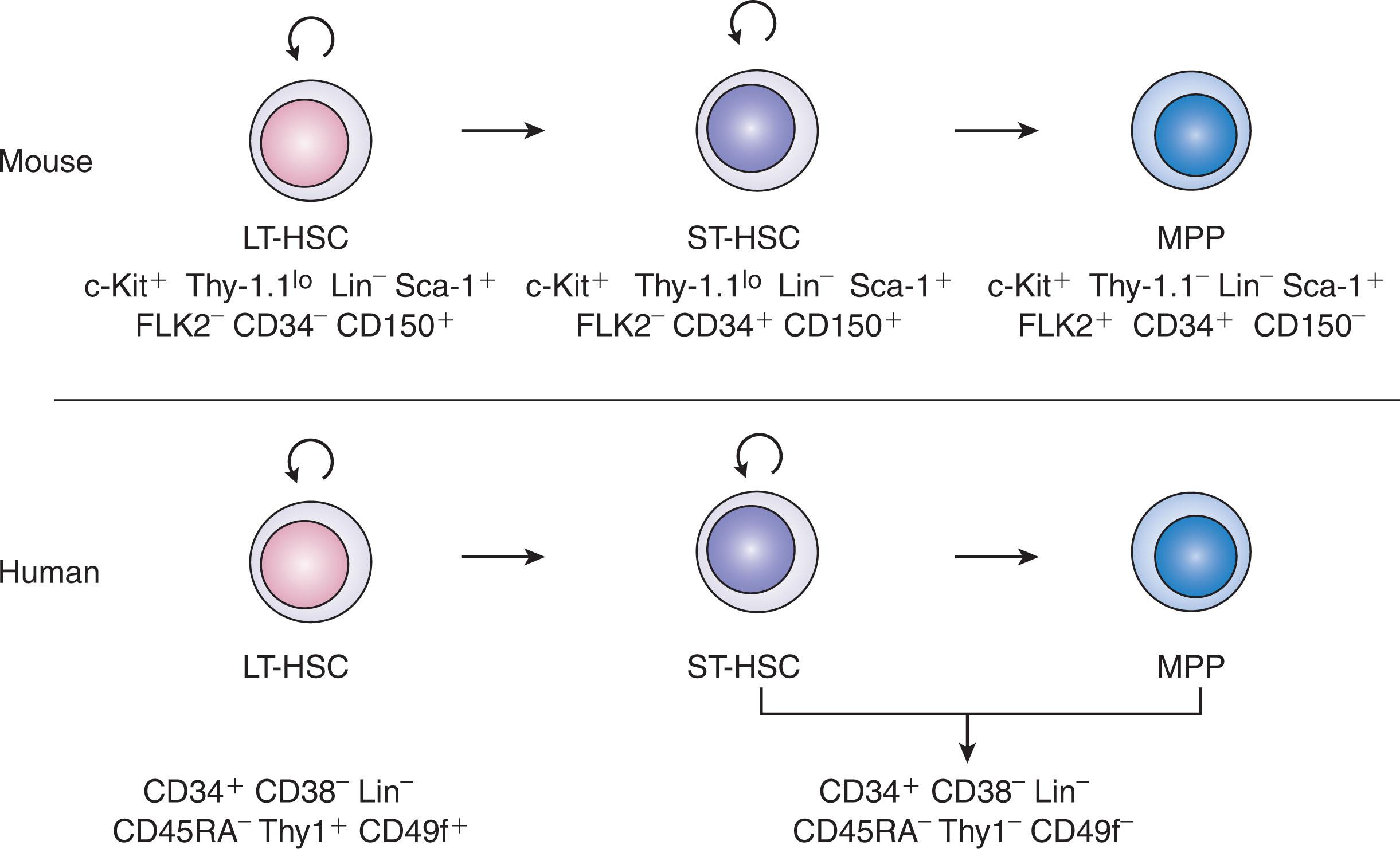
The HSC is the most well-defined somatic, multipotent stem cell in the body. With the emergence of monoclonal antibody technology and flow cytometry coupled with in vitro and in vivo functional assays, biologists have developed reproducible methods to analyze and isolate murine and human HSCs with a high level of enrichment. In mice, Weissman and colleagues were able to show that antibody-based depletion of BM cells expressing myeloid, B-cell, T-cell, and erythroid cells along with positive selection for cells expressing c-Kit, Sca-1, and Thy-1.1 lo (“KTLS” cells) allowed for enrichment of HSCs to approximately one of 10 to 30 cells as measured by the capacity to provide LT, multilineage hematopoietic reconstitution in a competitively transplanted, lethally irradiated congenic mouse. Because Thy-1.1 is not expressed in many strains of mice, additional markers were developed, including FLK2 (FLT3), the absence of which was shown to substantially enrich for murine LT-HSCs. Similarly, it has been demonstrated that the isolation of murine BM c-Kit + Sca-1 + Lin − (KSL) cells based upon the lack of expression of CD34 (34 − KSL) enriches for HSCs with LT reconstituting capability at the level of one of 5 to 10 cells ( Fig. 9.1 ).
An alternative and effective method for isolating BM HSCs involves the use of intravital dyes, Hoechst 33342 (Ho33342) and Rhodamine 123 (Rh123). HSCs, unlike more committed progenitor cells, efficiently efflux these dyes such that HSCs display low-intensity staining for these dyes. Li and Johnson demonstrated that HSCs capable of LT, multilineage repopulation in lethally irradiated mice were significantly enriched in the Rh123 lo Sca-1 + Lin − cells, but Rh123 hi Sca-1 + Lin − cells possessed little repopulating activity. Similarly, McAlister et al. showed that isolation of Ho33342 lo BM mononuclear cells significantly enriched for both the potential to produce colony-forming units in the spleen (CFU-S) on day 14 and cells capable of radioprotection and multilineage reconstitution in lethally irradiated mice. A subsequent and important refinement in the use of Ho33342 to isolate HSCs was made by Goodell et al., who showed that a Ho33342 side population (SP) can be identified via the emission of Ho33342 at two wavelengths, which yields a tail profile on flow cytometric analysis. Importantly, isolation of Ho33342 SP cells has been shown to yield variable enrichment for HSCs compared with CD34 − FLT3 − KSL cells, which may be caused by the sensitivity of the assay to variations in staining techniques and batch-to-batch differences in Ho33342 dye. However, Matsuzaki et al. demonstrated that transplantation of single Ho33342 SP 34 − KSL cells into lethally irradiated C57BL/6 mice yielded donor cell multilineage engraftment greater than 1% in more than 95% of transplanted mice. Therefore, the combination of Ho33342 SP cells with CD34 − KSL markers provides a basis for isolation of highly enriched LT-HSCs from mice.
A major advance in this field involved the discovery by Kiel et al. that the surface expression of CD150, a member of the signaling lymphocyte activation molecule (SLAM) family, significantly enriched for murine BM HSCs. It was also shown that the absence of CD41 and CD48 on CD150 + cells enriches further for the HSC population and that approximately half of CD150 + CD41 − CD48 − or CD150 + CD48 − KSL cells reconstitute lethally irradiated mice competitively transplanted with limiting numbers of cells. Taken together, combined isolation of SLAM- and KSL-enriched BM cells has become a reproducible and efficient strategy to isolate murine LT-HSCs with maximal enrichment (see Fig. 9.1 ).
Expression of the gene Ctnnal1, which encodes alpha-catulin, coupled with ckit expression enriches at a very high level for murine HSCs. Anatomic imaging of alpha-catulin-GFP+ckit+ cells revealed that the vast majority of these HSCs reside in the central marrow and diaphysis, in contact with leptin receptor+ and CXLC12+ perivascular stromal cells, and within 10 μm of sinusoidal blood vessels, not arterioles. Similarly, HoxB5 expression was shown to mark HSCs with long-term repopulating capacity in mice and greater than 94% of HoxB5+ long-term HSCs were found to reside in contact with VEcadherin+ ECs, suggesting a BM vascular/perivascular niche for adult HSCs. Conversely, combined analysis of three different HSC reporter lines and nine distinct niche populations in mice suggested that while HSCs were found in close association with leptin receptor+ stromal cells, sinusoidal ECs, and megakaryocytes, this distribution was not different than that of random dots. The authors concluded that the distribution of HSCs in the adult BM may simply reflect the frequency of different cell types in the BM niche, rather than true enrichment in association with a particular niche cell. A more detailed description of the niches for HSCs is presented in Chapter 33, Chapter 44 .
Through a combination of single cell gene expression analysis, flow cytometric index sorting, and single cell transplantation assays, Wilson et al. demonstrated that murine hematopoietic cells with high expression of endothelial protein C receptor (EPCR) combined with CD150+CD48-Sca-1 hi (EPCR hi CD48-CD150+Sca-1 hi HSCs) were the most highly enriched for long-term self-renewal capacity. Additional studies by Cohen et al. revealed that EPCR also regulates HSC retention in the BM via modulation of nitrous oxide production in coordination with protease activated receptor 1. Interestingly, EPCR surface expression on human cord blood (CB) HSCs has been shown to mark human cells with in vivo repopulating capacity following culture with the HSC expansion reagent, UM171. Ito et al. separately demonstrated that murine CD34-HSCs displayed high expression of the angiopoietin 1 (ANGPT1) receptor gene, Tie2, and that 68% of Tie2-GFP+CD34-CD150+CD38low/-Flt3-KSL cells from Tie2 reporter mice were capable of long-term reconstitution in single cell transplantation assays. Other markers recently described to enrich murine HSCs include leptin receptor, which is expressed on BM stromal cells, but has been used to isolate lepR+SLAM+HSCs which display enhanced repopulating capacity in vivo compared to lepR-SLAM+HSCs and an embryonic-like transcriptome. Gulati et al. reported that Hoxb5+ HSCs can be further purified using Neogenin surface expression such that Neogenin-Hoxb5+ HSCs are lineage balanced and provide long-term hematopoietic reconstitution, while Neogenin+Hoxb5+ HSCs are myeloid biased and display reduced reconstituting capacity. Finally, endothelial cell selective adhesion molecule (ESAM) is also expressed by murine HSCs and identifies a proliferative population of HSCs that resides near BM vasculature and retains in vivo repopulating capacity.
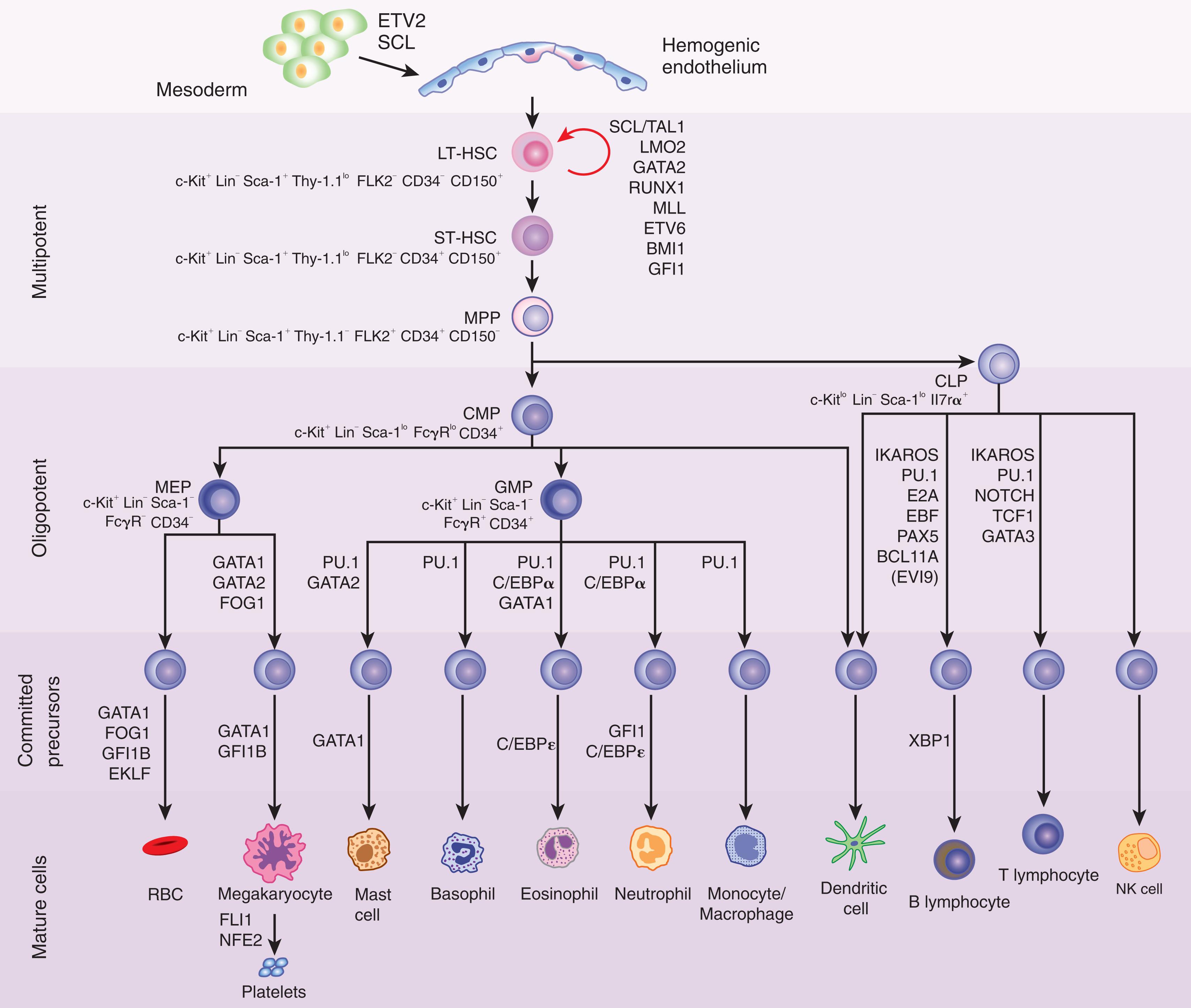
Although this chapter focuses on the phenotypic and functional characterization of HSCs, increasing evidence suggests that a subset of adult T-cell progenitors may possess myeloid potential. Using various methodologies, Bell et al. and Wada et al. first reported that adult T-cell thymic progenitors possessed myeloid differentiation potential. However, a subsequent study using in vivo transplantation models did not confirm the myeloid potential of adult T cells. De Obaldia et al. reported that the majority of resident granulocytes in the mouse thymus were derived from early thymic progenitors. Mechanistically, it has also been shown that the transcription factor, HES1, constrains myeloid gene expression in T-cell progenitors via repression of C/EPB-α. Taken together, these data suggest that a population of common lymphoid progenitors (CLPs) may indeed possess myeloid differentiation potential. Recent studies have also clarified the nature of CLPs and have dissected this population further into an all-lymphoid progenitor cell (ALP), which retains full lymphoid potential and thymic seeding capability, and B lymphoid progenitor cells (BLPs), which are restricted to the B-cell lineage. Whereas ALPs are characterized by the lack of surface expression of LY6D, BLPs express LY6D and upregulate the B-cell-specific factors, EBF1 and PAX5. The phenotypic markers of the hematopoietic hierarchy through myeloid and lymphoid differentiation are shown in Fig. 9.2 .
Significant progress has also been made in the phenotypic characterization of human HSCs via flow cytometric analysis combined with in vivo transplantation assays in immune-deficient mice. Of particular note, although murine HSCs can be characterized by the absence of CD34 expression on the cell surface, human HSCs are primarily enriched using CD34 surface expression, and this provides the basis for confirming sufficient HSC content to allow for successful hematopoietic cell transplantation in patients. There is also some controversy in this area because some investigations have suggested that LT-HSCs can be isolated from CD34 − human hematopoietic cells. Of note, only a small percentage (less than 0.1%) of CD34 + human hematopoietic cells possess the capacity to engraft following intravenous injection into nonobese diabetic/severe combined immune deficient (NOD/SCID) mice. Further enrichment of human HSCs has been demonstrated via negative selection for surface expression of CD38 and depletion of lineage surface markers. Thy-1 (CD90) surface expression also enriches for multilineage colony-forming ability and in vivo reconstituting capacity of human hematopoietic cells. Majeti et al. showed that the Lin − CD34 + CD38 − CD45RA − Thy-1 + population in human CB was enriched at the level of one in 10 cells for LT-HSCs. The authors also showed that candidate multipotent progenitor cells (MPPs) were demarcated by the Lin − CD34 + CD38 − CD45RA − Thy-1 − population, suggesting that the loss of Thy-1 reflects the transition of LT-HSCs to short-term (ST)-HSCs/MPPs.
Although it is possible to enrich murine BM HSCs to the level of nearly single-cell purity using various combinations of cell surface markers, isolation of human BM HSCs to the same level of purity has not been readily achieved. However, Notta et al. demonstrated that intrafemoral injection of a fluorescence-activated cell sorting (FACS)-purified population of human CB CD34 + CD38 − CD45RA − Thy-1 + cells that were additionally purified based on surface expression of the integrin α6 (CD49f) yielded 6.7-fold increased human donor chimerism at 20 weeks in NOD/SCID IL2Rγ −/− (NSG) mice compared with injection with the identical dose of CD34 + CD38 − CD45RA − Thy-1 + CD49f − cells. Only the Thy-1 + CD49f + cells could be serially transplanted in this study, and the enrichment for LT-HSCs via limiting dilution analysis was estimated to be approximately one in 11 CD34 + CD38 − CD45RA − Thy-1 + CD49f + cells. Further purification of this population of cells using Rh123 dye demonstrated that single-cell transplantation of Thy-1 + Rh123 lo CD49f + cells yielded LT, multilineage engraftment in five of 18 transplanted recipients. Serial transplantation was also successful in two of four secondary mice, suggesting that at least some of the Thy-1 + Rh123 lo CD49f + cells undergo self-renewal. Of note, because mice were transplanted via intrafemoral injection in these studies, it remained unknown whether this panel of markers equally identified human HSCs capable of homing properly to the BM after intravenous injection. Nonetheless, these studies revealed that the addition of CD49f + to the panel of human LT-HSC markers provided an improved capability to isolate human HSCs at a level of purity that was comparable to that applied to murine HSC isolation.
Two additional novel cell surface markers for human HSCs are CD166 (activated leukocyte adhesion molecule) and protein tyrosine phosphatase-sigma (PTPσ). Human Lin − CD34 + CD38 − CD49f + CD166 + cells engrafted in primary and secondary NSG mice at a significantly higher level than Lin − CD34 + CD38 − CD49f + CD166 − cells. Interestingly, CD166 is also expressed by BM osteoblasts and it was postulated that CD166 mediated HSC maintenance in vivo via homophilic interactions between CD166 expressed on HSCs and osteoblasts. Quarmyne et al. reported that NSG mice transplanted with human CB Lin − CD34 + CD38 − CD45RA − PTPσ − cells displayed 15-fold higher human hematopoietic cell engraftment at 16 weeks compared to mice transplanted with Lin − CD34 + CD38 − CD45RA − cells or Lin − CD34 + CD38 − CD45RA − PTPσ + cells. Protein tyrosine phosphatase–sigma (PTPσ) was shown to negatively regulate both murine and human HSC repopulation following transplantation, via inhibition of the RhoGTPase, RAC1. Subsequent studies showed that systemic administration of a small molecule, an allosteric inhibitor of PTPσ to irradiated- or chemotherapy-treated mice promoted the early regeneration of HSCs, white blood cells, and neutrophils in vivo, while ex vivo treatment of irradiated human CD34+ hematopoietic stem and progenitor cells (HSPCs) similarly promoted the rescue of human NSG mice repopulating cells.
Advances in methods to perform single cell transcriptomic, genomic, and proteomic analysis have led to several studies characterizing the molecular profile of single human HSCs. These studies have revealed remarkable heterogeneity within phenotypically identical human HSCs in steady state and in response to growth factor treatment and have shown that HSC fate determinations can be related directly to expression levels of lineage-specific transcription factors. Single cell analysis of HSCs from aging donors revealed age-associated epigenetic reprogramming within cancer and developmental pathways, suggesting the basis for increased incidence of acute myeloid leukemia (AML) with aging (see Chapter 13, Chapter 19 ). Continued progress in the molecular and functional characterization of single human HSCs will undoubtedly lead to much improved definition of the human hematopoietic hierarchy and the more optimized selection of purified human HSCs for transplantation.
The colony-forming cell (CFC) assay does not measure HSC content but rather committed myeloid progenitor cell content via a 14-day assay for colonies within methylcellulose media that is supplemented with specific growth factors. The CFC assay measures colony-forming unit-granulocyte/macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E), and CFU-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM). The CFU-GEMM, or CFU-mix colonies, represent a more immature progenitor cell population. B- and T-cell progenitor cell content can also be measured via in vitro assays but requires specialized coculture conditions, which are described elsewhere.
The LT culture-initiating cell (LTC-IC) assay is a 6-week in vitro assay in which BM cells are cocultured with murine stromal cells for four weeks followed by replating of the entire culture system into methylcellulose and additional two-week assay for colony formation. The LTC-IC, unlike the CFC, measures a more immature stem/progenitor cell population, although the results of the LTC-IC are inherently dependent and limited by technical variabilities in stromal coculture experiments. Importantly, the LTC-IC population lacks LT repopulating cells because transplantation of LTC-ICs into mice in a competitive transplantation assay does not result in any LT reconstitution.
The cobblestone area-forming cell (CAFC) assay also involves coculture of HSCs with pre-established stromal cell monolayers and relies on microscopic quantification of cobblestone-forming cells embedded underneath the stromal layer. It has been shown that CAFC content correlates well with CFU-S content on day 12 and marrow repopulating capacity. However, similarly to the LTC-IC, the CAFC assay does not measure LT-HSCs. An advantage of the CAFC and LTC-IC assays is that the estimate of stem/progenitor cell content is not confounded by the homing capacity of the cell population being tested. However, competitive transplantation assays provide a more physiologically relevant measure of functional HSC content and allow quantification of LT-HSC content as well as homing efficiency.
The first reproducible in vivo assay for hematopoietic progenitor cells (HPCs) was the CFU-S assay, which was developed by Till and McCullough. In this assay, BM cells are injected into lethally irradiated mice, and macroscopic spleen colonies are measured from one to three weeks after injection. These colonies represent ST repopulating cell and MPP activity but do not measure LT-HSC content.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here